Abstract
Physiological conditions that impinge on constitutive traffic and affect organelle structure are not known. We report that osmotically induced cell volume changes, which are known to occur under a variety of conditions, rapidly inhibited endoplasmic reticulum (ER)-to-Golgi transport in mammalian cells. Both ER export and ER Golgi intermediate compartment (ERGIC)-to-Golgi trafficking steps were blocked, but retrograde transport was active, and it mediated ERGIC and Golgi collapse into the ER. Extensive tubulation and relatively rapid Golgi resident redistribution were observed under hypo-osmotic conditions, whereas a slower redistribution of the same markers, without apparent tubulation, was observed under hyperosmotic conditions. The osmotic stress response correlated with the perturbation of COPI function, because both hypo- and hyperosmotic conditions slowed brefeldin A-induced dissociation of βCOP from Golgi membranes. Remarkably, Golgi residents reemerged after several hours of sustained incubation in hypotonic or hypertonic medium. Reemergence was independent of new protein synthesis but required PKC, an activity known to mediate cell volume recovery. Taken together these results indicate the existence of a coupling between cell volume and constitutive traffic that impacts organelle structure through independent effects on anterograde and retrograde flow and that involves, in part, modulation of COPI function.
INTRODUCTION
A number of pharmacological studies suggest that constitutive transport can be regulated by signal transduction pathways (Davidson et al., 1992; De Matteis et al., 1993; Hansen and Casanova, 1994; Ohashi and Huttner, 1994; Pimplikar and Simons, 1994; Muniz et al., 1996), but very little is known about the upstream activators or physiological stimuli that alter secretory traffic through the modulation of these signaling molecules. Regulation of secretory traffic can be expected to have dramatic effects on organelle structure if it alters the balance between membrane flow into and out of an organelle. This is clearly exemplified by the effects of the fungal metabolite brefeldin A (BFA)1 on the Golgi apparatus. BFA inhibits protein secretion, at least in part, by blocking transport from the endoplasmic reticulum (ER) to the Golgi (Fujiwara et al., 1988; Doms et al., 1989; Lippincott-Schwartz et al., 1989). Within minutes of BFA addition, membrane tubules that contain Golgi resident markers emanate from the Golgi out toward the cell periphery along microtubules (Lippincott-Schwartz et al., 1990). These membrane tubules form a dynamic network that persists for a brief period before abruptly fusing with the ER (Sciaky et al., 1997). This dramatic Golgi–ER fusion and redistribution of Golgi residents to the ER has been attributed to a block in ER-to-Golgi transport coupled with stimulation of retrograde trafficking out of the Golgi back to the ER (Doms et al., 1989; Lippincott-Schwartz et al., 1989, 1990). More recently, a variety of manipulations that inhibit ER-to-Golgi and/or intra-Golgi transport have been shown to cause a BFA-like redistribution of Golgi residents to the ER. These include high-level expression of an ERD2-like protein, ELP-1, a putative receptor for the retrieval of escaped ER proteins (Hsu et al., 1992); overexpression of RAB6, a GTPase implicated in intra-Golgi transport (Martinez et al., 1997); overexpression of an altered version of ARF1, a GTPase involved in COPI-coated vesicle formation whose GTP exchange factor is a target of BFA action (Dascher and Balch, 1994); and treatment of cells with nordihydroguaiaretic acid (NDGA), an inhibitor of lipoxygenase (Fujiwara et al., 1998). It is not clear whether the Golgi resident redistribution induced by these manipulations represents a normal retrograde pathway underlying Golgi stasis, but it is interesting to note that only BFA appears to elicit significant Golgi tubulation during the redistribution. These observations underscore the importance of balanced membrane flow for maintenance of Golgi structure and raise a question of whether regulation of constitutive transport in interphase cells, which clearly has the potential to profoundly affect organellar integrity, ever occurs in response to physiological conditions. Significantly, the alterations in Golgi structure induced by drug treatment (either BFA or NDGA) are reversible; removal of the drug results in the reappearance of Golgi marker staining indistinguishable from that in untreated cells. Hence, cells possess the capacity to recover from dramatic perturbations in organellar structure.
One possible physiological regulator of constitutive transport is osmotic stress and its associated cell volume changes. Cell volume changes accompany a wide variety of metabolic states, such as hormonal stimulation, and in turn cell volume changes modify a variety of metabolic functions (Lang et al., 1998). Indeed, cell volume changes induced by hypo- or hyperosmotic conditions have been shown to activate signaling cascades in a variety of cell types. Cell swelling, for example, leads to a rapid rise in intracellular cAMP and Ca2+ (Watson, 1989), as well as to the activation of a number of protein kinases, including phosphatidylinositol 3-kinase, PKC, and members of the MAPK family of protein kinases (Lang et al., 1998). At least a subset of these signaling pathways are involved in mediating cell volume regulatory mechanisms (Hoffmann and Simonsen, 1989; Lang et al., 1998; Roman et al., 1998) that allow regulatory volume decrease (RVD) in response to hypotonically induced cell swelling and regulatory volume increase in response to hypertonically induced cell shrinkage. These volume regulatory mechanisms are highly conserved, indicating their importance for cell survival. In fission yeast, osmotic stress leads to changes in vacuolar fission and fusion pathways that result in dramatic alterations in vacuolar morphology (Bone et al., 1998). In mammalian cells, hypertonic treatment is reported to inhibit ER-to-Golgi transport (Docherty and Snider, 1991), but it is not known whether alterations in Golgi structure accompany a hypertonically induced transport block or whether ER-to-Golgi transport is also affected by hypotonic treatment. Therefore, it remains to be tested whether osmotically induced cell volume increases and decreases represent physiological conditions that alter trafficking and organelle integrity in the early secretory pathway.
We observed that both hypo- and hyperosmotic conditions abruptly inhibited ER-to-Golgi transport in a variety of mammalian cultured cell lines. Both ER export and ER Golgi intermediate compartment (ERGIC)-to-Golgi transport steps were blocked, but backward transport from the ERGIC to the ER and from Golgi to ER took place, leading to collapse of the ERGIC and Golgi into the ER. Hypo-osmotically induced retrograde movement of Golgi residents was stimulated, relative to hypertonic, and was accompanied by extensive Golgi tubulation. The ability of COPI, a protein complex that forms transport vesicle coats at the ERGIC (Rowe et al., 1996) and Golgi (Orci et al., 1986; Malhotra et al., 1989; Serafini et al., 1991; Waters et al., 1991) and that is implicated in the prevention of Golgi tubulation (Scheel et al., 1997), to bind Golgi membranes was apparently unaffected by osmotic stress. However, the dissociation of COPI from Golgi membranes, assayed by inducing dissociation with BFA (Donaldson et al., 1990), was significantly slowed. This suggests that the osmotic stress response may be due, in part, to the inhibition of COPI dissociation. After several hours of sustained incubation in either hypo- or hypertonic medium, Golgi residents reemerged, even in the absence of protein synthesis. The reemergence appeared to correspond to recovery of normal cell volume by homeostatic mechanisms, because PKC inhibition, a condition that prevents cell volume recovery (Roman et al., 1998), prevented reemergence but not the initial Golgi collapse. These observations reveal an osmotic stress response, apparently coupled to volume changes, that dramatically affects membrane transport and organelle integrity in the early secretory pathway.
MATERIALS AND METHODS
Cell lines, Antibodies, and Reagents
COS-7 cells were maintained in Dulbecco’s modified Eagle’s medium (Life Technologies, Gaithersburg, MD) supplemented with 10% calf serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin (normal medium) at 37°C in a 5% CO2 incubator. HeLa cells were maintained similarly in minimum essential medium (MEM) (Life Technologies) supplemented with 10% FBS, and Chinese hamster ovary (CHO) cells were maintained in MEM-alpha (Life Technologies) supplemented with 10% FBS.
The following antibodies were used: a rabbit polyclonal antiserum against GM130 (provided by E. Sztul, University of Birmingham, Birmingham, AL); a mouse mAb against the cytoplasmic tail of vesicular stomatitis virus glycoprotein (VSV-G; provided by P. Weidman, St. Louis University, St. Louis, MO); a mouse mAb against ERGIC 53 and a mouse mAb against p63 (provided by H.-P. Hauri, Biocenter, Basel, Switzerland); a mouse mAb against βCOP (provided by V. Malhotra, University of San Diego, San Diego, CA, and also purchased from Sigma, St. Louis, MO [mAD]); an affinity-purified rabbit antiserum against SEC13 (provided by B.L. Tang and W. Hong, National University of Singapore, Singapore); a mouse mAb against the hemagglutinin (HA) epitope; a mouse mAb against GPP130 (A1/118); and a rabbit polyclonal antiserum against giantin. FITC- and rhodamine-conjugated secondary antibodies against mouse or rabbit immunoglobulins were purchased from Cappel (Organon Teknika, Durham, NC).
The cDNA encoding DPPIV was provided by A. Hubbard (Johns Hopkins Medical School, Baltimore, MD) and cloned into the PECE expression vector with an N-terminal HA epitope. Temperature-sensitive O45-VSV (ts-O45-VSV) was provided by P. Weidman.
BFA and calphostin C were purchased from Sigma and stored as 10 mg/ml and 1 mM stock solutions in DMSO at −20 and 4°C, respectively. Tran35S-label was purchased from DuPont NEN (Boston, MA). Cycloheximide was purchased from Sigma and added at 100 μg/ml directly to the medium just before use.
Osmotic Stress Treatments
HeLa or COS-7 cells were grown in 24-well plates on 12-mm glass coverslips in normal medium to 50–75% confluence. For the hypo- and hypertonic treatments in all figures except Figure 1B, a glass coverslip was removed from normal medium and placed in 1 ml of hypotonic medium (60 mM NaCl, 20 mM HEPES, pH 7.2, 2.5 mM MgOAc [210 mOsm]) or 1 ml of hypertonic (610 mOsm) medium (normal medium plus 25 mM KHEPES, pH 7.2, 0.4 M sucrose) in a 24-well plate, and the entire plate was placed in a 37°C water bath. In Figure 1B, media compositions were 90 mM NaCl, 20 mM HEPES, pH 7.2, 2.5 mM MgOAc (270 mOsm); 60 mM NaCl, 20 mM HEPES, pH 7.2, 2.5 mM MgOAc, 0.1 M sucrose (310 mOsm); and normal medium plus 0.2 M sucrose (410 mOsm).
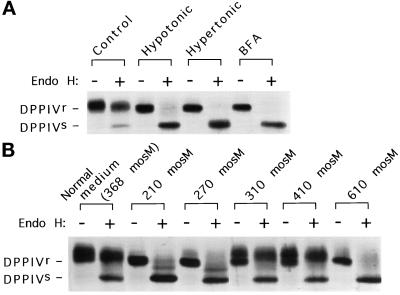
Figure 1.
ER-to-Golgi transport is sensitive to changes in extracellular osmolarity. (A) Both hypo- and hyperosmotic conditions block ER-to-Golgi transport. COS-7 cells transfected with HA epitope-tagged DPPIV were metabolically labeled for 30 min, chased in normal medium for 10 min, and placed in normal medium, hypotonic medium, hypertonic medium, or normal medium plus 10 μg/ml BFA for 110 min at 37°C. The DPPIV from each plate was isolated and analyzed for the acquisition of endo H resistance as described in MATERIALS AND METHODS. Endo H-sensitive (s) and resistant (r) forms of DPPIV are marked. (B) The response of ER-to-Golgi transport to extracellular osmolarity is threshold in nature. The assay in A was performed under a range of osmotic (media compositions as detailed in MATERIALS AND METHODS) conditions.
Immunofluorescence Microscopy
Cells were fixed for 20 min in 3% paraformaldehyde, and antibody staining was performed according to the method of Linstedt et al. (1997). Cells were analyzed using a fluorescence microscope (Nikon, Melville, NY) equipped with a Hamamatsu black-and-white cooled charge-coupled device camera (Hamamatsu Photonics, Hamamatsu City, Japan). All images were taken with a 40× objective. Digital images were acquired in the program Photoshop (Adobe Systems, Mountain View, CA).
Transport Assay
COS-7 cells grown on 35-mm plates to 75% confluence were transiently transfected with HA epitope-tagged dipeptidyl peptidase IV (DPPIV) in the PECE vector as described previously (Linstedt et al., 1997). Two days after transfection, cells were starved for 30 min in Dulbecco’s modified Eagle’s medium–methionine–cysteine (Sigma) at 37°C, followed by the addition of 160 μCi of Tran35S-label in 1 ml of starvation medium to each plate. After 30 min at 37°C, the label was removed from each plate and replaced with normal medium. After 10 min at 37°C, the normal medium was replaced with normal medium, normal medium in the presence of 10 μg/ml BFA, hypotonic medium (described above), or hypertonic medium (described above). After 110 min at 37°C, each plate was washed three times with cold PBS and scraped into 0.2 ml of 1% SDS, 20 mM Tris, pH 8.0, 150 mM NaCl. The lysates were boiled 5 min, passed through a 25-gauge needle, and spun in a microfuge at 14,000 × g for 15 min at room temperature. The supernatant from each was diluted fivefold into 1% Triton X-100, 20 mM Tris, pH 8.0, 150 mM NaCl, 1 mM EDTA, and added to 10 μl of protein A beads and rotated at 4°C for 30 min. The unbound material was added to 10 μl of protein A beads precoated with rabbit anti-mouse antibodies and the mouse mAb against the HA epitope. After rotation at 4°C for 12 h, the beads were collected and washed five times with radioimmunoprecipitation assay buffer. Each sample was split in half and either left untreated or digested with endoglycosidase H (endo H; obtained from NEB, Beverly, MA, and according to their protocol). Treated and untreated samples were boiled in sample buffer, resolved by SDS-PAGE on a 7% gel, and exposed to autoradiography.
ts045-VSV Infection
CHO cells were grown on 12-mm glass coverslips in a 24-well plate to 75% confluence. ts-O45-VSV (∼25 pfu/cell) in 0.2 ml of MEM-alpha, buffered with 25 mM KHEPES, pH 7.4, was added to each well, and the entire plate was placed at 32°C for 45 min; 0.8 ml of MEM-alpha plus 10% FBS was then added to each well, and the plate was placed at 39.5°C. After 3.5 h, the medium in each well was replaced with MEM-alpha plus 10% FBS containing 100 μg/ml cycloheximide and placed at 39.5°C for an additional 30 min.
Density Gradient Fractionation
HeLa cells, grown to confluence on 15-cm plates, were incubated in serum-free normal medium, serum-free normal medium containing 10 μg/ml BFA, or hypotonic medium (210 mOsm) at 37°C for 60 min. Each plate was washed twice with ice-cold PBS and scraped into 20 ml of PBS. All subsequent steps were at 4°C and essentially as described by Jesch and Linstedt (1998). In brief, the cells were pelleted and washed with 10 ml of sucrose wash buffer (0.25 M sucrose, 10 mM triethanolamine, pH 7.4, 1 mM EDTA), and resuspended with 0.25 ml of homogenization buffer (50 mM NaCl, 10 mM triethanolamine, pH 7.4, 1 mM EDTA) containing 1 mM PMSF and 10 μg/ml leupeptin, pepstatin, and aprotinin. Cells were homogenized by 20 passages through a 25-gauge needle and centrifuged at 1000 × g for 1 min. Each postnuclear supernatant was adjusted to 1.6 M sucrose (from a 2 M stock), and 1.5 ml of each sample was overlayed with 1.1 ml each of 1.4, 1.2, and 0.8 sucrose solutions in 5 mM KHEPES, pH 7.4. Gradients were centrifuged in an SW50.1 rotor (Beckman Instruments, Palo Alto, CA) at 100,000 × g for 105 min. Fractions (0.4 ml) were collected from the top, and each fraction was precipitated with 20% TCA with 0.5% Triton X-100 added as a carrier. Pellets were dissolved in sample buffer and resolved by SDS-PAGE on a 7% gel. Immunoblotting, using antibodies against GPP130 and p63, was performed as previously described.
Quantitation of βCOP Binding to Golgi Membranes
HeLa cells grown on 12-mm coverslips were treated as described in the figure legends and fixed in 3% paraformaldehyde for double immunofluorescent staining with the mouse mAb against βCOP and the rabbit polyclonal antibody against giantin. Images taken with a 40× objective were acquired as described above and analyzed in NIH Image. The mean fluorescence intensity per pixel of βCOP staining in a fixed circle approximately enclosing the Golgi region was measured. In BFA-treated cells, which have no apparent Golgi staining, the brightest juxtanuclear region was chosen.
RESULTS
Osmotic Stress Inhibits ER-to-Golgi Transport
Cell volume changes induced by changes in extracellular osmolarity lead to the activation of multiple signal transduction cascades and alterations in various metabolic pathways, resulting in an overall change in cellular physiology (Lang et al., 1998). We therefore asked whether the early constitutive secretory pathway might also be responsive to changes in osmotic conditions. The general efficacy of constitutive transport between the ER and Golgi, under hypo- or hyperosmotic conditions, was assessed by monitoring the acquisition of endo H resistance, a standard indicator of the arrival of newly synthesized proteins in the Golgi (Kornfeld and Kornfeld, 1985). COS-7 cells that had been transiently transfected with DPPIV, a plasma membrane marker (Hong et al., 1989), were pulse labeled with [35S]methionine, chased for a brief period in normal medium, and then further chased in normal medium, hypotonic medium, hypertonic medium, or normal medium in the presence of BFA. The latter condition is known to block ER-to-Golgi transport (Fujiwara et al., 1988; Doms et al., 1989; Lippincott-Schwartz et al., 1989), but it is also known that ER-localized DPPIV eventually (≥4 h) acquires endo H resistance in BFA-treated cells because of the redistribution of Golgi glycosylation enzymes to the ER (Fujiwara et al., 1998). Therefore, we performed the experiment with a 2-h chase time to assess ER-to-Golgi transport independent of the effects of Golgi redistribution. After 2 h of chase, the cells were lysed, and DPPIV was immunoprecipitated. Each immunoprecipitate was split in half and either left untreated or digested with endo H. Figure 1A shows that although DPPIV acquired endo H resistance in normal medium, it remained completely sensitive to endo H in either hypo- or hypertonic medium or in the presence of BFA, indicating that ER-to-Golgi transport was potently inhibited under hypotonic as well as hypertonic conditions. Figure 1B illustrates the threshold response of ER-to-Golgi transport to changes in medium osmolarity. Transport occurred efficiently between 310 and 410 mOsm. However, relatively small changes either below or above the permissive osmolarity range, 13 and 33% respectively, greatly inhibited ER-to-Golgi transport. It should be noted that the 310-mOsm medium (permissive for transport) was identical to the 210-mOsm medium (nonpermissive for transport) except for the addition of 0.1 M sucrose, arguing that the hypotonity of the 210-mOsm medium, rather than the absence of an unidentified component of normal medium, was responsible for the block in forward transport. Altogether, these results indicated that osmotically induced cell volume changes, a physiological regulator of a variety of metabolic pathways, regulates the early constitutive secretory pathway.
ER Export and ERGIC-to-Golgi Transport Steps Are Blocked
Transport between the ER and Golgi consists of two steps: an ER-to-ERGIC step followed by an ERGIC-to-cis-Golgi step (Bannykh and Balch, 1997; Presley et al., 1997; Scales et al., 1997). Thus the osmotically induced block in ER-to-Golgi transport could be due to a block in either step or both. To dissect the response of the early secretory pathway to osmotically induced cell volume changes, we used ts-O45-VSV-G (Gallione and Rose, 1985) to monitor the transport steps. CHO cells infected with ts-O45-VSV were first maintained at 39.5°C to trap newly synthesized VSV-G in the ER (Doms et al., 1987) and then shifted to the permissive temperature in either normal or hypotonic medium (210 mOsm). Cycloheximide was added during the last 30 min of the 39.5°C incubation in normal medium as well as all subsequent incubations to prevent new synthesis of VSV-G. As expected, VSV-G was in the ER in cells maintained at 39.5°C (Figure 2A). Upon shift to 32°C in normal medium, VSV-G exited the ER and was transported to the Golgi by 15 min (Figure 2B). However, when cells were shifted to 32°C in hypotonic medium, VSV-G was detected only in the ER at 15 min (Figure 2C) and also at the earlier 5- and 10-min time points (our unpublished results), suggesting that the protein failed to exit the ER. Similar results were obtained under hyperosmotic (610-mOsm) conditions (our unpublished results). Next, we examined the effect of osmotic stress on ERGIC-to-Golgi transport. Again, VSV-infected cells were maintained at 39.5°C for 4 h but subsequently shifted to 15°C for 1.5 h to allow the accumulation of VSV-G in the ERGIC (Schweizer et al., 1988, 1990; Lotti et al., 1992) and then shifted to 32°C in either normal or hypotonic medium (210 mOsm). Again, cycloheximide was added during the last 30 min of the 39.5°C incubation as well as in all subsequent incubations to prevent new synthesis of VSV-G. As expected, VSV-G exhibited an ERGIC-like pattern in cells incubated at 15°C for 1.5 h (Figure 3, A and D). Upon shift to 32°C in normal medium, VSV-G was successfully transported to the Golgi by 20 min (Figure 3B) and to the cell surface by 90 min (Figure 3C). In contrast, at 20 min in hypotonic medium, VSV-G remained in the ERGIC, and some also appeared to be in the ER (Figure 3E). Clearly, transport between the ERGIC and the Golgi was perturbed in hypotonic medium. Closer examination of the hypo-osmotically treated cells suggested that the block in ERGIC-to-Golgi transport might be due, at least in part, to the redistribution of the ERGIC itself to the ER: a longer incubation in hypotonic medium led to the redistribution of the bulk of the VSV-G, which had started out in the ERGIC, to the ER (see Figure 5F). These observations suggested that, in addition to blocking export out of the ER and transport between the ERGIC and the Golgi, hypo-osmotic conditions were inducing the collapse of the ERGIC itself into the ER.
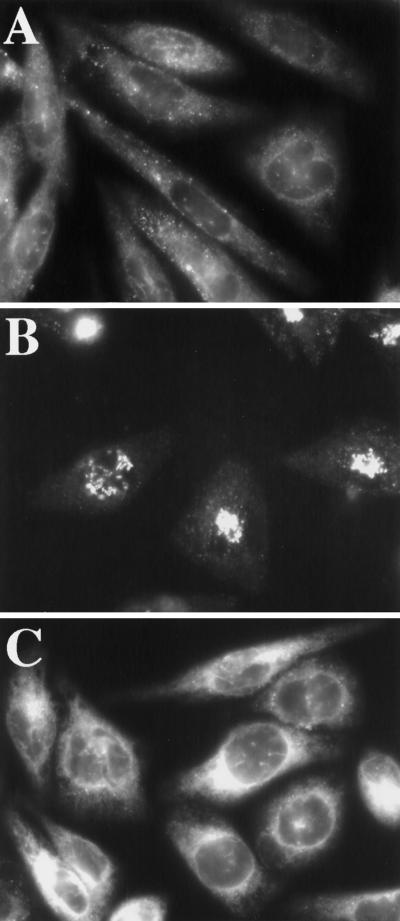
Figure 2.
Hypo-osmotic conditions block the export of VSV-G from the ER. CHO cells were infected with ts-O45-VSV as described in MATERIALS AND METHODS and maintained at 39.5°C for 4 h to trap VSV-G in the ER. During the last 30 min of the 39.5°C incubation and in all subsequent incubations, 100 μg/ml cycloheximide was added to prevent new protein synthesis. Cells were shifted to 32°C in normal medium (B) or hypotonic (210-mOsm)medium (C) or maintained at 39.5°C (A) for 15 min, after which cells were fixed and processed for immunofluorescence using a mAb against VSV-G.
Figure 3.
Hypo-osmotic conditions block transport of VSV-G from the ERGIC to the Golgi and causes VSV-G to redistribute from the ERGIC to the ER. CHO cells were infected with ts-O45-VSV and maintained at 39.5°C for 4 h. During the last 30 min of the 39.5°C incubation and in all subsequent incubations, 100 μg/ml cycloheximide was added to prevent new protein synthesis. Cells were then shifted to 15°C in normal medium to allow VSV-G to move into but not out of the ERGIC. After 1.5 h (see A), cells were shifted to 32°C in normal medium (B and C) or hypotonic (210-mOsm) medium (E and F) for 20 min (B and E) or 90 min (C and F) or maintained in normal medium at 15°C for 90 min (D). Cells were fixed and processed for immunofluorescence using a mAb against VSV-G.
Figure 5.
Hypo-osmotic conditions induce the tubulation and redistribution of GPP130 to the ER. COS-7 cells, grown to 50% confluence on 12-mm glass coverslips, were removed from normal medium and placed in 1 ml of hypotonic (210-mOsm) medium for 30 min (B) or 120 min (C) or hypotonic medium plus 0.1 M sucrose (D) for 30 min. Cells maintained in normal medium are shown in A. Cells were fixed and processed for immunofluorescence using a mAb against GPP130.
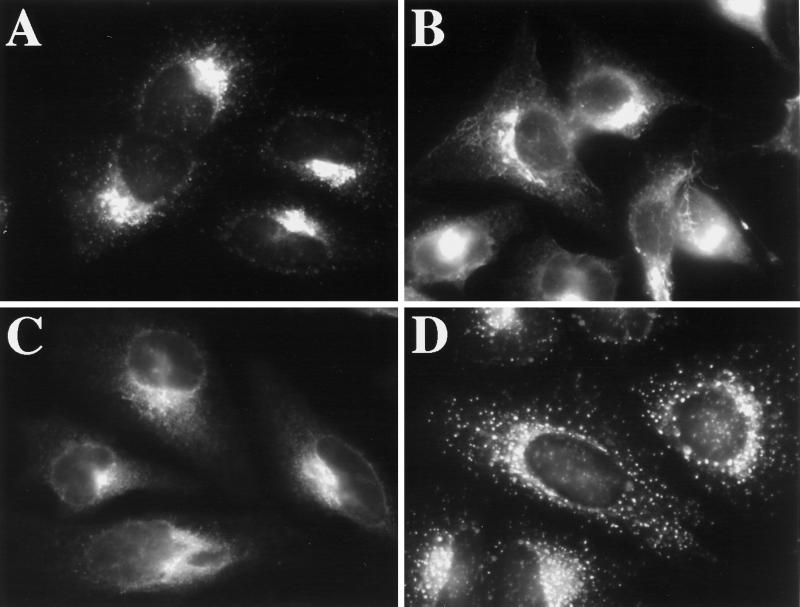
Osmotic Stress Causes ERGIC 53 to Redistribute to the ER
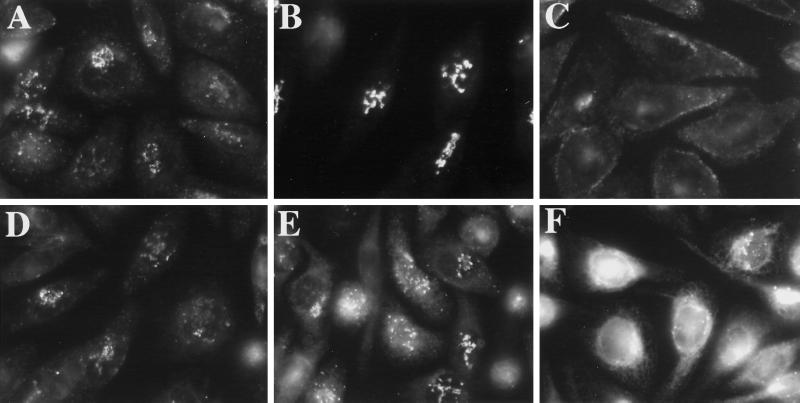
To directly examine the effect of hypo-osmotic conditions on the ERGIC, we looked, by indirect immunofluorescence, at the distribution of the ERGIC marker ERGIC 53 (Schweizer et al., 1988) upon incubation in hypotonic medium (210 mOsm). Figure 4 shows that hypo-osmotic treatment indeed induced the redistribution of the bulk of ERGIC 53 from its normal punctate distribution (Figure 4A) to a reticular ER distribution (Figure 4C) after 30 min at 37°C. At an intermediate time point of 15 min, ERGIC 53 was present, in most cells, in extensive tubules, suggesting that its redistribution might be mediated, at least in part, by tubules (Figure 4B). Interestingly, the distribution of ERGIC 53 under hypo-osmotic conditions (Figure 4, B or C) clearly differed from its distribution in BFA (Figure 4D). This is significant because the ERGIC is thought to be maintained in the presence of BFA as a result of ongoing ER export (Lippincott-Schwartz et al., 1990). The hypotonically induced redistribution of the majority of ERGIC 53 to the ER is consistent with our observation that ER export was blocked under hypo-osmotic conditions. Hyperosmotic conditions (610 mOsm) induced a similar redistribution of ERGIC 53 to the ER (our unpublished results).
Figure 4.
Hypo-osmotic conditions induce the redistribution of ERGIC 53 to the ER. HeLa cells were grown to 50% confluence on 12-mm glass coverslips. Coverslips were removed from normal medium and placed in 1 ml of hypotonic (210-mOsm) medium for 15 min (B) or 30 min (C). Cells maintained in normal medium are shown in A, and cells incubated in normal medium with 10 μg/ml BFA for 60 min are shown in D. Cells were fixed and processed for immunofluorescence using a mAb against ERGIC 53.
Hypo-osmotic Conditions Cause Tubulation of the Golgi and Redistribution of Golgi Residents to the ER
Based on previous studies, if retrograde traffic were to persist during osmotic stress, we would expect the block in anterograde transport to cause Golgi residents to redistribute to the ER. To address whether Golgi residents, in common with ERGIC 53, redistribute to the ER, we examined the distribution of a cis-Golgi integral membrane marker, GPP130 (Linstedt et al., 1997) in COS-7 cells that had been exposed to hypotonic medium (210 mOsm). In interphase cells maintained in normal medium, few if any deviations from a normal perinuclear Golgi pattern were observed (Figure 5A). After a 30-min incubation in hypotonic medium, GPP130 relocalized to a reticular network of long membrane tubules that emanated from the Golgi region (Figure 5B). These extensive membrane tubules, observable in 15% of the cells at this time (another 10% of the cells exhibited only a few tubules), were reminiscent of the tubules that mediate the BFA-induced movement of Golgi residents into the ER (Lippincott-Schwartz et al., 1990; Sciaky et al., 1997). Consistent with the idea that these hypotonically induced tubules were intermediates in the redistribution of GPP130 to the ER, at 60 min, the percentage of cells with tubule-localized GPP130 decreased, and 20% of the cells now exhibited ER-localized GPP130 (Figure 5C). Complete GPP130 redistribution was never observed; after 120 min in hypotonic medium, only 43% of the cells exhibited significant ER localization (see below for an explanation). Inclusion of 100 mM sucrose in the hypotonic medium prevented the redistribution of GPP130 (Figure 5D), arguing that the hypotonicity of the medium and not the depletion of an unidentified component(s) of normal medium was the stimulus for the dramatic redistribution of GPP130.
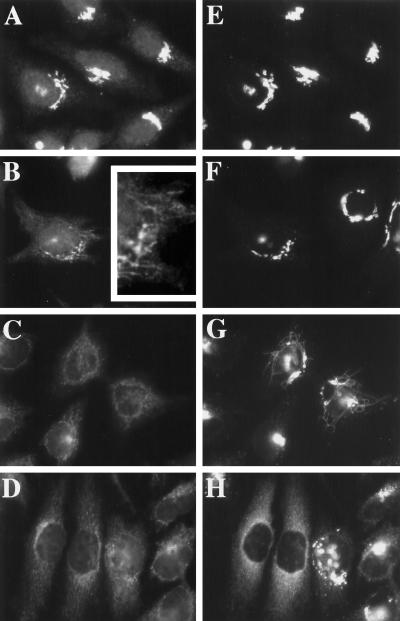
The tubulation and redistribution response was not cell type specific or specific for GPP130. Figure 6 compares the response of GM130 (Nakamura et al., 1995), another cis-Golgi resident (Figure 6, A–D), with that of GPP130 (Figure 6, E–H) in double-labeled HeLa cells at 0, 5, 30, and 120 min of hypo-osmotic treatment. As expected, GM130 and GPP130 patterns were indistinguishable in normal medium (Figure 6, A and E). Upon hypotonic treatment, both GM130 and GPP130 redistributed with time from the Golgi to the ER. There were, however, notable differences in the behavior of the two markers. First, the redistribution of GM130 occurred with much faster kinetics (t½, ∼5–10 min) than GPP130 (t½, ∼60–120 min) such that, at 30 min, most cells exhibited ER-localized GM130 (Figure 6C), whereas a significant fraction of GPP130 was still Golgi and/or vesicle and tubule localized (Figure 6G). GM130 tubules were shorter and observed only at the earliest, 5 min time point (Figure 6B, inset), before the appearance of GPP130 tubules (Figure 6F). The second difference in the behavior of the two markers was the extent to which each marker redistributed to the ER. GM130 was completely redistributed in nearly every cell by 30 min (Figure 6C). In contrast, similar to what we observed in COS-7 cells, only 10–20% of the cells exhibited completely ER-localized GPP130 at 120 min of hypotonic treatment (Figure 6H; see below for an explanation). Other Golgi markers tested, including mannosidase II (Novikoff et al., 1983) and giantin (Linstedt and Hauri, 1993), also redistributed to the ER under hypo-osmotic conditions (our unpublished results). Golgi residents fell into two classes: the fast class, comprising GM130 and mannosidase II, redistributed rapidly and completely; and the slow class, comprising GPP130 and giantin, redistributed more slowly and incompletely. Hyperosmotic conditions also induced the redistribution of Golgi residents in both HeLa and COS-7 cells (our unpublished results), but strikingly, tubulation was only observed under hypo-osmotic conditions, and overall, the hypertonically induced Golgi-to-ER redistribution was considerably slower for each marker (t½, ∼30 min for GM130) compared with the hypotonically induced redistribution (t½, ∼5–10 min for GM130). Altogether, these observations suggested that although anterograde transport was completely inhibited under osmotic stress, retrograde traffic was active, leading to the apparent redistribution of Golgi residents to the ER.
Figure 6.
Hypo-osmotic conditions induce the redistribution of other Golgi residents to the ER. HeLa cells, grown to 50% confluence on 12-mm glass coverslips, were removed from normal medium and placed in 1 ml of hypotonic medium for 5 min (B and F), 30 min (C and G), or 120 min (D and H). The inset in B shows an enlargement of a portion of the cell shown in B. Cells maintained in normal medium are shown in A and E. Cells were fixed and processed for double-label immunofluorescence using a polyclonal antiserum against GM130 (A–D) and a mAb against GPP130 (E–H).
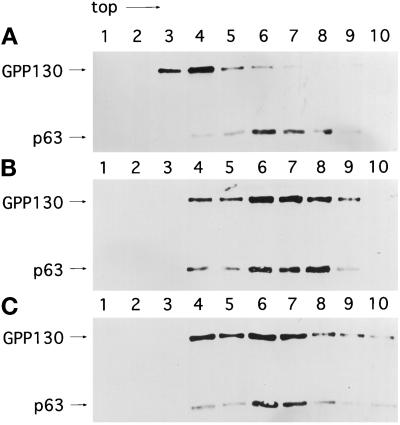
As a further test of whether hypotonic conditions induced the redistribution of Golgi residents to the ER, we performed density gradient fractionation to compare the relative distribution of the Golgi, marked by GPP130, and the ER, marked by p63 (Schweizer et al., 1995), in untreated and hypotonically treated cells, with BFA-treated cells as a control. As expected, the Golgi and ER were resolved after fractionation of untreated cells. The Golgi marker was recovered primarily in fractions 3 and 4, and the ER marker was recovered mostly in fractions 6 and 7 (Figure 7A). In contrast, the Golgi exhibited a significant redistribution to the ER-containing fractions in BFA-treated cells (Figure 7B) and in hypotonically treated cells (Figure 7C), as would be expected for Golgi redistribution into the ER. In correspondence with the morphological data presented above, the redistribution of the Golgi marker GPP130 was extensive but not complete after 60 min of hypotonic treatment. Altogether, our observations strongly suggest that the cell volume changes that accompany hypo-osmotic stress induce the redistribution of Golgi residents to the ER.
Figure 7.
Density gradient fractionation of ER and Golgi in untreated (A), BFA-treated (B), and hypotonically treated (C) cells. Fifteen-centimeter cm plates of confluent HeLa cells were incubated in normal medium without serum (A), normal medium without serum in the presence of 10 μg/ml BFA (B), or hypotonic medium (210 mOsm) (C) for 60 min at 37°C. The postnuclear supernatant obtained from each plate was fractionated in a sucrose step flotation gradient (as described in MATERIALS AND METHODS) and probed with antibodies against GPP130 (Golgi) and p63 (ER).
Osmotic Stress Slows the BFA-induced Rapid Dissociation of βCOP from Golgi Membranes
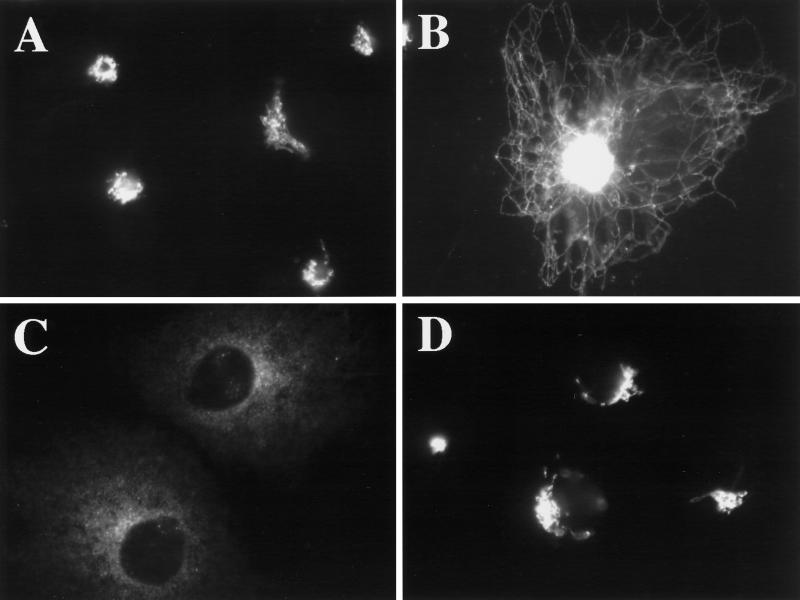
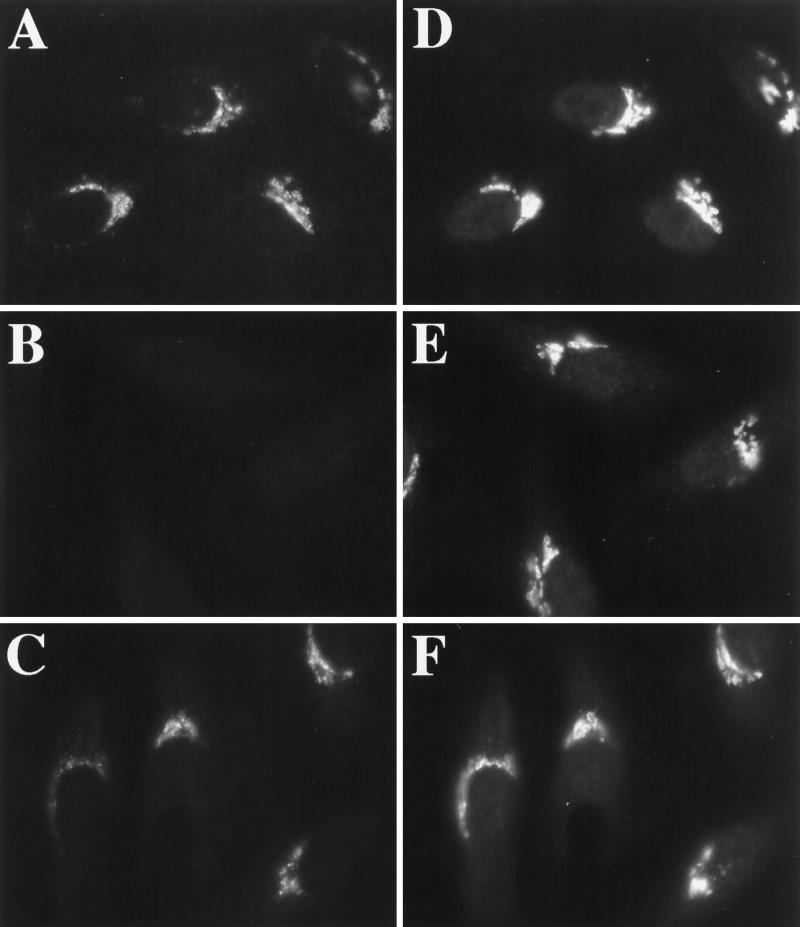
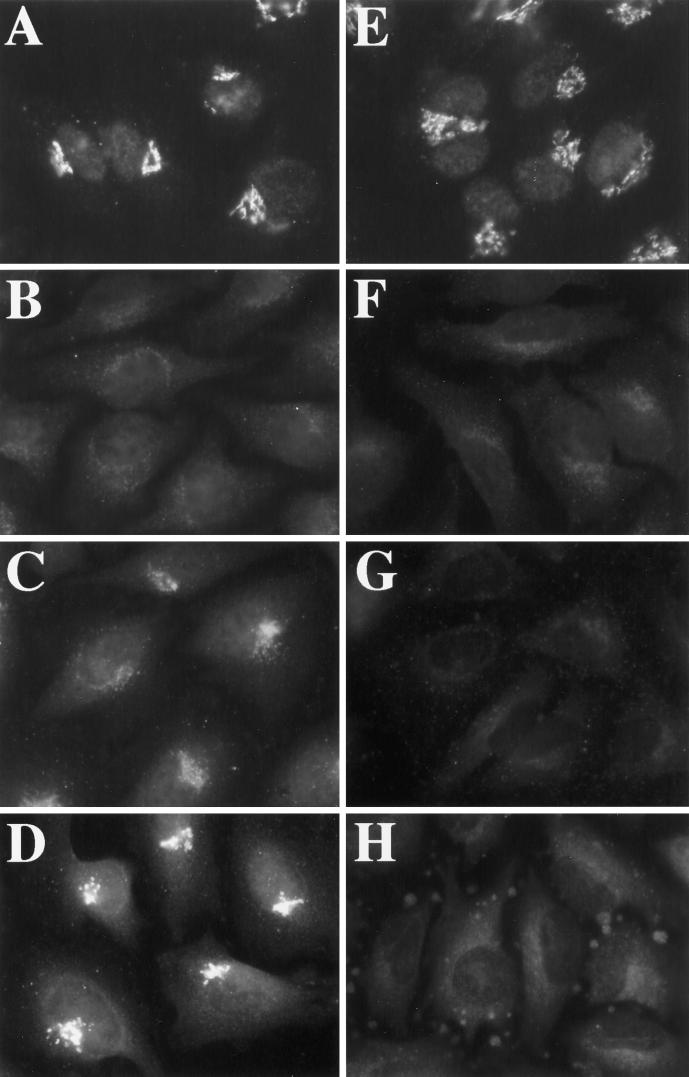
Osmotic stress caused an ER export block but allowed (or induced) retrograde trafficking of ERGIC and Golgi proteins. This suggested that vesicle production but not membrane fusion was inhibited. Given the critical role that the recruitment of vesicle coats (COPI and COPII) play in budding reactions between the ER and Golgi (Barlowe et al., 1994; Aridor and Balch, 1996; Schekman and Orci, 1996), and given that dramatic tubulation of the Golgi is known to be associated with perturbation of COPI function (Allan and Kreis, 1986; Donaldson et al., 1990; Lippincott-Schwartz et al., 1990; Duden et al., 1991; Elazar et al., 1994) we asked whether, like BFA, hypo- or hyperosmotic conditions might prevent the association of COPI with Golgi membranes (Donaldson et al., 1990). Contrary to our expectations, COPI (assayed by βCOP immunoreactivity) remained clearly associated with Golgi membranes under both hypo- and hyperosmotic conditions, at least out to 30 min (our unpublished results). Therefore, the dramatic tubulation of the Golgi and concomitant redistribution of Golgi residents to the ER under hypo-osmotic conditions could not be ascribed simply to the dissociation of COPI from the Golgi. However, osmotic stress perturbed COPI function, because it prevented the rapid, BFA-induced dissociation of COPI from Golgi membranes (Donaldson et al., 1990). Figure 8, A and D, shows the approximately coincident localization of βCOP (Figure 8A) and giantin (Figure 8D) in untreated cells maintained in normal medium. Two minutes after BFA addition, βCOP could no longer be detected in the Golgi region (Figure 8B) in cells marked by giantin staining (Figure 8E). In contrast, βCOP was easily detected on Golgi membranes after 2 min of BFA if cells were first incubated in hypotonic (210 mOsm) or hypertonic (610 mOsm; Figure 9A) medium for 1 min before the addition of BFA (Figure 8C). βCOP remained localized in a pattern approximately similar to giantin (Figure 8D). Longer times of incubation in hypotonic or hypertonic medium resulted in the diminution of βCOP staining in the Golgi region (our unpublished results), as observed for GPP130 and giantin. However, βCOP staining of membrane tubules was never observed.
Figure 8.
Hypotonic treatment prevents the rapid, BFA-induced dissociation of βCOP from the Golgi. HeLa cells, grown on 12-mm glass coverslips, were left in normal medium (A, B, D, and E) or placed in 1 ml of hypotonic medium for 1 min (C and F) before the addition of 10 μg/ml BFA. Two minutes after BFA addition, cells were fixed and processed for double-label immunofluorescence using a mAb against βCOP (A–C) and a polyclonal antiserum against giantin (D–F).
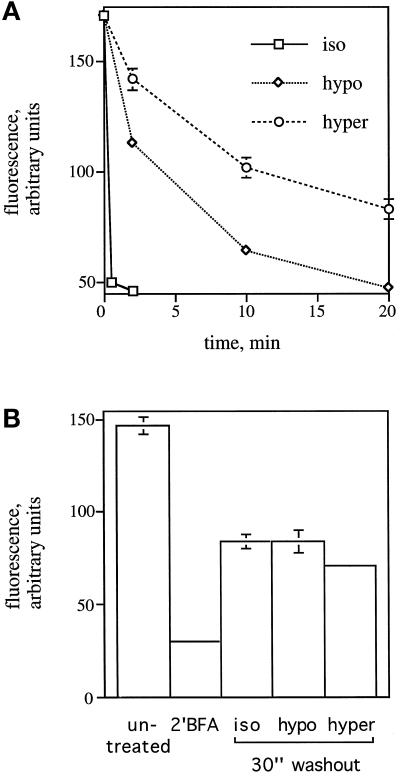
Figure 9.
Effect of hypotonic and hypertonic treatment on the rate of βCOP dissociation (A) and association (B). (A) HeLa cells, grown on 12-mm glass coverslips, were left in normal medium without serum or placed in hypotonic medium (210 mOsm) or hypertonic medium (610 mOsm) for 1 min before the addition of 10 μg/ml BFA. At each time point indicated, cells were fixed and stained using a mAb against βCOP and a polyclonal antiserum against giantin. The average of the mean fluorescence intensity of βCOP staining in a fixed circle (2782 pixels) enclosing the Golgi region (as indicated by giantin staining) in 50 cells was measured. (B) HeLa cells on coverslips were incubated with 2.5 μg/ml BFA in HEPES-buffered normal medium, pH 7.2, without serum for 2 min at room temperature to induce the dissociation of βCOP from the Golgi. Rebinding of βCOP to the Golgi was assessed by washing cells four times with 1 ml of normal medium without serum, hypotonic medium (210 mOsm), or hypertonic medium (610 mOsm) and placing them at 37°C for 30 s. The average of the mean fluorescence intensity of βCOP staining was determined as in A. Vertical bars represent the SEM as determined from 25 measurements.
A quantitative analysis of the fluorescence intensity attributable to βCOP staining in the Golgi region over time after BFA addition indicated that the dissociation of βCOP from the Golgi was not blocked but significantly slowed (Figure 9A). In iso-osmotic medium, the t½ for dissociation was <30 s (Figure 9A; Donaldson et al., 1990). However, in hypo-osmotic medium the t½ was 5–10 min, and in hyperosmotic medium the t½ was ∼20 min. This result suggested that both hypo- and hyperosmotic stress conditions reduced the rate of COPI dissociation from the Golgi. To rule out the possibility that hypotonic and hypertonic media might exert their effects simply by reducing the potency of BFA, we examined the rate of βCOP dissociation under hypo-osmotic conditions in response to BFA concentrations ranging from 1 to 15 μg/ml. The maximally effective concentration of BFA has been previously demonstrated to be 1 μg/ml (Donaldson et al., 1990). The t½ of COPI dissociation was 5–10 min, regardless of the BFA concentration used (our unpublished results). Next, we determined the effect of osmotic stress conditions on the rate of association of COPI with Golgi membranes. For this, we first treated cells with 2.5 μg/ml BFA in normal medium to induce the complete dissociation of COPI from Golgi membranes. After 2 min at room temperature, which was sufficient to reduce the Golgi-associated βCOP staining to background levels (Figure 9B, compare 2′BFA with untreated) without significantly perturbing the morphology of the Golgi as assayed by giantin staining, the BFA was washed out with iso-osmotic, hypo-osmotic, or hyperosmotic medium and placed at 37°C to allow the reassociation of COPI with the Golgi. After 30 s, the amount of βCOP staining reappearing in the Golgi region was quantitated. As shown in Figure 9B, osmotic stress conditions had no significant effect on the initial rate of association of COPI with the Golgi. The sum of the results suggested that hypo- and hyperosmotic conditions led to an alteration of COPI function subsequent to its association with, and before its dissociation from, the Golgi.
Adaptation Leads to Reemergence of Golgi Residents from the ER
As described above, hypotonic treatment led to a rapid and complete dispersal of certain markers (GM130 and mannosidase II), whereas the redistribution of other markers (GPP130 and giantin) was slower and less complete. To better understand the fate of the more slowly redistributing markers, we extended the time course of hypotonic treatment. To our surprise, rather than leading to an increase in the extent of GPP130 and giantin redistribution, long-term, sustained incubation in hypotonic medium resulted in the reversal of redistribution and a return of GPP130 and giantin to an apparently normal perinuclear distribution (our unpublished results). The reversal of redistribution is presented for GM130, whose initial redistribution was more complete (Figure 10, A–D). Figure 10A shows the pattern of GM130 in cells maintained in normal medium. By 30 min in hypotonic medium, the redistribution of GM130 to the ER was complete (Figure 10B). However, at 3 h of incubation in hypotonic medium, GM130 began to reappear in its normal perinuclear location (Figure 10C) and returned completely to its normal pattern by 6 h (Figure 10D). The reversal (as indicated by GM130 staining) occurred between 2 and 6 h of incubation and in the presence or absence of 100 μg/ml cycloheximide, indicating that the return of these proteins to the Golgi region was due not to new synthesis but, rather, to the return of preexisting protein to the Golgi. Furthermore, the insensitivity of both the redistribution and the reversal of redistribution to 100 μg/ml cycloheximide indicated that in response to hypo-osmotic conditions, the Golgi could, at least in part, be disassembled and reassembled with no new protein synthesis. Finally, the reversal of the osmotically induced response (i.e., Golgi resident redistribution) suggested a possible explanation for the incomplete redistribution observed for the more slowly redistributing markers GPP130 and giantin: by the time the bulk of GPP130 and giantin began to redistribute to the ER as a consequence of hypo-osmotic treatment, cells were already on the path to recovery. A similar recovery was also observed in cells that were incubated for several hours in hypertonic medium (our unpublished results).
Figure 10.
Golgi residents reemerge after long-term incubation in hypotonic medium, and reemergence is blocked by calphostin C. COS-7 cells, grown on 12-mm coverslips and either untreated (A–D) or pretreated with 100 nM calphostin C for 30 min (E–H), were removed from normal medium and placed in hypotonic (210-mOsm) medium (B–D) or hypotonic (210-mOsm) medium plus 100 nM calphostin C (F–H). Cells were fixed at 30 min (B and F), 3 h (C and G), or 6 h (D and H) and processed for immunofluorescence using a polyclonal antiserum against GM130. Cells maintained in normal medium in the absence (A) or presence of 100 nM calphostin C (E) were also fixed and stained.
Adaptation Requires PKC
Physiological increases in cell volume are known to activate multiple signal transduction cascades, at least a subset of which are likely to mediate an RVD back to the original cell volume (Hoffmann and Simonsen, 1989; Lang et al., 1998). In light of this established response, it seemed reasonable that the reemergence of Golgi residents from the ER upon long-term, sustained incubation in hypotonic medium was a consequence of a return to normal cell volume. A test of this hypothesis would be the inhibition of Golgi resident reemergence by an agent that is known to block cell volume homeostasis. Primary among the RVD responses to cell swelling are the efflux of inorganic solutes triggered by the opening of chloride and potassium channels (Hoffmann and Simonsen, 1989; Lang et al., 1998). Recently, selective inhibitors of PKC, including calphostin C (Kobayashi et al., 1989), have been shown to prevent cell volume recovery from swelling through the inhibition of volume-dependent chloride channels (Roman et al., 1998). To test whether the reemergence of Golgi residents upon sustained incubation in hypotonic medium was due to RVD-mediated restoration of normal cell volume, we asked whether calphostin C would prevent the reemergence of Golgi residents. As described above (Figure 10, A–D), GM130 redistributed to the ER by 30 min in hypotonic medium but reemerged and returned to its normal Golgi pattern after 3–6 h. As shown in Figure 10, E–H, pretreatment of cells with 100 nM calphostin C had no effect on GM130 in normal medium (Figure 10E) or on the initial GM130 redistribution response to hypo-osmotic conditions (Figure 10F), but it prevented the reemergence of GM130 from the ER (Figure 10, G and H). The inhibition by calphostin C was reversible; removal of the drug after 2 h of treatment resulted in the reemergence of GM130 (our unpublished results). Significantly, calphostin C pretreatment led to a near complete redistribution of GPP130 to the ER by 2–4 h (our unpublished results). Consistent with a role for PKC in RVD rather than a more direct role in preventing reemergence, calphostin C had no detectable effect on its own either on Golgi morphology or on ER-to-Golgi transport (our unpublished results). These results indicate that PKC-mediated adaptation to osmotic stress reactivates the trafficking steps that produce and maintain normal Golgi structure.
DISCUSSION
The abundance of certain subcellular compartments, such as ER, peroxisomes, and mitochondria, is dramatically altered by changes in metabolic state (for review, see Nunnari, 1996); therefore it seems likely that organelle biogenesis in general would be responsive to changes in cell physiology. We demonstrated that changes in the medium osmolarity of a variety of cultured cell lines affected constitutive transport and influenced the integrity of organelles in the early secretory pathway. Because the plasma membrane is freely permeable to water, cells undergo swelling or shrinking, within minutes of transfer to hypo- or hyperosmotic medium, respectively. Changes in cell volume, in turn, alter a number of metabolic pathways (for review, see Lang et al., 1998), as well as activate volume regulatory mechanisms that function to restore normal cell volume (for review, see Hoffmann and Simonsen, 1989). We report that cell volume increase abruptly inhibited ER-to-Golgi transport and induced extensive tubulation of the ERGIC and Golgi followed by the apparent collapse of these organelles into the ER. Cell volume decrease also abruptly inhibited ER-to-Golgi transport and, without detectable tubulation, induced a slower apparent collapse of the ERGIC and Golgi into the ER. Redistribution of Golgi residents into the ER occurs under a variety of experimental conditions, including BFA treatment (Doms et al., 1989; Lippincott-Schwartz et al., 1989; Fujiwara et al., 1988), NDGA treatment (Fujiwara et al., 1998), overexpression of RAB6 (Martinez et al., 1997), ARF1-T31N overexpression (Dascher and Balch, 1994), and overexpression of an ERD-2–like protein (Hsu et al., 1992). However, to our knowledge this is the first report of a physiological stimulus that induced redistribution of the Golgi and/or ERGIC into the ER.
The fact that such dramatic alterations in ERGIC and Golgi resident distribution can occur in response to physiologically relevant changes in cell volume raises the intriguing possibility that the ERGIC and Golgi might be lost and regained under a variety of physiological conditions that lead to alterations in cell volume. Certain cell types, such as those of the kidney medulla and intestine, encounter a wide range of extracellular osmolarity, and many other cell types are exposed to changes in extracellular osmolarity during a variety of disorders (Lang et al., 1998). Volume changes of the magnitude reported here also occur when intracellular osmolarity is altered by a variety of metabolic pathways (Lang et al., 1998). For instance, β-adrenergic agonists and dibutyrl cAMP cause lung epithelial cells to undergo swelling and/or shrinking in a concentration-dependent manner (Nakahari and Marunaka, 1996). Similarly, insulin causes swelling of hepatocytes (Lang et al., 1998). Significantly, hypo-osmotically induced swelling of hepatocytes mimics the insulin response: glycogen synthesis and protein synthesis are stimulated, whereas glycogen breakdown and proteolysis are inhibited (Lang et al., 1998). In fact, at least a subset of the metabolic changes that are insulin induced appear to be mediated by cell swelling, leading to the suggestion that cell swelling itself may act as a second messenger (Al-Habori et al., 1992; Lang et al., 1998).
Remarkably, the Golgi reemerged from the ER, even when cells were maintained in hypo- or hypertonic medium, and even in the absence of new protein synthesis. When applied to cells during hypo-osmotic stress, calphostin C, a selective inhibitor of PKC (Kobayashi et al., 1989), blocks recovery of normal cell volume (Roman et al., 1998) and blocked Golgi reemergence from the ER. Because calphostin C had no apparent effect on forward transport in nonstressed cells, it is likely that the role of PKC in Golgi reemergence was to promote cell volume recovery. That is, in the absence of PKC function, cell volume could not be restored, and thus transport remained inactive. The ability of cells to restore normal cell volume and then reactivate transport explains the apparent incomplete redistribution of the more slowly moving markers such as GPP130: forward transport was reactivated, at least in some percentage of the cells, before a time point when all cells could exhibit complete GPP130 redistribution. Consistent with this explanation, complete ER redistribution of GPP130 during osmotic stress was achieved by using calphostin C to inhibit reemergence. The adaptive response of cells to osmotic stress suggests that cell volume and transport are tightly coupled. The lack of a requirement for new protein synthesis for this response indicates that the coupling is mediated by posttranslational mechanisms.
A simple explanation for the apparent collapse of the ERGIC and Golgi into the ER that accompanied the osmotically induced forward transport block is that resident proteins of the ERGIC and Golgi constitutively cycle through the ER; thus when ER-to-Golgi transport is blocked, each resident accumulates in the ER at the rate at which it normally cycles to the ER (Cole et al., 1998). Unfortunately a direct comparison of osmotically induced redistribution rates to normal recycling rates cannot be made, because the normal rates of Golgi resident recycling are presently unknown. Nevertheless, the evidence argues that hypo-osmotic conditions did not simply block forward transport, but rather, retrograde transport must also have been activated. First, although both hypo- and hyperosmotic conditions were equally potent inhibitors of forward transport, Golgi residents redistributed to the ER much more rapidly under hypo-osmotic conditions (t½, ∼5–10 min for GM130) than under hyperosmotic conditions (t½, ∼30 min for GM130). Second, the tubules that appeared to carry ERGIC and Golgi residents to the ER were clearly induced, or at least greatly exaggerated, compared with what can be observed in nonstressed cells. Third, other conditions that block ER-to-Golgi transport, such as microinjection of SAR1-H79G protein (Shima et al., 1998) or anti-βCOP antibody (Pepperkok et al., 1993), do not cause extensive tubulation or rapid redistribution of Golgi residents into the ER. The finding that physiologically important stresses, such as those caused by hypo- and hyperosmotic conditions, block ER export and stimulate tubulation of the ERGIC and Golgi implies that anterograde and retrograde transport between the ER and the Golgi have the potential to be regulated independently. Differential regulation of anterograde and retrograde pathways would provide a mechanism for restructuring of the early constitutive secretory pathway according to the demands of changes in cell physiology.
Elucidation of the pathway linking osmotic changes to the arrest of anterograde transport and the stimulation of retrograde transport is an important area for future research. It is possible that volume changes during cell swelling (1.2- to 1.4-fold increase at most under the conditions used in this study (see Watson, 1989) or shrinking (down to 0.5 of the original cell volume) could directly inactivate a factor(s) that is required for anterograde transport between the ER and Golgi and, at the same time, directly stimulate a rate-limiting factor(s) involved in tubulation and retrograde transport. This could occur through a change in cell volume, per se, if the transport factors were concentration or dilution sensitive. Or, it could occur as a consequence of swelling or shrinking-induced changes in cytoplasmic pH (for review, see Graf and Haussinger, 1996) that alter the activities of pH-sensitive transport factors. Another possibility is that osmotic stress lowers the level of ATP to generate the affects documented herein, although several lines of evidence argue against this possibility. First, although lowering ATP levels in intact cells by sodium azide and 2-deoxyglucose treatment is known to induce low levels of tubulation of Golgi membranes (Cluett et al., 1993), redistribution of Golgi residents to the ER is not observed (Donaldson et al., 1990; our unpublished results). Second, lowering ATP levels in intact cells is known to cause the release of βCOP from Golgi membranes (Donaldson et al., 1990), but we observed that the association of βCOP with Golgi membranes was maintained during osmotic stress. Third, we have measured the ratio of ATP to protein in cells extracted at various times after exposure to hypotonic medium and have observed no significant variation (our unpublished results). Regardless of the exact cause(s) of the osmotically induced changes in anterograde and retrograde transport, the changes in the activities of the various transport factors would have to be reversible upon restoration of normal cell volume in the absence of new protein synthesis.
An alternative explanation to those listed above is that a cell volume change could differentially affect anterograde and retrograde constitutive transport pathways through a classical signal transduction–mediated process. Cell swelling leads to rapid increases in intracellular Ca2+ and cAMP (Watson, 1989) and stimulates PKC, phosphatidylinositol 3-kinase, and MAPK cascades leading to the activation of Jun-NH2-terminal kinase as well as extracellular signal-regulated kinases 1 and 2 (for review, see Lang et al., 1998). A straightforward approach to investigate the role of classic signaling molecules in coupling cell volume changes to the forward transport block and Golgi-resident redistribution response would be to specifically inhibit or activate the signaling molecules and test whether the response of the secretory pathway to osmotic stress is lost. Unfortunately, some of the agents that might be used are already known either to alter cell volume, e.g., dibutyrl cAMP and forskolin (Nakahari and Marunaka, 1996), or to interfere with cell volume regulatory mechanisms, e.g., inhibitors of PKC (Roman et al., 1998), NDGA (Light et al., 1997), inhibitors of phospholipase A2 (PLA2) (Light et al., 1997), calmodulin antagonists (Lang et al., 1998), and toxins that inhibit trimeric G proteins (Hoffmann and Dunham, 1995). Thus it will be important in future studies to determine whether an inhibition of the response is a consequence of actual uncoupling or whether it is an indirect consequence of a primary effect on cell volume which subsequently feeds back on the secretory pathway. Indeed, it is a noteworthy possibility that some of the previously described effects of signaling molecule modulators on transport kinetics (Hansen and Casanova, 1994; Pimplikar and Simons, 1994; Muniz et al., 1996) may have been due to the role of the corresponding signaling molecules in cell volume control rather than their role in modifying transport factors.
Our observations suggested that anterograde transport was blocked at more than one step under both hypo- and hyperosmotic conditions. One impairment was apparently in COPI function: although BFA normally causes the rapid dissociation of COPI from Golgi and ERGIC membranes (t½, <30 s) (Donaldson et al., 1990), COPI remained firmly Golgi associated if BFA was added under hypo- or hyperosmotic conditions (t½, ∼10 min or greater). This suggested that COPI could bind to Golgi and ERGIC membranes under hypo- or hyperosmotic conditions but was slowed significantly in its ability to dissociate from the Golgi and ERGIC. This slowed dissociation of COPI in the presence of BFA mimics the behavior of COPI in cells overexpressing ARF1-Q71I, an altered version of ARF1 that is slowed in it ability to hydrolyze GTP (Teal et al., 1994). That cells overexpressing ARF1-Q71I accumulate VSV-G in the ERGIC is consistent with a requirement for COPI dissociation (as well as association) in ERGIC-to-Golgi transport (Dascher and Balch, 1994). Based on these data, we suggest that hypo- and hyperosmotic conditions blocked ERGIC-to-Golgi transport by inhibiting COPI function, presumably after its association with, and before its dissociation from, Golgi membranes.
In addition to a block in ERGIC-to-Golgi transport, ER export of VSV-G was rapidly inhibited. Although it is formally possible that the block in ER export under hypo-osmotic conditions was an indirect consequence of a primary block in COPI function (Pelham, 1995), the redistribution of ERGIC 53 to the ER under hypo-osmotic conditions mimicked the redistribution of ERGIC 53 to the ER observed in cells microinjected with SAR1-H79G protein to block ER export (Shima et al., 1998). Furthermore, cells overexpressing ARF1-Q71I, and consequently exhibiting a perturbation of COPI function that parallels the hypo-osmotically induced perturbation of COPI function, are not blocked in ER export (Dascher and Balch, 1994). These data altogether indicate a direct inhibition of ER export under hypo-osmotic conditions. The inhibition of ER export could be due to a block in either the recruitment of COPII components to sites of budding or the inability of COPII, once assembled, to carry out vesicle formation (Barlowe et al., 1994; Aridor and Balch, 1996; Schekman and Orci, 1996). Our observations indicated that presumed ER export sites, as marked by immunofluorescent staining with an antibody against the COPII subunit SEC13 (Shaywitz et al., 1995; Tang et al., 1997), although reduced in number, were not completely lost during hypo-osmotic conditions (our unpublished results). It is tempting to speculate that the function of COPII is perturbed in a manner analogous to that of COPI function, at a step subsequent to its association with but before its dissociation from membranes.
Osmotic stress appeared to selectively regulate anterograde and retrograde pathways, because the effects of hypo- and hyperosmotic treatment on anterograde transport were indistinguishable, whereas their effects on retrograde transport were kinetically and morphologically distinct. Very little is known at present about the factors that participate in Golgi tubulation. One candidate for such a factor is PLA2. PLA2 inhibitors prevent BFA-induced tubulation and redistribution of Golgi residents to the ER (de Figueiredo et al., 1998). Although the exact role of PLA2 in tubulation is unclear, it is tempting to speculate that PLA2, whose activity is stimulated by cell swelling (Lang et al., 1998), somehow mediates the extensive tubulation of the Golgi under hypo-osmotic conditions.
Why would osmotic stress cause the Golgi to redistribute to the ER? In fission yeast, regulation of vacuolar fission and fusion pathways by osmotic stress may function to restore normal cell volume (Bone et al., 1998). It seems unlikely, though, that the redistribution of Golgi residents to the ER in mammalian cells would function to alleviate both hypo- and hyperosmotically induced changes in cell volume. Rather, we speculate that sending Golgi proteins back to the ER might function as a protective response to stress. It has been proposed that periodic constitutive recycling of Golgi residents through the ER would provide a quality control mechanism ensuring that the modification enzymes and structural proteins that reside in the Golgi, many of which have half-lives that span an entire cell division cycle, are periodically monitored for whether they are properly folded by the protein-folding machinery residing in the ER (Cole et al., 1998). Osmotic changes induce the expression of certain cytoplasmic heat shock proteins (Lang et al., 1998), but whether fluctuations in cell volume result in higher rates of protein misfolding, and targeted degradation, has not been reported. Similarly, it remains to be determined whether the Golgi residents that reemerge, presumably as a consequence of an adjustment back to a normal cell volume, have been in contact with the folding machinery in the ER.
ACKNOWLEDGMENTS
These experiments were made possible by the generous contributions of antibodies and other reagents by the following investigators: E. Sztul (University of Birmingham, Birmingham, AL), P. Weidman (St. Louis University, St. Louis, MO), H.-P. Hauri (Biocenter, Basel, Switzerland), V. Malhotra (University of San Diego, San Diego, CA), B.L. Tang and W. Hong (National University of Singapore, Singapore), and A. Hubbard (Johns Hopkins Medical School, Baltimore, MD). This work was supported by National Institutes of Health grant GM-56779-02 (to A.D.L.). T.H.L. was supported in part by a postdoctoral fellowship from the American Cancer Society.
Abbreviations used:
- BFA
brefeldin A
- CHO
Chinese hamster ovary
- endo H
endoglycosidase H
- ER
endoplasmic reticulum
- HA
hemagglutinin
- MEM
minimum essential medium
- NDGA
nordihydroguaiaretic acid
- PKC
protein kinase C
- PLA2
phospholipase A2
- RVD
regulatory volume decrease
- ts
temperature-sensitive
- VSV-G
vesicular stomatitis virus glycoprotein
REFERENCES
- Al-Habori M, Peak M, Thomas TH, Agius L. The role of cell swelling in the stimulation of glycogen synthesis by insulin. Biochem J. 1992;282:789–296. doi: 10.1042/bj2820789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan VJ, Kreis TE. A microtubule-binding protein associated with membranes of the Golgi apparatus. J Cell Biol. 1986;103:2229–2239. doi: 10.1083/jcb.103.6.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Balch WE. Principles of selective transport: coat complexes hold the key. Trends Cell Biol. 1996;6:315–320. doi: 10.1016/0962-8924(96)10027-1. [DOI] [PubMed] [Google Scholar]
- Bannykh SI, Balch WE. Membrane dynamics at the endoplasmic-reticulum Golgi interface. J Cell Biol. 1997;138:1–4. doi: 10.1083/jcb.138.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach M, Razzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by SEC proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Bone N, Millar JB, Toda T, Armstrong J. Regulated vacuole fusion and fission in Schizosaccharomyces pombe: an osmotic response dependent on MAP kinases. Curr Biol. 1998;8:135–144. doi: 10.1016/s0960-9822(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Cluett EB, Wood SA, Banta M, Brown WJ. Tubulation of Golgi membranes in vivo and in vitro in the absence of brefeldin A. J Cell Biol. 1993;120:15–24. doi: 10.1083/jcb.120.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole NB, Ellenberg J, Song J, DiEuliis D, Lippincott-Schwartz J. Retrograde transport of Golgi-localized proteins to the ER. J Cell Biol. 1998;140:1–15. doi: 10.1083/jcb.140.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher C, Balch W. Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J Biol Chem. 1994;269:1437–1448. [PubMed] [Google Scholar]
- Davidson HW, McGowan CH, Balch WE. Evidence for the regulation of exocytic transport by protein phosphorylation. J Cell Biol. 1992;116:1343–1355. doi: 10.1083/jcb.116.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Figueiredo P, Drecktrah D, Katzenellenbogen JA, Strang M, Brown WJ. Evidence that phospholipase A2 activity is required for Golgi complex and trans Golgi network membrane tubulation. Proc Natl Acad Sci USA. 1998;95:8642–8647. doi: 10.1073/pnas.95.15.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Matteis MA, Santini G, Kahn RA, Di Tullio G, Luini A. Receptor and protein kinase C-mediated regulation of ARF binding to the Golgi complex. Nature. 1993;26:818–821. doi: 10.1038/364818a0. [DOI] [PubMed] [Google Scholar]
- Docherty PA, Snider MD. Effects of hypertonic and sodium-free medium on transport of a membrane glycoprotein along the secretory pathway in cultured mammalian cells. J Cell Physiol. 1991;146:34–42. doi: 10.1002/jcp.1041460106. [DOI] [PubMed] [Google Scholar]
- Doms RW, Keller DS, Helenius A, Balch WE. Role for adenosine triphosphate in regulating the assembly and transport of vesicular stomatitis virus G protein trimers. J Cell Biol. 1987;105:1957–1969. doi: 10.1083/jcb.105.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms RW, Russ G, Yewdell JW. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989;109:61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Lippincott-Schwartz J, Bloom GS, Kreiss TE, Klausner RD. Dissociation of a 100 kDa peripheral membrane protein from the Golgi apparatus is an early event in brefeldin A action. J Cell Biol. 1990;111:2295–2306. doi: 10.1083/jcb.111.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duden R, Griffiths G, Argos P, Kreiss TE. A 110 kd protein associated with nonclathrin-coated vesicles and the Golgi complex, shows homology to β-adaptins. Cell. 1991;64:649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Elazar Z, Orci L, Ostermann J, Amherdt M, Tanigawa G, Rothman JE. ADP-ribosylation factor and coatomer couple fusion to vesicle budding. J Cell Biol. 1994;124:415–424. doi: 10.1083/jcb.124.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988;263:18545–18552. [PubMed] [Google Scholar]
- Fujiwara T, Takami N, Misumi Y, Ikehara Y. Nordihydroguaiaretic acid blocks protein transport in the secretory pathway causing redistribution of Golgi proteins into the endoplasmic reticulum. J Biol Chem. 1998;273:3068–3075. doi: 10.1074/jbc.273.5.3068. [DOI] [PubMed] [Google Scholar]
- Gallione CJ, Rose JK. A single amino acid substitution in a hydrophobic domain causes temperature-sensitive cell-surface transport of a mutant viral glycoprotein. J Virol. 1985;54:374–382. doi: 10.1128/jvi.54.2.374-382.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J, Haussinger D. Ion transport in hepatocytes: mechanisms and correlations to cell volume, hormone actions and metabolism. J Hepatol. 1996;24(suppl 1):53–77. [PubMed] [Google Scholar]
- Hansen SH, Casanova JE. Gsalpha stimulates transcytosis and apical secretion in MDCK cells through cAMP and protein kinase A. J Cell Biol. 1994;126:677–687. doi: 10.1083/jcb.126.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann EK, Dunham PB. Membrane mechanisms and intracellular signaling in cell volume regulation. Int Rev Cytol. 1995;161:173–262. doi: 10.1016/s0074-7696(08)62498-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Simonsen LO. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989;67:315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Hong WJ, Piazza GA, Hixson DC, Doyle D. Expression of enzymatically active rat dipeptidyl peptidase IV in Chinese hamster ovary cells after transfection. Biochemistry. 1989;28:8474–8479. doi: 10.1021/bi00447a030. [DOI] [PubMed] [Google Scholar]
- Hsu VW, Shah N, Klausner RD. A brefeldin A-like phenotype is induced by the overexpression of a human ERD-2-like protein, ELP-1. Cell. 1992;69:625–635. doi: 10.1016/0092-8674(92)90226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesch SA, Linstedt AD. The Golgi and endoplasmic reticulum remain independent during mitosis in HeLa cells. Mol Biol Cell. 1998;9:623–635. doi: 10.1091/mbc.9.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. Assembly of asparagine linked oligosaccharides. Annu Rev Biochem. 1985;52:631–634. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Light DB, Mertins TM, Belongia JA, Witt CA. 5-Lipoxygenase metabolites of arachidonic acid regulate volume decrease by mudpuppy red blood cells. J Membr Biol. 1997;158:229–239. doi: 10.1007/s002329900260. [DOI] [PubMed] [Google Scholar]
- Linstedt AD, Hauri H-P. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol Biol Cell. 1993;4:679–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt AD, Mehta A, Suhan J, Reggio H, Hauri H-P. Sequence and overexpression of GPP130/GIMPc: evidence for saturable pH-sensitive targeting of a type II early Golgi membrane. Mol Biol Cell. 1997;8:1073–1087. doi: 10.1091/mbc.8.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Donaldson JG, Schweizer A, Berger EG, Hauri H-P, Yuan LC, Klausner RD. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti V, Torrisi M, Pascale M, Bonatti S. Immunocytochemical analysis of the transfer of vesicular stomatitis virus glycoprotein from the intermediate compartment to the Golgi complex. J Cell Biol. 1992;118:43–50. doi: 10.1083/jcb.118.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V, Serafini T, Orci L, Shepherd JC, Rothman JE. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- Martinez O, Antony C, Pehau-Arnaudet G, Berger EG, Salamero J, Goud B. GTP-bound forms of rab6 induce the redistribution of Golgi proteins into the endoplasmic reticulum. Proc Natl Acad Sci USA. 1997;94:1828–1833. doi: 10.1073/pnas.94.5.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz M, Alonso M, Hidalgo J, Velasco A. A regulatory role for cAMP-dependent protein kinase in protein traffic along the exocytic route. J Biol Chem. 1996;271:30935–30941. doi: 10.1074/jbc.271.48.30935. [DOI] [PubMed] [Google Scholar]
- Nakahari T, Marunaka Y. Regulation of cell volume by β2-adrenergic stimulation in rat fetal distal lung epithelial cells. J Membr Biol. 1996;151:91–100. doi: 10.1007/s002329900060. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreiss TE, Warren G. Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff PM, Tulsiani DR, Touster O, Yam A, Novikoff AB. Immunocytochemical localization of alpha-d-mannosidase II in the Golgi apparatus of rat liver. Proc Natl Acad Sci USA. 1983;80:4364–4368. doi: 10.1073/pnas.80.14.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari J. Regulation of organelle biogenesis. Cell. 1996;84:389–394. doi: 10.1016/s0092-8674(00)81283-0. [DOI] [PubMed] [Google Scholar]
- Ohashi M, Huttner WB. An elevation of cytosolic protein phosphorylation modulates trimeric G-protein regulation of secretory vesicle formation from the trans-Golgi network. J Biol Chem. 1994;269:24897–24905. [PubMed] [Google Scholar]
- Orci L, Glick BS, Rothman JE. A new type of coated vesicular carrier that appears not to contain clathrin: its possible role in protein transport through the Golgi stack. Cell. 1986;51:1053–1062. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- Pelham HR. Sorting and retrieval between the endoplasmic reticulum and Golgi apparatus. Curr Opion Cell Biol. 1995;7:530–535. doi: 10.1016/0955-0674(95)80010-7. [DOI] [PubMed] [Google Scholar]
- Pepperkok R, Scheel J, Horstmann H, Hauri HP, Griffiths G, Kreis TE. βCOP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 1993;74:71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- Pimplikar SW, Simons K. Activators of protein kinase A stimulate apical but not basolateral transport in epithelial Madin-Darby canine kidney cells. J Biol Chem. 1994;269:19054–19059. [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJM, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Roman RM, Bodily KO, Wang Y, Raymond JR, Fitz JG. Activation of protein kinase C alpha couples cell volume to membrane Cl− permeability in HTC hepatoma and Mz-ChA-1 cholangiocarcinoma cells. Hepatology. 1998;28:1073–1080. doi: 10.1002/hep.510280423. [DOI] [PubMed] [Google Scholar]
- Rowe T, Aridor M, McCaffery JM, Plutner H, Nuoffer C, Balch WE. COPII vesicles derived from mammalian endoplasmic reticulum microsomes recruit COPI. J Cell Biol. 1996;135:895–911. doi: 10.1083/jcb.135.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales SJ, Pepperkok R, Kreis TE. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Scheel J, Pepperkok R, Lowe M, Griffiths G, Kreis TE. Dissociation of coatomer from membranes is required for brefeldin A-induced transfer of Golgi enzymes to the endoplasmic reticulum. J Cell Biol. 1997;137:319–333. doi: 10.1083/jcb.137.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Fransen JA, Bachi T, Ginsel L, Hauri H-P. Identification, by a monoclonal antibody, of a 53-kDa protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi complex. J Cell Biol. 1988;107:1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A, Fransen JA, Matter K, Kreis TE, Ginsel L, Hauri H-P. Identification of an intermediate compartment involved in protein transport from endoplasmic reticulum to Golgi apparatus. Eur J Cell Biol. 1990;53:185–196. [PubMed] [Google Scholar]
- Schweizer A, Rohrer J, Slot JW, Geuze HJ, Kornfeld S. Reassessment of the subcellular localization of p63. J Cell Sci. 1995;108:2477–2485. doi: 10.1242/jcs.108.6.2477. [DOI] [PubMed] [Google Scholar]
- Sciaky N, Presley PJ, Smith C, Zaal KA, Cole N, Moreira JE, Terasaki M, Siggia E, Lippincott-Schwartz J. Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J Cell Biol. 1997;139:1137–1155. doi: 10.1083/jcb.139.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA, Rothman JE. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- Shaywitz DA, Orci L, Ravazzola M, Swaroop A, Kaiser CA. Human SEC13Rp functions in yeast and is located on transport vesicles budding from the endoplasmic reticulum. J Cell Biol. 1995;128:769–777. doi: 10.1083/jcb.128.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima DT, Cabrera-Poch N, Pepperkok R, Warren G. An ordered inheritance strategy for the Golgi apparatus: visualization of mitotic disassembly reveals a role for the mitotic spindle. J Cell Biol. 1998;141:955–966. doi: 10.1083/jcb.141.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL, Peter F, Krijnse-Locker J, Low SH, Griffiths G, Hong W. The mammalian homolog of yeast Sec13p is enriched in the intermediate compartment and essential for protein transport from the endoplasmic reticulum to the Golgi apparatus. Mol Cell Biol. 1997;17:256–266. doi: 10.1128/mcb.17.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teal SB, Hsu VW, Peters PJ, Klausner RD, Donaldson JG. An activating mutation in ARF1 stabilizes coatomer binding to Golgi membranes. J Biol Chem. 1994;269:3135–3138. [PubMed] [Google Scholar]
- Waters MG, Serafini T, Rothman JE. “Coatomer”: a cytosolic protein complex containing subunits of nonclathrin-coated Golgi transport vesicles. Nature. 1991;349:248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]
- Watson PA. Accumulation of cAMP and calcium in S49 mouse lymphoma cells following hyposmotic swelling. J Biol Chem. 1989;264:14735–14740. [PubMed] [Google Scholar]