Abstract
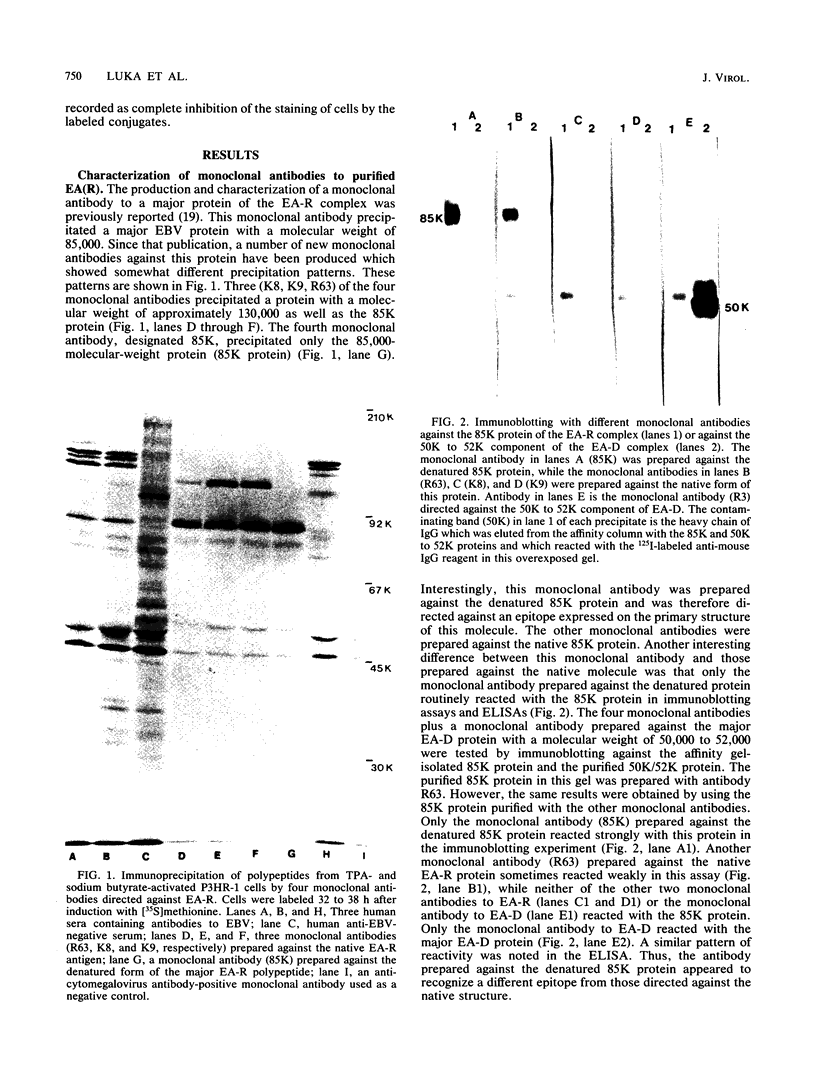
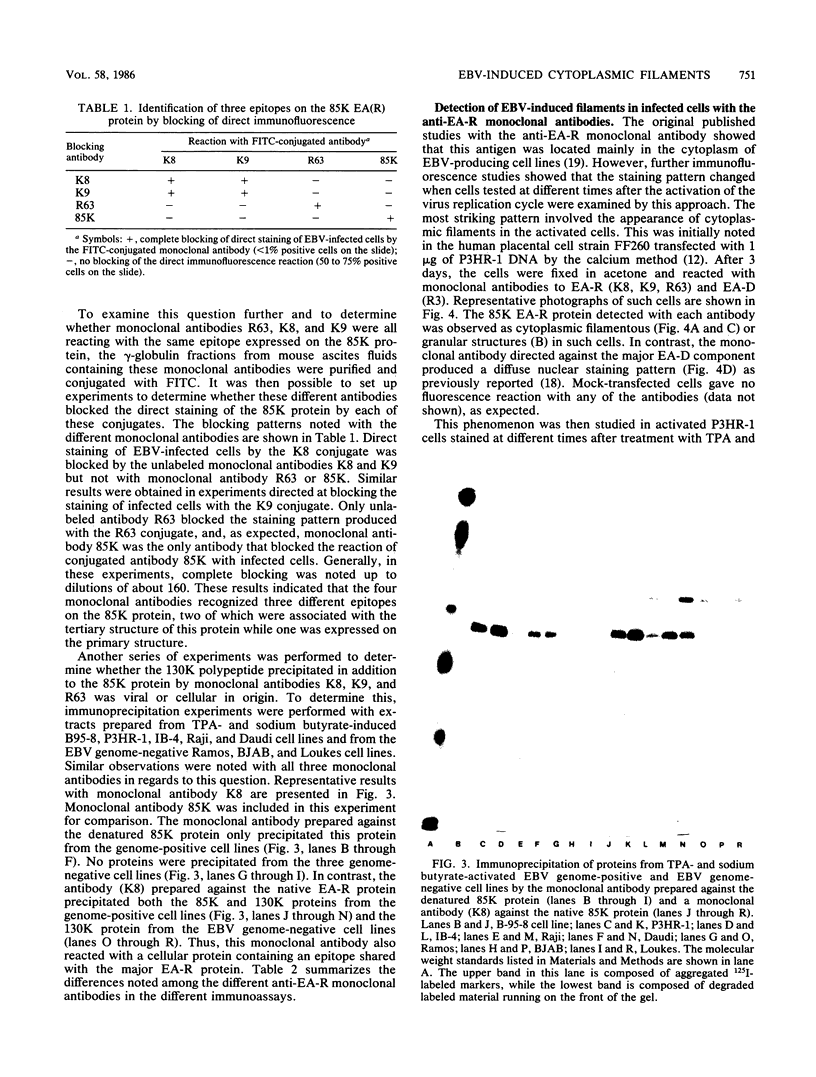
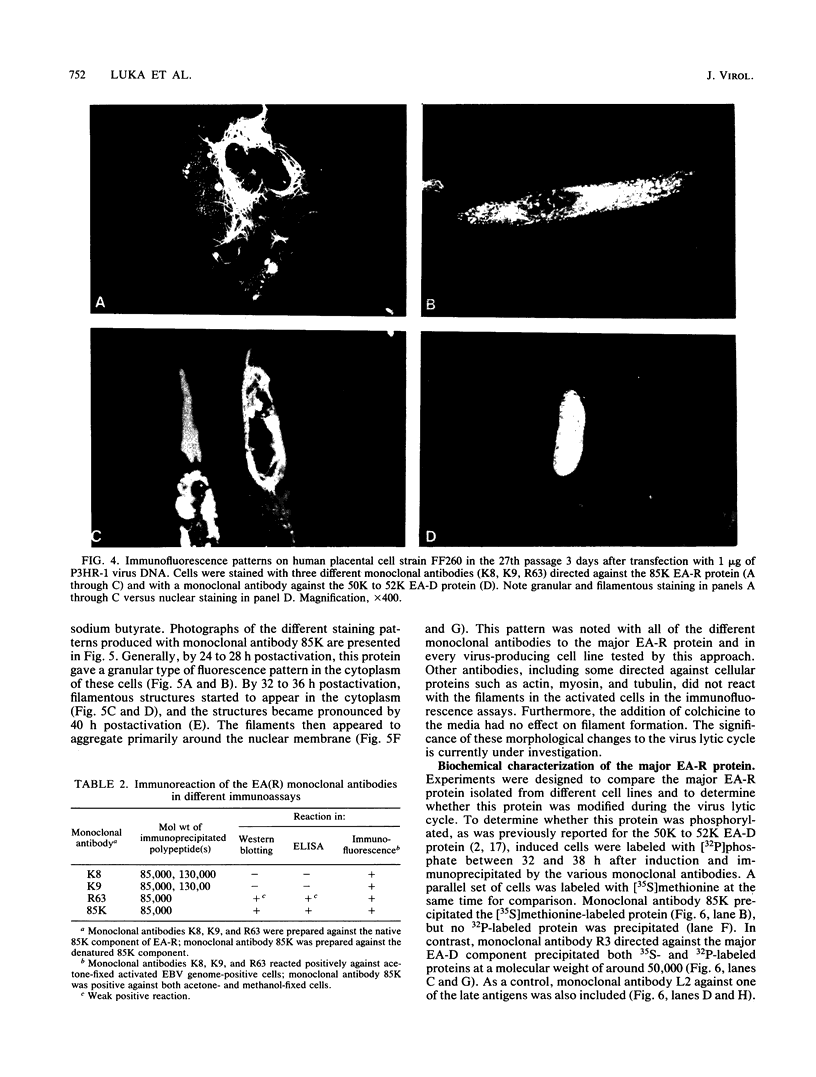
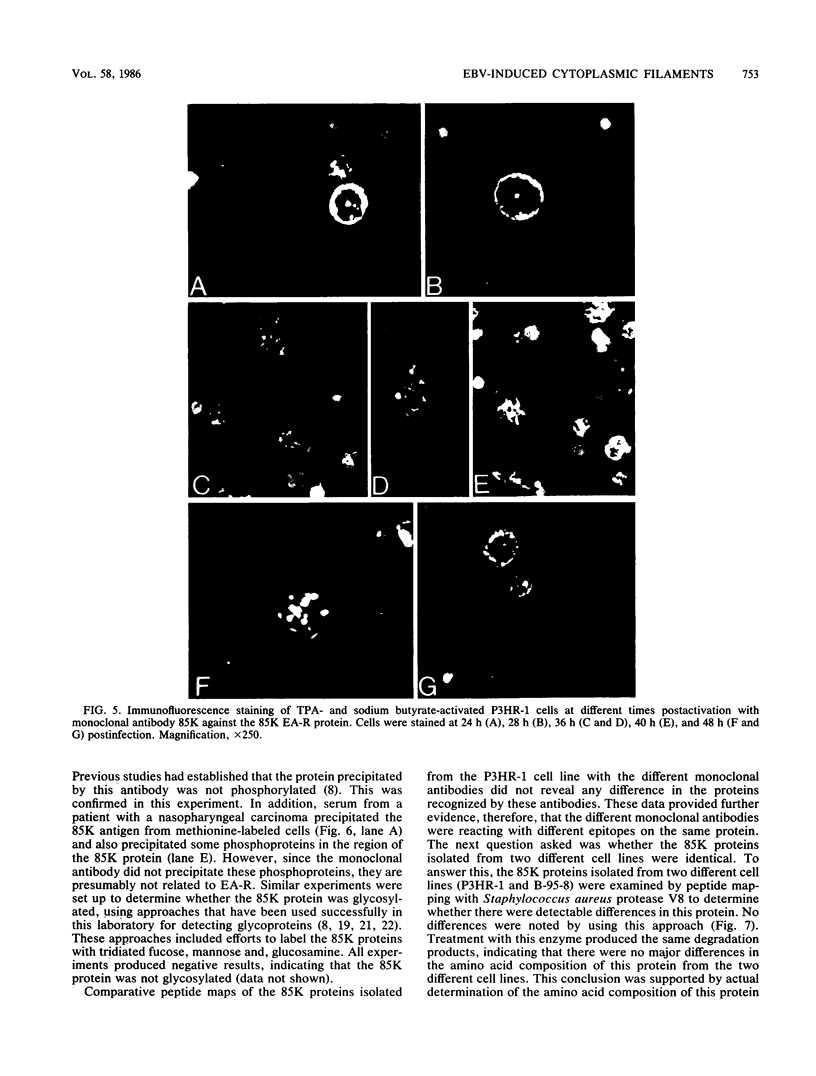
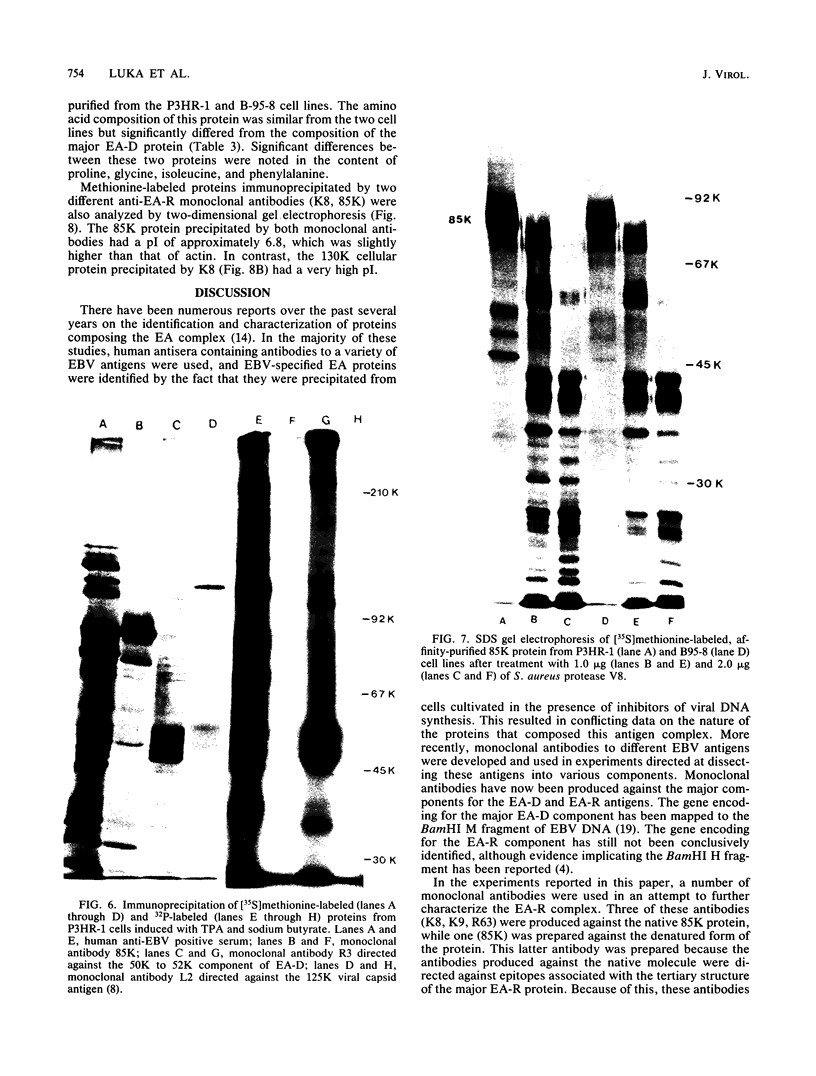
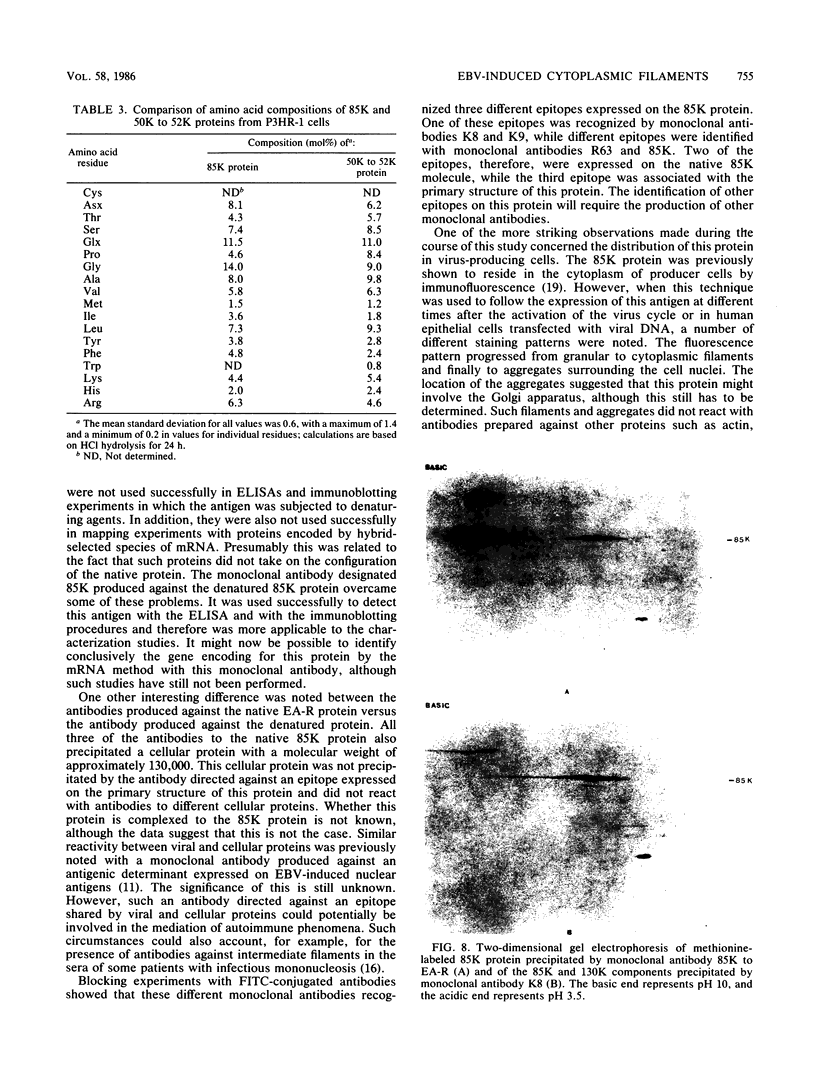
Four monoclonal antibodies produced against the restricted component of the Epstein-Barr virus (EBV) early antigen (EA-R) precipitated a polypeptide with an approximate molecular weight of 85,000. Three of these antibodies prepared against the native 85,000-molecular-weight protein (85K protein) reacted by immunofluorescence with acetone-fixed smears but not methanol-fixed smears of EBV-producing cells activated with tumor-promoting agent and sodium butyrate. The fourth monoclonal antibody which was produced against the denatured 85K protein reacted with both acetone-fixed cells and methanol-fixed cells. Blocking of direct immunofluorescence by the different monoclonal antibodies established that these monoclonal antibodies were directed against three different epitopes expressed on the 85K protein. The cytoplasmic staining pattern produced by each antibody was granular during the first 24 to 28 h after induction, developed into filamentous structures about 36 h after induction, and then began to aggregate after 48 h. Similar structures were observed in human placental cells transfected by EBV DNA and stained with three of the monoclonal antibodies. These results suggest that the EA-R polypeptide is assembled into filaments during the EBV lytic cycle. The significance of this in regards to replication has yet to be determined. Biochemical characterization of this major EA-R component did not reveal any major differences in this protein isolated from different cell lines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Couch E. F., Nahmias A. J. Filamentous structures of type 2 Herpesvirus hominis infection of the chorioallantoic membrane. J Virol. 1969 Feb;3(2):228–232. doi: 10.1128/jvi.3.2.228-232.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein A. L. Immunobiochemical characterization with monoclonal antibodies of Epstein-Barr virus-associated early antigens in chemically induced cells. J Virol. 1984 May;50(2):372–379. doi: 10.1128/jvi.50.2.372-379.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B., Wroblewska Z., Frankel M. E., Koprowski H. Molecular mimicry in virus infection: crossreaction of measles virus phosphoprotein or of herpes simplex virus protein with human intermediate filaments. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2346–2350. doi: 10.1073/pnas.80.8.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., Boyd A., Stoerker J., Holliday J. Functional mapping of the Epstein-Barr virus genome: identification of sites coding for the restricted early antigen, the diffuse early antigen, and the nuclear antigen. Virology. 1983 Aug;129(1):188–198. doi: 10.1016/0042-6822(83)90405-1. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W., Klein G. Demonstration of two distinct components in the early antigen complex of Epstein-Barr virus-infected cells. Int J Cancer. 1971 Sep 15;8(2):272–282. doi: 10.1002/ijc.2910080212. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W., Klein G., Gunven P., Clifford P., Morrow R. H., Ziegler J. L. Antibodies to early Epstein-Barr virus-induced antigens in Burkitt's lymphoma. J Natl Cancer Inst. 1971 Apr;46(4):861–871. [PubMed] [Google Scholar]
- Henle W., Ho H. C., Henle G., Kwan H. C. Antibodies to Epstein-Barr virus-related antigens in nasopharyngeal carcinoma. Comparison of active cases with long-term survivors. J Natl Cancer Inst. 1973 Aug;51(2):361–369. [PubMed] [Google Scholar]
- Kishishita M., Luka J., Vroman B., Poduslo J. F., Pearson G. R. Production of monoclonal antibody to a late intracellular Epstein-Barr virus-induced antigen. Virology. 1984 Mar;133(2):363–375. doi: 10.1016/0042-6822(84)90402-1. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Choi E. I., Pagano J. S. Qualitative and quantitative analyses of Epstein-Barr virus early antigen diffuse component by western blotting enzyme-linked immunosorbent assay with a monoclonal antibody. J Virol. 1985 Mar;53(3):793–799. doi: 10.1128/jvi.53.3.793-799.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka J., Chase R. C., Pearson G. R. A sensitive enzyme-linked immunosorbent assay (ELISA) against the major EBV-associated antigens. I. Correlation between ELISA and immunofluorescence titers using purified antigens. J Immunol Methods. 1984 Feb 24;67(1):145–156. doi: 10.1016/0022-1759(84)90093-0. [DOI] [PubMed] [Google Scholar]
- Luka J., Kreofsky T., Pearson G. R., Hennessy K., Kieff E. Identification and characterization of a cellular protein that cross-reacts with the Epstein-Barr virus nuclear antigen. J Virol. 1984 Dec;52(3):833–838. doi: 10.1128/jvi.52.3.833-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Grogan E., Heston L., Robinson J., Smith D. Epstein-Barr viral DNA: infectivity for human placental cells. Science. 1981 Apr 24;212(4493):452–455. doi: 10.1126/science.6259735. [DOI] [PubMed] [Google Scholar]
- Moore C. L., Griffith J. D., Shaw J. E. Filamentous structures associated with Epstein-Barr virus-infected cells. J Virol. 1982 Jul;43(1):305–313. doi: 10.1128/jvi.43.1.305-313.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pearson G. R., Henle G., Henle W. Production of antigens associated with Epstein-Barr virus in experimentally infected lymphoblastoid cell lines. J Natl Cancer Inst. 1971 Jun;46(6):1243–1250. [PubMed] [Google Scholar]
- Pearson G. R., Vroman B., Chase B., Sculley T., Hummel M., Kieff E. Identification of polypeptide components of the Epstein-Barr virus early antigen complex with monoclonal antibodies. J Virol. 1983 Jul;47(1):193–201. doi: 10.1128/jvi.47.1.193-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualtiere L. F., Chase R., Pearson G. R. Purification and biologic characterization of a major Epstein Barr virus-induced membrane glycoprotein. J Immunol. 1982 Aug;129(2):814–818. [PubMed] [Google Scholar]
- Qualtiere L. F., Pearson G. R. Epstein-Barr virus-induced membrane antigens: immunochemical characterization of Triton X-100 solubilized viral membrane antigens from EBV-superinfected Raji cells. Int J Cancer. 1979 Jun 15;23(6):808–817. doi: 10.1002/ijc.2910230612. [DOI] [PubMed] [Google Scholar]
- Qualtiere L. F., Pearson G. R. Radioimmune precipitation study comparing the Epstein-Barr virus membrane antigens expressed on P3HR-1 virus-superinfected Raji cells to those expressed on cells in a B-95 virus-transformed producer culture activated with tumor-promoting agent (TPA). Virology. 1980 Apr 30;102(2):360–369. doi: 10.1016/0042-6822(80)90103-8. [DOI] [PubMed] [Google Scholar]
- Takaki K., Polack A., Bornkamm G. W. Expression of a nuclear and a cytoplasmic Epstein-Barr virus early antigen after DNA transfer: cooperation of two distant parts of the genome for expression of the cytoplasmic antigen. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4568–4572. doi: 10.1073/pnas.81.14.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroman B., Luka J., Rodriguez M., Pearson G. R. Characterization of a major protein with a molecular weight of 160,000 associated with the viral capsid of Epstein-Barr virus. J Virol. 1985 Jan;53(1):107–113. doi: 10.1128/jvi.53.1.107-113.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. K., Rowe N. H., Sanderlin K. C. Herpes simplex virus types 1 and 2 in clinical infections: differences observed by electron microscopy. J Infect Dis. 1977 Mar;135(3):486–489. doi: 10.1093/infdis/135.3.486. [DOI] [PubMed] [Google Scholar]











