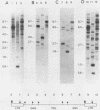
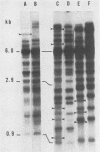
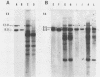
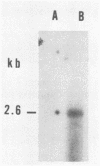
Abstract
A pedigreed breeding population of feral Mus cervicolor popaeus with a high incidence of mammary tumors, arising between 6 and 14 months of age, is described. These mice were chronically infected with a type B retrovirus which is distantly related to the mouse mammary tumor virus (MMTV) of inbred strains of Mus musculus. MMTV-induced mammary tumors in inbred mice frequently (80%) contained an insertion of the viral genome into the int-1 or int-2 loci of the tumor cellular genome. These two cellular genetic loci were also altered by viral insertion in 11 of 20 M. cervicolor popaeus mammary tumor cellular DNAs tested. Results of our study of mammary tumorigenesis in feral mice demonstrate that viral-induced rearrangement and activation of the int loci are not limited to the genetic background of inbred mice selected for highly infectious MMTV and a high incidence of mammary tumors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERVONT H. B. Biological studies on the mammary tumor inciter in mice. Ann N Y Acad Sci. 1952 Jul 10;54(6):1004–1011. doi: 10.1111/j.1749-6632.1952.tb39975.x. [DOI] [PubMed] [Google Scholar]
- ANDERVONT H. B., DUNN T. B. Occurrence of tumors in wild house mice. J Natl Cancer Inst. 1962 May;28:1153–1163. [PubMed] [Google Scholar]
- Alitalo K., Schwab M., Lin C. C., Varmus H. E., Bishop J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1707–1711. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Drohan W., Gallahan D., D'Hoostelaere L., Potter M. Novel class of mouse mammary tumor virus-related DNA sequences found in all species of Mus, including mice lacking the virus proviral genome. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4113–4117. doi: 10.1073/pnas.79.13.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Colcher D., Horan Hand P., Teramoto Y. A., Wunderlich D., Schlom J. Use of monoclonal antibodies to define the diversity of mammary tumor viral gene products in virions and mammary tumors of the genus Mus. Cancer Res. 1981 Apr;41(4):1451–1459. [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dickson C., Smith R., Brookes S., Peters G. Tumorigenesis by mouse mammary tumor virus: proviral activation of a cellular gene in the common integration region int-2. Cell. 1984 Jun;37(2):529–536. doi: 10.1016/0092-8674(84)90383-0. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Hand P. H., Teramoto Y. A., Callahan R., Schlom J. Interspecies radioimmunoassay for the major internal protein of mammary tumor viruses. Virology. 1980 Feb;101(1):61–71. doi: 10.1016/0042-6822(80)90483-3. [DOI] [PubMed] [Google Scholar]
- Morris V. L., Medeiros E., Ringold G. M., Bishop J. M., Varmus H. E. Comparison of mouse mammary tumor virus-specific DNA in inbred, wild and Asian mice, and in tumors and normal organs from inbred mice. J Mol Biol. 1977 Jul;114(1):73–91. doi: 10.1016/0022-2836(77)90284-4. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Nusse R., van Ooyen A., Cox D., Fung Y. K., Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984 Jan 12;307(5947):131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- Peters G., Brookes S., Smith R., Dickson C. Tumorigenesis by mouse mammary tumor virus: evidence for a common region for provirus integration in mammary tumors. Cell. 1983 Jun;33(2):369–377. doi: 10.1016/0092-8674(83)90418-x. [DOI] [PubMed] [Google Scholar]
- Peters G., Kozak C., Dickson C. Mouse mammary tumor virus integration regions int-1 and int-2 map on different mouse chromosomes. Mol Cell Biol. 1984 Feb;4(2):375–378. doi: 10.1128/mcb.4.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rongey R. W., Hlavackova A., Lara S., Estes J., Gardner M. B. Types B and C RNA virus in breast tissue and milk of wild mice. J Natl Cancer Inst. 1973 Jun;50(6):1581–1589. doi: 10.1093/jnci/50.6.1581. [DOI] [PubMed] [Google Scholar]
- Schlom J., Hand P. H., Teramoto Y. A., Callahan R., Todaro G., Schidlovsky G. Characterization of a new virus from Mus cervicolor immunologically related to the mouse mammary tumor virus. J Natl Cancer Inst. 1978 Dec;61(6):1509–1519. [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Varmus H. E., Bishop J. M., George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983 Jun 9;303(5917):497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stewart T. A., Pattengale P. K., Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell. 1984 Oct;38(3):627–637. doi: 10.1016/0092-8674(84)90257-5. [DOI] [PubMed] [Google Scholar]
- Teramoto Y. A., Hand P. H., Callahan R., Schlom J. Detection of novel murine mammary tumor viruses by interspecies immunoassays. J Natl Cancer Inst. 1980 Apr;64(4):967–975. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]