Abstract
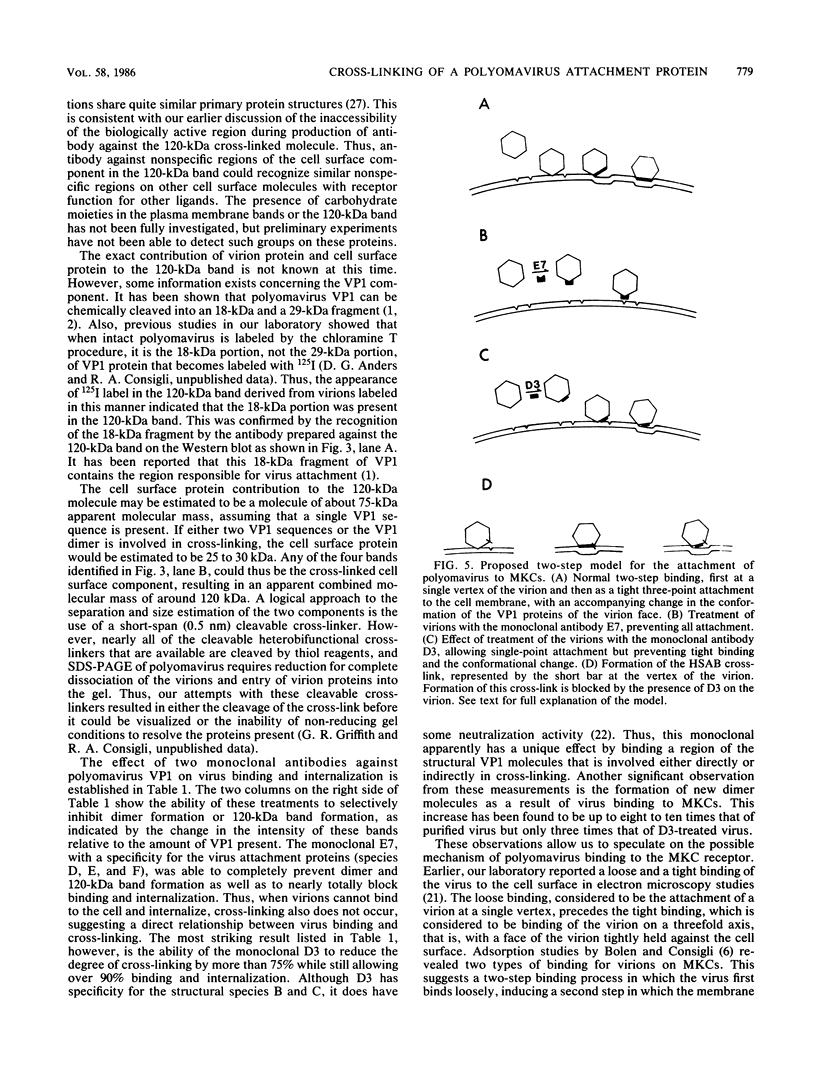
We used photoaffinity cross-linking with the heterobifunctional cross-linker N-hydroxysuccinimidyl 4-azidobenzoate (HSAB) to covalently link polyomavirus to a mouse kidney cell surface component. The virus-HSAB combination was adsorbed to the cells and then cross-linked and isolated in monopinocytotic vesicles from the cells after endocytosis. The cross-linked product was identified on sodium dodecyl sulfate-polyacrylamide gels by the presence of a new band carrying 125I-labeled virion protein with a higher molecular mass than the normal virion protein bands. A single new band, with an apparent molecular mass of 120 kilodaltons (120 kDa), was identified by this procedure. This band was formed only in the presence of the HSAB cross-linker when virions were bound to the cells. The band also copurified with cross-linked virions when virion-containing vesicles were treated with detergent to remove the cell membrane. Antibody treatments that blocked up to 100% of virus binding and internalization also blocked cross-linking, as measured by the formation of the 120-kDa band. The 120-kDa band was characterized by preparation of antibody against the excised band from the gel. This antibody was shown to have the expected dual specificity for polyomavirus VP1 sequences and plasma membrane proteins, as analyzed on Western blots. The anti-120-kDa antibody was also shown by immunofluorescence to bind to the surface of mouse kidney cells. These data have demonstrated that molecules of possible biological significance in the binding of polyomavirus to mouse kidney cells have been cross-linked and that cell surface molecules have been identified that may be characterized further for possible receptor function in polyomavirus attachment.
Full text
PDF

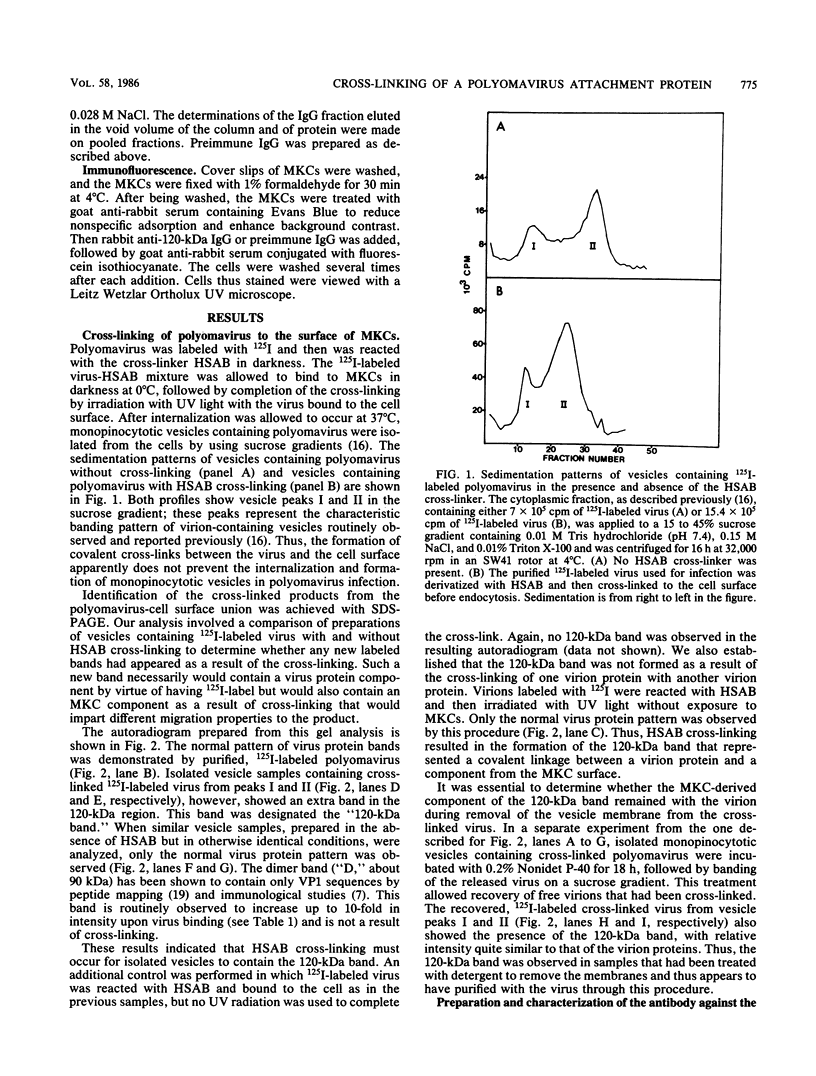
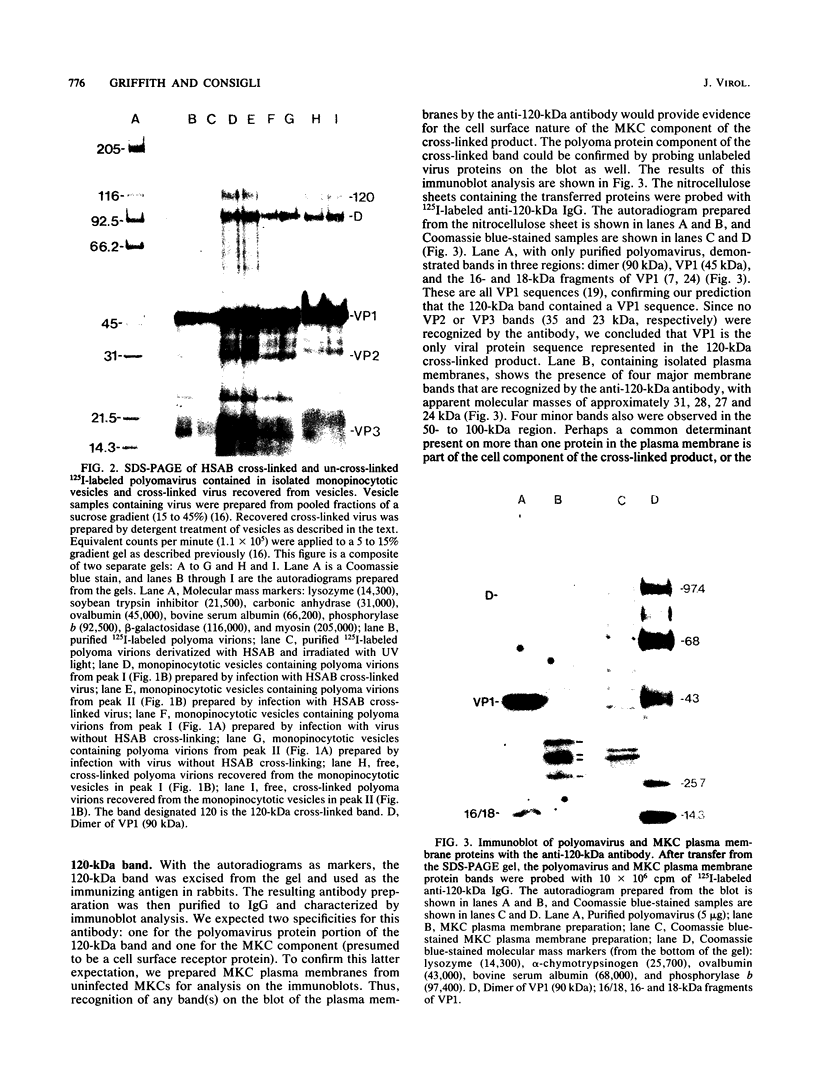




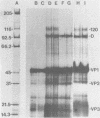
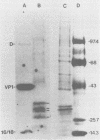
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders D. G., Consigli R. A. Chemical cleavage of polyomavirus major structural protein VP1: identification of cleavage products and evidence that the receptor moiety resides in the carboxy-terminal region. J Virol. 1983 Oct;48(1):197–205. doi: 10.1128/jvi.48.1.197-205.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders D. G., Consigli R. A. Comparison of nonphosphorylated and phosphorylated species of polyomavirus major capsid protein VP1 and identification of the major phosphorylation region. J Virol. 1983 Oct;48(1):206–217. doi: 10.1128/jvi.48.1.206-217.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Bittner M., Kupferer P., Morris C. F. Electrophoretic transfer of proteins and nucleic acids from slab gels to diazobenzyloxymethyl cellulose or nitrocellulose sheets. Anal Biochem. 1980 Mar 1;102(2):459–471. doi: 10.1016/0003-2697(80)90182-7. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Anders D. G., Trempy J., Consigli R. A. Differences in the subpopulations of the structural proteins of polyoma virions and capsids: biological functions of the multiple VP1 species. J Virol. 1981 Jan;37(1):80–91. doi: 10.1128/jvi.37.1.80-91.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Consigli R. A. Differential adsorption of polyoma virions and capsids to mouse kidney cells and guinea pig erythrocytes. J Virol. 1979 Nov;32(2):679–683. doi: 10.1128/jvi.32.2.679-683.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Consigli R. A. Separation of neutralizing and hemagglutination-inhibiting antibody activities and specificity of antisera to sodium dodecyl sulfate-derived polypeptides of polyoma virions. J Virol. 1980 Apr;34(1):119–129. doi: 10.1128/jvi.34.1.119-129.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Characterization of a DNA-protein complex and capsomere subunits derived from polyoma virus by treatment with ethyleneglycol-bis-N,N'-tetraacetic acid and dithiothreitol. J Virol. 1978 Jul;27(1):193–204. doi: 10.1128/jvi.27.1.193-204.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consigli R. A., Zabielski J., Weil R. Plaque assay for polyoma virus on primary mouse kidney cell cultures. Appl Microbiol. 1973 Oct;26(4):627–628. doi: 10.1128/am.26.4.627-628.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M., Fox C. F. Chemical cross-linking in biology. Annu Rev Biophys Bioeng. 1979;8:165–193. doi: 10.1146/annurev.bb.08.060179.001121. [DOI] [PubMed] [Google Scholar]
- Edwards J., Mann E., Brown D. T. Conformational changes in Sindbis virus envelope proteins accompanying exposure to low pH. J Virol. 1983 Mar;45(3):1090–1097. doi: 10.1128/jvi.45.3.1090-1097.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Ostapchuk P., Wimmer E. Bivalent attachment of antibody onto poliovirus leads to conformational alteration and neutralization. J Virol. 1983 Nov;48(2):547–550. doi: 10.1128/jvi.48.2.547-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko J. D., Gilmore J. R., Glaser M. Use of a fluorescent probe to determine the viscosity of LM cell membranes with altered phospholipid compositions. Biochemistry. 1977 May 3;16(9):1881–1890. doi: 10.1021/bi00628a019. [DOI] [PubMed] [Google Scholar]
- Frost E., Bourgaux P. Decapsidation of polyoma virus: identification of subviral species. Virology. 1975 Nov;68(1):245–255. doi: 10.1016/0042-6822(75)90165-8. [DOI] [PubMed] [Google Scholar]
- Griffith G. R., Consigli R. A. Isolation and characterization of monopinocytotic vesicles containing polyomavirus from the cytoplasm of infected mouse kidney cells. J Virol. 1984 Apr;50(1):77–85. doi: 10.1128/jvi.50.1.77-85.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Ek B., Rönnstrand L. Characterization of the receptor for platelet-derived growth factor on human fibroblasts. Demonstration of an intimate relationship with a 185,000-Dalton substrate for the platelet-derived growth factor-stimulated kinase. J Biol Chem. 1983 Aug 25;258(16):10054–10061. [PubMed] [Google Scholar]
- Hennache B., Boulanger P. Biochemical study of KB-cell receptor for adenovirus. Biochem J. 1977 Aug 15;166(2):237–247. doi: 10.1042/bj1660237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Fried M., Waterfield M. D. Nonhistone virion proteins of polyoma: characterisation of the particle proteins by tryptic peptide analysis by use of ion-exchange columns. Virology. 1975 Aug;66(2):408–419. doi: 10.1016/0042-6822(75)90213-5. [DOI] [PubMed] [Google Scholar]
- Ji T. H. The application of chemical crosslinking for studies on cell membranes and the identification of surface reporters. Biochim Biophys Acta. 1979 Apr 23;559(1):39–69. doi: 10.1016/0304-4157(79)90007-8. [DOI] [PubMed] [Google Scholar]
- Mackay R. L., Consigli R. A. Early events in polyoma virus infection: attachment, penetration, and nuclear entry. J Virol. 1976 Aug;19(2):620–636. doi: 10.1128/jvi.19.2.620-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott S. J., Consigli R. A. Production and characterization of monoclonal antibodies to polyomavirus major capsid protein VP1. J Virol. 1985 Nov;56(2):365–372. doi: 10.1128/jvi.56.2.365-372.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Center M. S., Consigli R. A. Origin of the polyoma virus-associated endonuclease. J Virol. 1975 Jan;17(1):127–131. doi: 10.1128/jvi.17.1.127-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. Immunological reactivity of antisera to sodium dodecyl sulfate-derived polypeptides of polyoma virions. J Virol. 1977 Mar;21(3):1113–1120. doi: 10.1128/jvi.21.3.1113-1120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Bayley P. M., Brown E. B., Martin S. R., Waterfield M. D., White J. M., Wilson I. A., Wiley D. C. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci U S A. 1982 Feb;79(4):968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Consigli R. A. Transient inhibition of polyoma virus synthesis by Sendai virus (parainfluenza I). I. Demonstration and nature of the inhibition by inactivated virus. J Virol. 1972 Dec;10(6):1091–1097. doi: 10.1128/jvi.10.6.1091-1097.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C., Goldstein J. L., Brown M. S., Russell D. W. The LDL receptor gene: a mosaic of exons shared with different proteins. Science. 1985 May 17;228(4701):815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip C. C., Yeung C. W., Moule M. L. Photoaffinity labeling of insulin receptor of rat adiopocyte plasma membrane. J Biol Chem. 1978 Mar 25;253(6):1743–1745. [PubMed] [Google Scholar]
- Yip C. C., Yeung C. W., Moule M. L. Photoaffinity labeling of insulin receptor proteins of liver plasma membrane preparations. Biochemistry. 1980 Jan 8;19(1):70–76. doi: 10.1021/bi00542a011. [DOI] [PubMed] [Google Scholar]
- Zarling D. A., Miskimen J. A., Fan D. P., Fujimoto E. K., Smith P. K. Association of Sendai virion envelope and a mouse surface membrane polypeptide on newly infected cells: lack of association with H-2K/D or alteration of viral immunogenicity. J Immunol. 1982 Jan;128(1):251–257. [PubMed] [Google Scholar]