Abstract
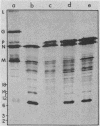
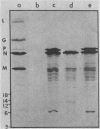
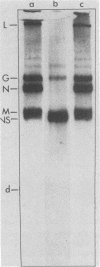
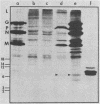
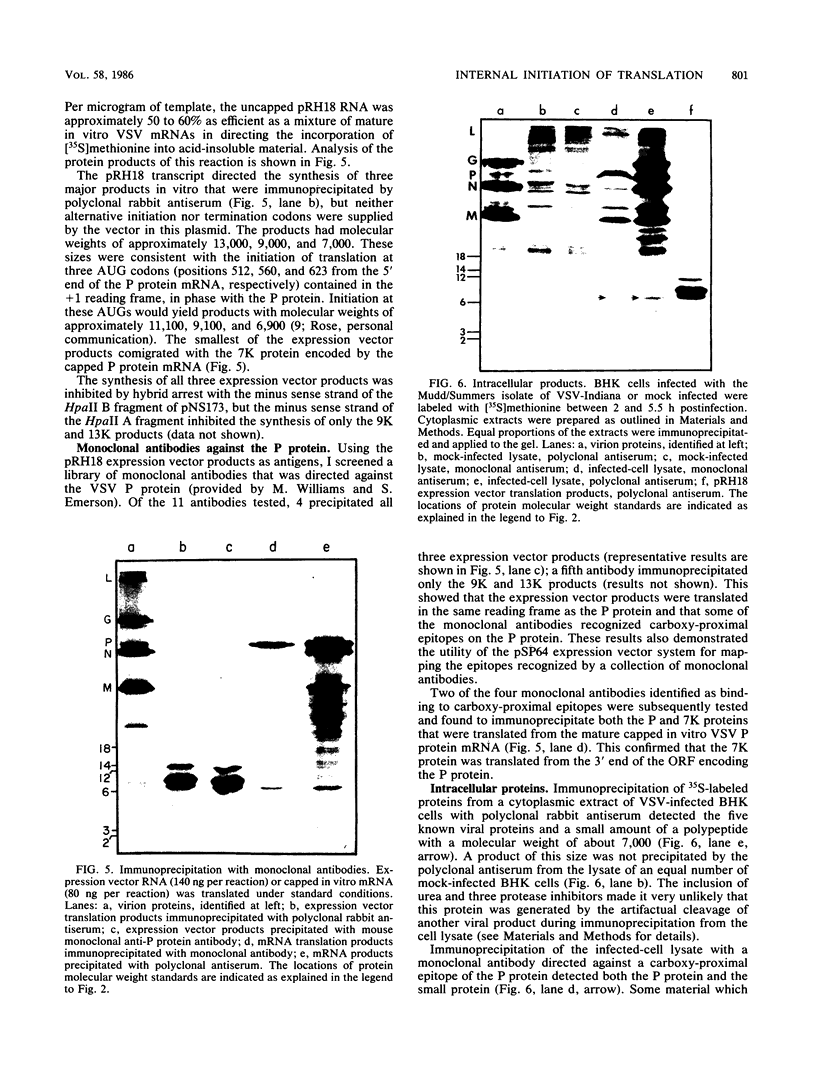
In vitro translation of a mixture of the vesicular stomatitis virus (VSV) polyadenylated mRNAs yielded a previously undetected protein with a molecular weight of approximately 7,000 (7K protein). Hybrid-arrested translation demonstrated that both the 7K protein and the VSV phosphoprotein (P protein) were encoded by the P protein message. Immunoprecipitation of the 7K protein with monoclonal antiserum directed against the P protein indicated that the two products were encoded in the same open reading frame. A protein of approximately the same size was immunoprecipitated from cytoplasmic extracts of VSV-infected cells by both the polyclonal and monoclonal antisera, and it is likely that it was a previously unrecognized viral gene product. Translational mapping of the P protein mRNA in vitro indicated that the 7K protein was encoded in the 3' one-third of the sequence. The synthesis of the 7K protein in vitro was unaffected by hybrid arrest conditions which blocked the 5' two-thirds of the mRNA and inhibited synthesis of the P protein. These results imply that the ribosomes bind and initiate translation internally on the P protein mRNA at a site located hundreds of nucleotides downstream from the capped 5' end.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. D., Abraham G., Colonno R. J. Vesicular stomatitis virus: mode of transcription. J Gen Virol. 1977 Jan;34(1):1–8. doi: 10.1099/0022-1317-34-1-1. [DOI] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Rozenblatt S., Arnheiter H., Richardson C. D. Measles virus P gene codes for two proteins. J Virol. 1985 Mar;53(3):908–919. doi: 10.1128/jvi.53.3.908-919.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Gould K. G., Akashi H., Clerx-van Haaster C. M. The complete sequence and coding content of snowshoe hare bunyavirus small (S) viral RNA species. Nucleic Acids Res. 1982 Jun 25;10(12):3703–3713. doi: 10.1093/nar/10.12.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W., Ball L. A., Hightower L. E. Coding assignments of the five smaller mRNAs of Newcastle disease virus. J Virol. 1982 Sep;43(3):1024–1031. doi: 10.1128/jvi.43.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch V., Muel B., Brun G. Temperature-sensitive mutant TS O82 of vesicular stomatitis virus. I. Rescue at nonpermissive temperature by uv-irradiated virus. Virology. 1979 Feb;93(1):286–290. doi: 10.1016/0042-6822(79)90301-5. [DOI] [PubMed] [Google Scholar]
- Emerson S. U. Reconstitution studies detect a single polymerase entry site on the vesicular stomatitis virus genome. Cell. 1982 Dec;31(3 Pt 2):635–642. doi: 10.1016/0092-8674(82)90319-1. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. S., Banerjee A. K. Vesicular stomatitis virus NS proteins: structural similarity without extensive sequence homology. J Virol. 1985 Jul;55(1):60–66. doi: 10.1128/jvi.55.1.60-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B. M., Kolakofsky D. Sendai virus contains overlapping genes expressed from a single mRNA. Cell. 1983 Dec;35(3 Pt 2):829–836. doi: 10.1016/0092-8674(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Herman R. C. Conditional synthesis of an aberrant glycoprotein mRNA by the internal deletion mutant of vesicular stomatitis virus. J Virol. 1983 Jun;46(3):709–717. doi: 10.1128/jvi.46.3.709-717.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. C., Lazzarini R. A. Aberrant glycoprotein mRNA synthesized by the internal deletion mutant of vesicular stomatitis virus. J Virol. 1981 Oct;40(1):78–86. doi: 10.1128/jvi.40.1.78-86.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J., Choppin P. W. Sequences of mRNAs derived from genome RNA segment 7 of influenza virus: colinear and interrupted mRNAs code for overlapping proteins. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4170–4174. doi: 10.1073/pnas.78.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert M., Rittenhouse L., Perrault J., Summers D. F., Kolakofsky D. Plus and minus strand leader RNAs in negative strand virus-infected cells. Cell. 1979 Nov;18(3):735–747. doi: 10.1016/0092-8674(79)90127-2. [DOI] [PubMed] [Google Scholar]
- Masters P. S., Samuel C. E. Detection of in vivo synthesis of polycistronic mRNAs of vesicular stomatitis virus. Virology. 1984 Apr 30;134(2):277–286. doi: 10.1016/0042-6822(84)90297-6. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
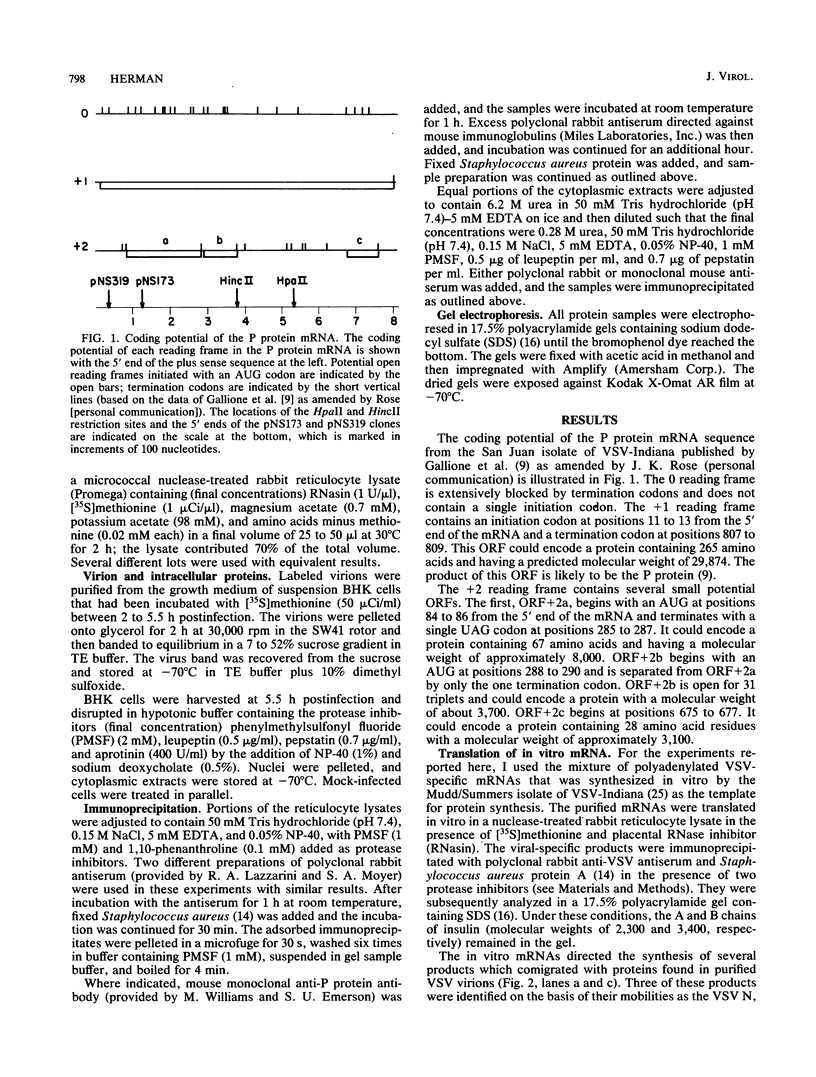
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Moyer S. A., Banerjee A. K. Messenger RNA species synthesized in vitro by the virion-associated RNA polymerase of vesicular stomatitis virus. Cell. 1975 Jan;4(1):37–43. doi: 10.1016/0092-8674(75)90131-2. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Grubman M. J., Ehrenfeld E., Banerjee A. K. Studies on the in vivo and in vitro messenger RNA species of vesicular stomatitis virus. Virology. 1975 Oct;67(2):463–473. doi: 10.1016/0042-6822(75)90447-x. [DOI] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Oct;42(2):328–340. doi: 10.1016/0042-6822(70)90277-1. [DOI] [PubMed] [Google Scholar]
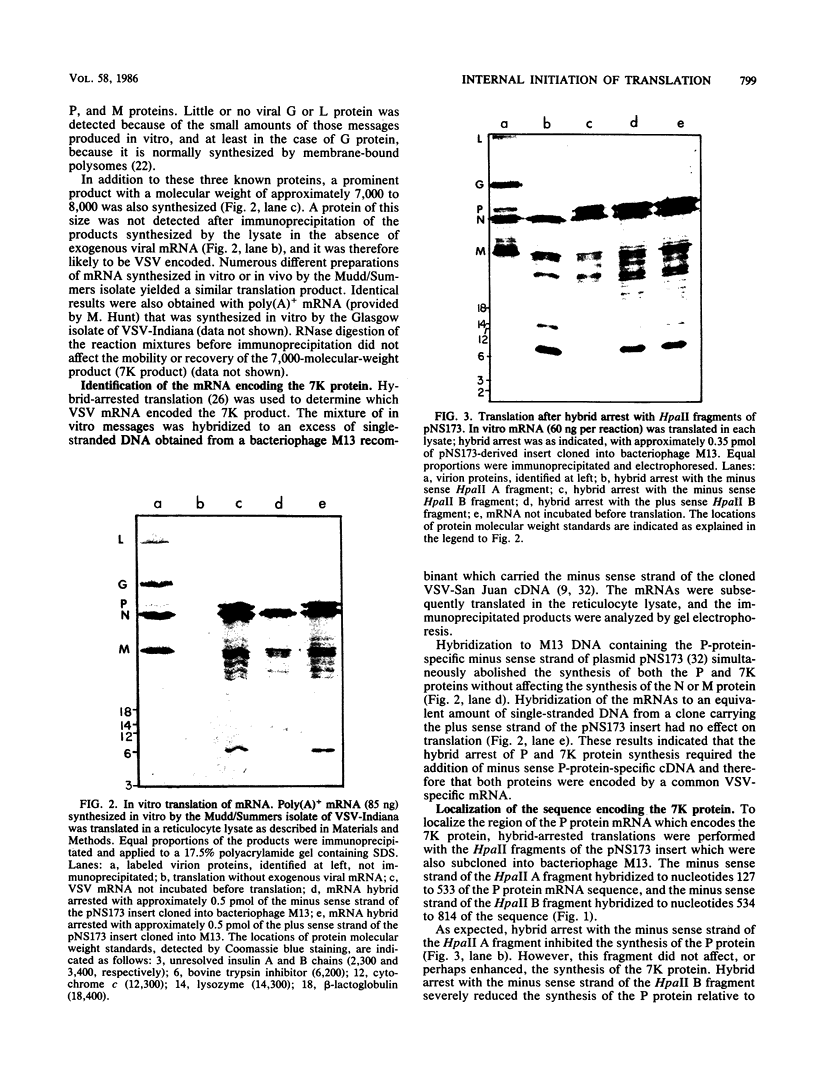
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. G., Harris T. J., Lamb R. A. Analysis and gene assignment of mRNAs of a paramyxovirus, simian virus 5. Virology. 1984 Oct 30;138(2):310–323. doi: 10.1016/0042-6822(84)90354-4. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Dumont R., Baltimore D. Screening procedure for complementation-dependent mutants of vesicular stomatitis virus. J Virol. 1975 Jan;15(1):41–49. doi: 10.1128/jvi.15.1.41-49.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Complete sequences of the ribosome recognition sites in vesicular stomatitis virus mRNAs: recognition by the 40S and 80S complexes. Cell. 1978 Jun;14(2):345–353. doi: 10.1016/0092-8674(78)90120-4. [DOI] [PubMed] [Google Scholar]
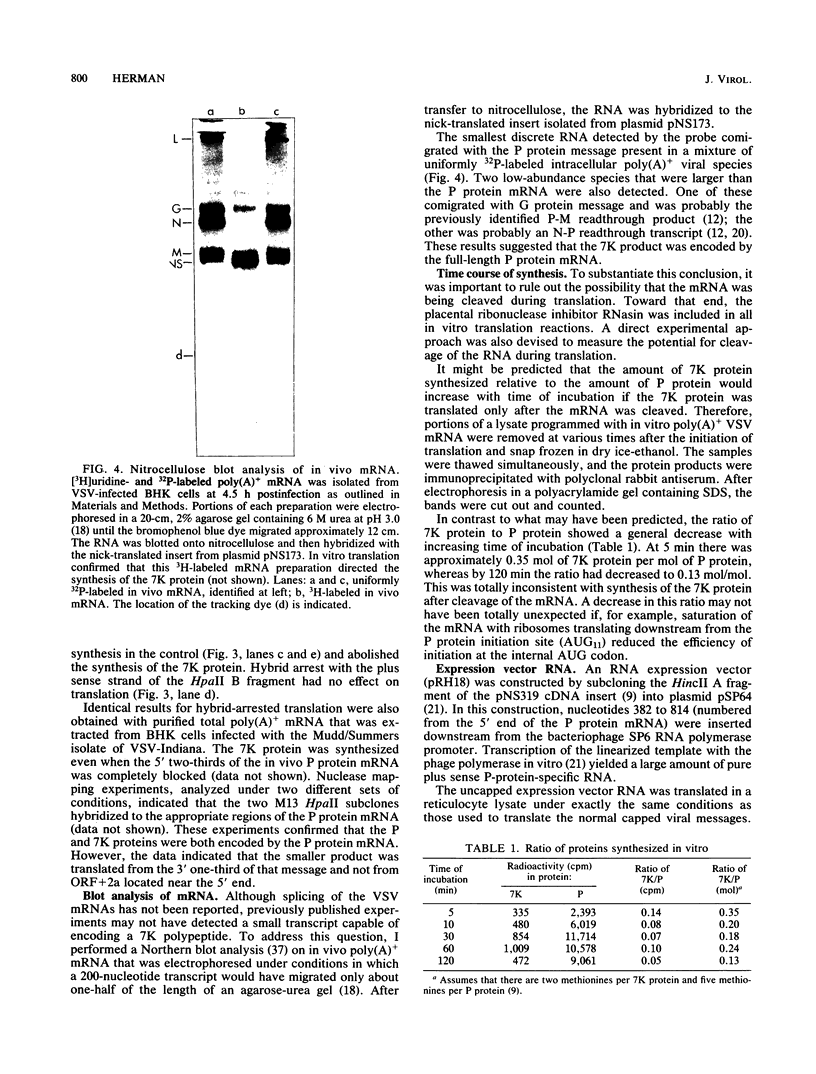
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Iverson L. Nucleotide sequences from the 3'-ends of vesicular stomatitis virus mRNA's as determined from cloned DNA. J Virol. 1979 Nov;32(2):404–411. doi: 10.1128/jvi.32.2.404-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K. Nucleotide sequences of ribosome recgonition sites in messenger RNAs of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3672–3676. doi: 10.1073/pnas.74.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Identical 3'-terminal octanucleotide sequence in 18S ribosomal ribonucleic acid from different eukaryotes. A proposed role for this sequence in the recognition of terminator codons. Biochem J. 1974 Sep;141(3):609–615. doi: 10.1042/bj1410609a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. Unique mode of transcription in vitro by Vesicular stomatitis virus. Cell. 1980 Aug;21(1):267–275. doi: 10.1016/0092-8674(80)90134-8. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber L. A., Hickey E. D., Baglioni C. Influence of potassium salt concentration and temperature on inhibition of mRNA translation by 7-methylguanosine5'-monophosphate. J Biol Chem. 1978 Jan 10;253(1):178–183. [PubMed] [Google Scholar]