Abstract
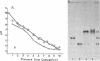
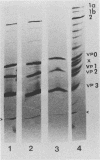
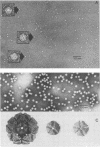
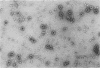
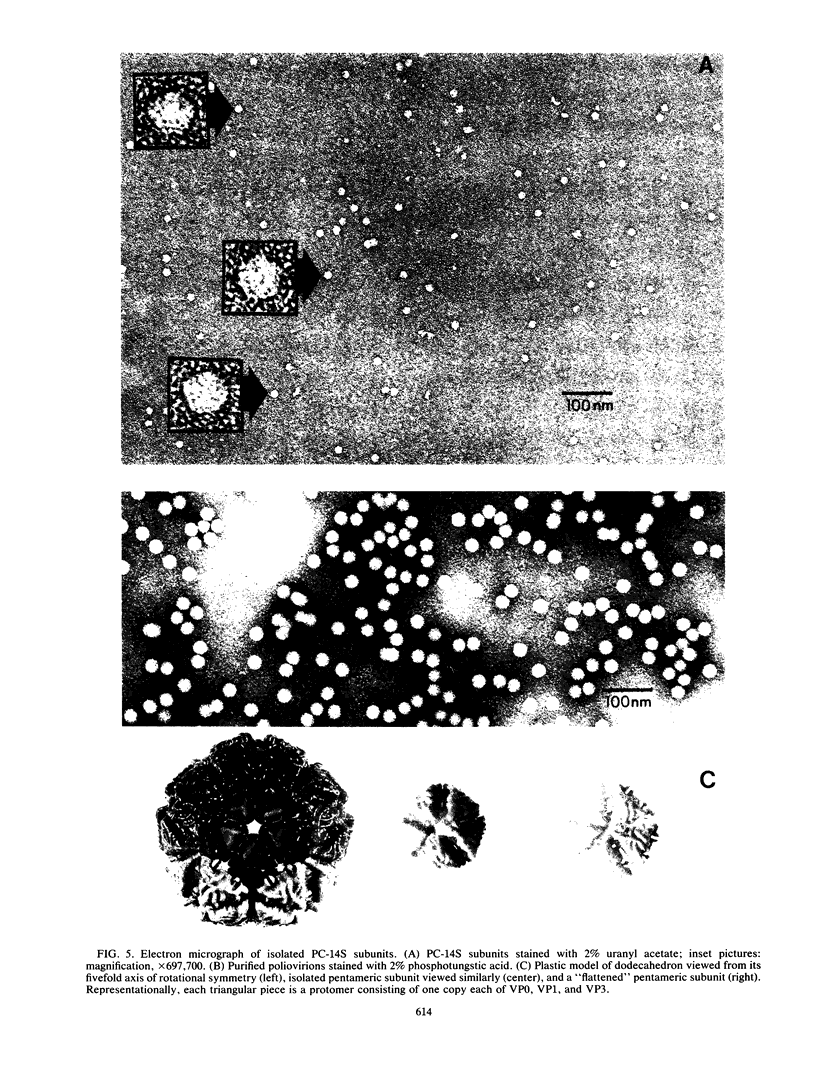
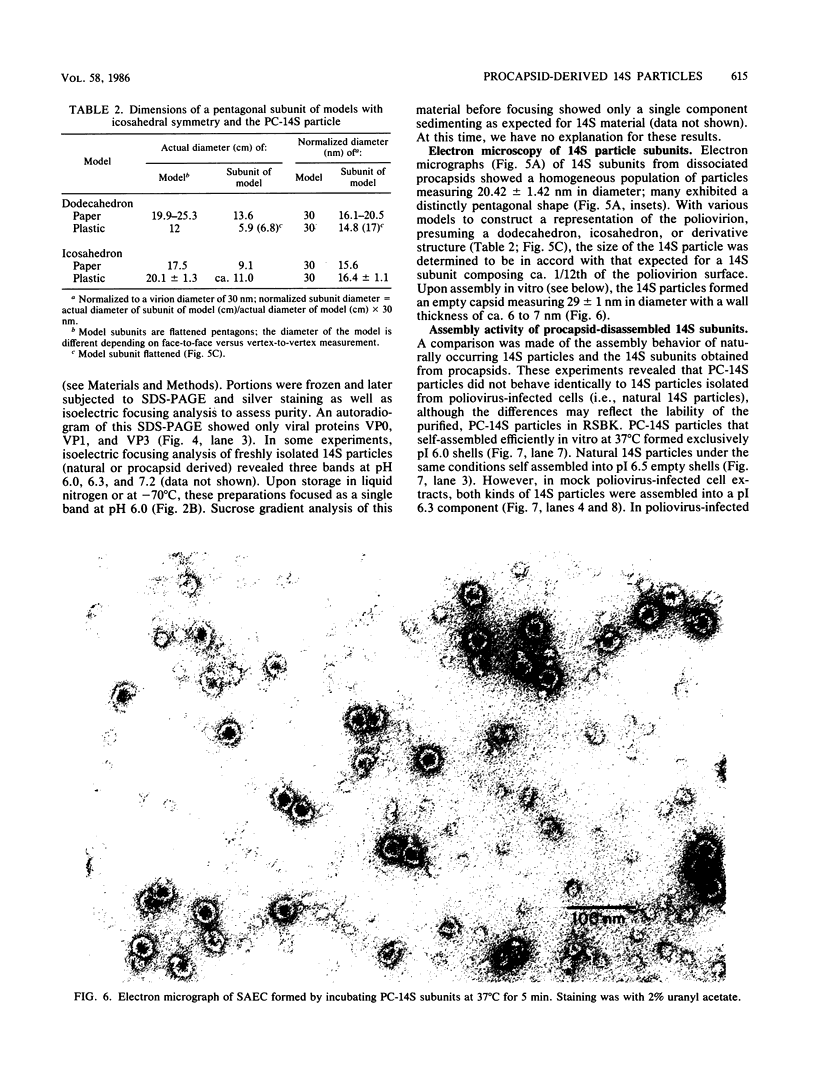
Highly purified 14S subunit particles were obtained from alkali-dissociated poliovirus type 1 procapsids (naturally occurring empty capsids in poliovirus-infected cells) to compare their morphological and biophysical properties with those of naturally occurring 14S particles. Procapsid-derived 14S particles (PC-14S), like naturally occurring 14S particles, were capable of self-assembly into an empty shell in buffer or extracts from uninfected cells. These empty capsids always exhibited pIs more acidic than those of procapsids but were themselves distinguishable by their respective pIs. Nevertheless, if PC-14S or naturally occurring 14S particles were incubated with extracts made from poliovirus-infected cells, procapsidlike empty shells were formed. This clearly showed that the 14S particle, however obtained, possesses the information to form an empty shell of correct dimensions but of improper conformation, unless a factor present in poliovirus-infected cells is present. With the electron microscope, the PC-14S subunit frequently was seen as a pentagonal structure with a diameter of 20.4 +/- 1.4 nm, a size somewhat larger than expected for a subunit composing 1/12th of the poliovirus surface. Upon self-assembly in vitro, the empty shell formed exhibited a diameter of 29 +/- 1 nm and a wall thickness of ca. 6 to 7 nm. It was necessary to avoid CsCl banding of procapsids in their preparation as this treatment altered both their pI and their sensitivity to alkali dissociation into 14S subunits. The relevance of these findings to the nature and role of procapsids and the requirement for a morphopoietic factor in poliovirus morphogenesis is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Drzeniek R., Bilello P. Reconstitution of poliovirus. Biochem Biophys Res Commun. 1972 Jan 31;46(2):719–724. doi: 10.1016/s0006-291x(72)80199-2. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- Marongiu M. E., Pani A., Corrias M. V., Sau M., La Colla P. Poliovirus morphogenesis. I. Identification of 80S dissociable particles and evidence for the artifactual production of procapsids. J Virol. 1981 Aug;39(2):341–347. doi: 10.1128/jvi.39.2.341-347.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Perlin M., Phillips B. A. In vitro assembly of polioviruses. 3. Assembly of 14 S particles into empty capsids by poliovirus-infected HeLa cell membranes. Virology. 1973 May;53(1):107–114. doi: 10.1016/0042-6822(73)90469-8. [DOI] [PubMed] [Google Scholar]
- Phillips B. A. In vitro assembly of poliovirus. II. Evidence for the self-assembly of 14 S particles into empty capsids. Virology. 1971 May;44(2):307–316. doi: 10.1016/0042-6822(71)90262-5. [DOI] [PubMed] [Google Scholar]
- Phillips B. A. In vitro assembly of polioviruses. I. Kinetics of the assembly of empty capsids and the role of extracts from infected cells. Virology. 1969 Dec;39(4):811–821. doi: 10.1016/0042-6822(69)90018-x. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Lundquist R. E., Maizel J. V., Jr Absence of subviral particles and assembly activity in HeLa cells infected with defective-interfering (DI) particles of poliovirus. Virology. 1980 Jan 15;100(1):116–124. doi: 10.1016/0042-6822(80)90557-7. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Summers D. F., Maizel J. V., Jr In vitro assembly of poliovirus-related particles. Virology. 1968 Jun;35(2):216–226. doi: 10.1016/0042-6822(68)90262-6. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Wiemert S. In vitro assembly of poliovirus. V. Evidence that the self-assembly activity of 14 S particles is independent of extract assembly factor(s) and host proteins. Virology. 1978 Jul 1;88(1):92–104. doi: 10.1016/0042-6822(78)90113-7. [DOI] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Differences between poliovirus empty capsids formed in vivo and those formed in vitro: a role for the morphopoietic factor. J Virol. 1981 Oct;40(1):173–183. doi: 10.1128/jvi.40.1.173-183.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Picornaviral structure and assembly. Microbiol Rev. 1981 Jun;45(2):287–315. doi: 10.1128/mr.45.2.287-315.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Poliovirus empty capsid morphogenesis: evidence for conformational differences between self- and extract-assembled empty capsids. J Virol. 1982 Mar;41(3):792–800. doi: 10.1128/jvi.41.3.792-800.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombaut B., Vrijsen R., Boeyé A. Epitope evolution in poliovirus maturation. Arch Virol. 1983;76(4):289–298. doi: 10.1007/BF01311196. [DOI] [PubMed] [Google Scholar]
- Rombaut B., Vrijsen R., Brioen P., Boeyé A. A pH-dependent antigenic conversion of empty capsids of poliovirus studied with the aid of monoclonal antibodies to N and H antigen. Virology. 1982 Oct 15;122(1):215–218. doi: 10.1016/0042-6822(82)90393-2. [DOI] [PubMed] [Google Scholar]
- WATANABE Y., WATANABE K., HINUMA Y. Synthesis of poliovirus-specific proteins in HeLa cells. Biochim Biophys Acta. 1962 Dec 31;61:976–977. doi: 10.1016/0926-6550(62)90015-4. [DOI] [PubMed] [Google Scholar]
- Yafal A. G., Palma E. L. Morphogenesis of foot-and-mouth disease virus. I. Role of procapsids as virion Precursors. J Virol. 1979 Jun;30(3):643–649. doi: 10.1128/jvi.30.3.643-649.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]