Abstract
bEND.3 cells are polyoma middle T-transformed mouse brain endothelial cells that express very little or no thrombospondin-1, a natural inhibitor of angiogenesis, but express high levels of platelet endothelial cell adhesion molecule-1 (PECAM-1) that localizes to sites of cell–cell contact. Here, we have examined the role of PECAM-1 in regulation of bEND.3 cell proliferation, migration, morphogenesis, and hemangioma formation. We show that down-regulating PECAM-1 expression by antisense transfection of bEND.3 cells has a dramatic effect on their morphology, proliferation, and morphogenesis on Matrigel. There is an optimal level for PECAM-1 expression such that high levels of PECAM-1 inhibit, whereas moderate levels of PECAM-1 stimulate, endothelial cell morphogenesis. The down-regulation of PECAM-1 in bEND.3 cells resulted in reexpression of endogenous thrombospondin-1 and its antiangiogenic receptor CD36. The expression of the vascular endothelial growth factor receptors flk-1 and flt-1, as well as integrins and metalloproteinases (which are involved in angiogenesis), were also affected. These observations are consistent with the changes observed in proliferation, migration, and adhesion characteristics of the antisense-transfected bEND.3 cells as well as with their lack of ability to form hemangiomas in mice. Thus, a reciprocal relationship exists between thrombospondin-1 and PECAM-1 expression, such that these two molecules appear to be constituents of a “switch” that regulates in concert many components of the angiogenic and differentiated phenotypes of endothelial cells.
INTRODUCTION
Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) is a member of the immunoglobulin (Ig) superfamily that is expressed on endothelial cells (ECs) of large and small vessels, as well as on platelets, leukocytes, and hematopoietic precursors. It contains six Ig-like domains, a short hydrophobic transmembrane domain, and a cytoplasmic tail of variable length due to alternative splicing of exons 10 through 16 (Newman et al., 1990; Albelda et al., 1991; Xie and Muller, 1993; DeLisser et al., 1994b; Kirschbaum et al., 1994; Newman, 1997). PECAM-1 has been implicated in leukocyte–EC interactions, transendothelial migration, endothelial cell–cell adhesion, angiogenesis, and development of the early cardiovascular system (Bogen et al., 1992; Horak et al., 1992; Baldwin et al., 1994; DeLisser et al., 1994b; Berman and Muller, 1995; Bischoff, 1995; Muller, 1995). In some cultured ECs, such as the human umbilical vein endothelial cell (HUVEC), PECAM-1 is normally localized to the sites of cell–cell contact in regions distinct from adherens junctions or tight junctions (Ayalon et al., 1994; DeLisser et al., 1994b; Dejana et al., 1995; Romer et al., 1995). Differences in the detergent extractability of PECAM-1 compared with cadherins also suggest differences in the mode of association with cytoskeletal proteins (Ayalon et al., 1994; Romer et al., 1995). PECAM-1 mediates cell–cell adhesion via both homophilic and heterophilic mechanisms. Homophilic adhesion requires Ig domains 1 and 2 (Sun et al., 1996). Heterophilic cell–cell adhesion is dependent on divalent cations and is inhibited by antibodies that bind either Ig domain 2 or 6 through a mechanism that is not yet understood (Muller et al., 1992; DeLisser et al., 1993). αvβ3 integrin has been implicated as a heterotypic ligand for PECAM-1, which may mediate leukocyte–EC interactions facilitating transmigration of the endothelium (Piali et al., 1995; Buckley et al., 1996). Integrin-associated protein, which is a receptor for thrombospondin-1 (TS1; Gao et al., 1996b) and associates with αvβ3, is also essential for EC transmigration (Cooper et al., 1995). However, the role of these molecules in EC interactions remains elusive. Finally, PECAM-1-mediated cellular interactions may be affected by deletion or alternative splicing of the PECAM-1 cytoplasmic domain (Baldwin et al., 1994; DeLisser et al., 1994a; Kirschbaum et al., 1994; Yan et al., 1995), suggesting an important role for the intracellular domain in regulating PECAM-1-dependent cellular interactions. This is perhaps mediated by specific interactions of signal-transducing molecules with cytoplasmic domains of various PECAM-1 isoforms. PECAM-1 was recently found to be phosphorylated on tyrosine residues in ECs (Lu et al., 1996; Osawa et al., 1997; Pinter et al., 1997), providing potential docking sites for signal-transducing molecules. Phosphorylated PECAM-1 interacts with src-like kinases (Lu et al., 1997) and with the tyrosine phosphatase SH-PTP2 (Jackson et al., 1997b; Masuda et al., 1997) via their SH-2 domains. These interactions may be mediated by phosphorylated tyrosine residues in exons 13 and 14 of the PECAM-1 cytoplasmic domain (Lu et al., 1996; Famiglietti et al., 1997; Jackson et al., 1997a). However, the role of these molecules and the signaling pathways involved in modulating the adhesive function of PECAM-1 in an “inside-out” direction are not understood.
Mouse brain endothelial cells (MBECs) are very susceptible to transformation by polyoma middle T oncogenes (Williams et al., 1989). Such transformed cells (e.g., bEND.3 cells) grow rapidly in culture, express little or no TS1 (a natural inhibitor of angiogenesis; RayChaudhury et al., 1994; Sheibani and Frazier, 1995), and form hemangiomas in mice (Montesano et al., 1990). We have demonstrated that constitutive reexpression of TS1 in bEND.3 (bEND/TS) cells restores many aspects of a normal EC phenotype. The bEND/TS cells exhibit an altered morphology, a slower growth rate, a normal proteolytic balance (i.e., PAI-1>>uPA), and an ability to organize better on Matrigel. Most dramatically, the TS1-transfected cells lose the ability to form tumors in mice (Sheibani and Frazier, 1995). These data suggest that TS1, and perhaps other TS isoforms, have a major role in the regulation of EC phenotype. Furthermore, concomitant changes in the expression of many genes as part of the response to TS1 expression suggested that there is a switch that coordinates components of the two phenotypic states of EC: the quiescent, differentiated state versus the migratory, proliferative, and hence angiogenic state.
To better understand the mechanisms of TS1 action on ECs, we examined the expression of several genes in TS1-transfected bEND.3 cells whose products have been implicated in regulation of the EC phenotype. We showed that bEND.3 cells express very high levels of PECAM-1 localized to sites of cell–cell contact. However, bEND/TS cells completely lacked PECAM-1 expression (Sheibani et al., 1997). This may affect the interaction with and recruitment of host EC that is an essential part of hemangioma formation (Montesano et al., 1990). We have recently reported that expression of murine or human PECAM-1 cDNA in these cells significantly enhanced their ability to differentiate on Matrigel (Sheibani et al., 1997). Thus, PECAM-1 can modulate EC interactions that are essential during angiogenesis. The purpose of the present study was to further investigate the role of PECAM-1 in regulation of bEND.3 cell–cell interactions during angiogenesis and tumorigenesis.
MATERIALS AND METHODS
Construction of Expression Vectors
The antisense PECAM-1 expression plasmids were generated by ligating a 0.7-kb pair BamHI fragment of murine PECAM-1 (mPECAM-1) cDNA containing approximately one-third of the 5′ coding sequences in an antisense orientation with either pREP8 (constitutive) or pMEP4 (inducible) expression vectors (Invitrogen, San Diego, CA) digested with BamHI and dephosphorylated. The size and orientation of all inserts were confirmed by restriction digestion. The pREP8 vector provides constitutive expression and l-histidinol (his D) selection. Cells were initially selected in the presence of 2.5 mM l-histidinol and then increased to 10 mM. The pMEP4 vector provides inducible expression in the presence of heavy metals (zinc or cadmium) and hygromycin (hyg) selection. Cells were selected in the presence of 50 μg/ml hygromycin. However, the promoter was leaky enough to result in sufficient levels of expression that no induction was essential.
Cell Lines and DNA Transfection
bEND.3 cells were maintained as described previously (Sheibani and Frazier, 1995). The vectors used to express antisense PECAM-1, the pREP8/BAMAS and the pMEP4/BAMAS, encoded the his gene (which allows growth in medium containing l-histidinol) and the hyg gene (which allows growth in the presence of hygromycin), respectively. Cells were transfected by lipofectin as described previously (Sheibani and Frazier, 1995). Transfected cells were grown in the presence of from 2.5 to 10 mM l-histidinol or 50 μg/ml hygromycin. After 2–3 wk, resistant colonies were either cloned directly or were expanded, enriched by cell sorting, and then individual clones were isolated as described below. Individual clones were expanded and screened by Western blotting the total cell lysates. Several representative clones were obtained for additional studies.
Fluorescence-activated Cell-sorting Analysis
Cells grown on 100-mm tissue culture plates were removed by 0.04% EDTA, 0.05% bovine serum albumin (BSA) in phosphate-buffered saline (Dulbecco’s PBS, Life Technologies, Gaithersburg, MD), washed with Tris-buffered saline (TBS; 20 mM Tris-HCl, pH 7.6, 137 mM NaCl), resuspended in TBS with 1% goat serum, and kept on ice for 20 min. Cells were pelleted, resuspended in TBS with 1% BSA containing anti-PECAM-1 antibody (10 μg/ml; Mab390), and kept on ice for 30 min. Cells were washed twice with TBS with 1% BSA, resuspended in TBS with 1% BSA containing a 1:100 dilution of fluorescein isothiocyanate-conjugated goat anti-rat antibody (Pierce, Rockford, IL) and kept on ice for 30 min. Cells were washed twice with TBS with 1% BSA, resuspended in TBS with 1% BSA, and flow cytometry was performed using a FACScan (Becton Dickinson, San Jose, CA). To enrich for population of antisense transfected cells that lacked PECAM-1 expression, magnetic Dynabeads conjugated with sheep anti-rat IgG (Dynal, Lake Success, NY) were used. Cells were stained with anti-PECAM-1 antibody as described above and then incubated with magnetic beads conjugated with a sheep anti-rat IgG to separate the cells that express PECAM-1 from those that do not. This was performed twice to enrich for antisense-transfected bEND.3 cells that express little or no PECAM-1. These cells were then plated at low density for isolation of individual clones.
Northern Blot Analysis
Poly(A+) RNA was isolated from logarithmically growing cells as described (Sheibani et al., 1991). RNA (5 μg) was electrophoresed in a 1.2% agarose gel containing formaldehyde, transferred to ζ-probe membrane (Bio-Rad, Hercules, CA), prehybridized, and then hybridized in the presence of random primer 32P-labeled cDNA probes. The cDNA probes used were the mPECAM-1 entire coding regions (from Dr. S.M. Albelda, University of Pennsylvania, Philadelphia, PA), the 1.4-kb pair BamHI 5′ fragment of human TS1 cDNA (Sheibani and Frazier, 1995), the full-length murine CD36 cDNA (from Dr. G. Endemann, Scios Nova, Sunnyvale, CA), the flk-1 (a 2.6-kb pair fragment of the murine flk-1 cDNA encoding part of the extracellular domain), and flt-1 (a 2.1-kb pair fragment of the murine flt-1 cDNA encoding part of the extracellular domain) cDNA probes (from Dr. W. Risau, Max-Planck Institute, Bad Nauheim, Germany), the cDNA probe for rat αv (from Dr. M. Hammerman, Washington University, St. Louis, MO), the cDNA probes for mouse β1 (from Dr. D. Dean, Washington University, St. Louis, MO), the cDNA probe for mouse β3, and β5 integrins (from Dr. P. Ross, Washington University, St. Louis, MO), the cDNA probe for human c-jun (from Dr. R. Tjian, University of California, Berkeley, CA), and a 1.3-kb pair PstI fragment of rat glyceraldehyde-3-phosphate dehydrogenase cDNA to control for loading (Sheibani and Frazier, 1995).
Western Blot Analysis
Cells were removed from the plate by trypsin-EDTA and washed with TBS. Approximately 5 × 105 cells were resuspended in 0.1 ml of lysis buffer (20 mM Tris-HCl, pH 7.6, 2 mM EDTA) and stored at −70°C until ready for analysis. Cell lysates were thawed, mixed with 6× SDS sample buffer, boiled, and analyzed by SDS-PAGE and blotting as described previously (Sheibani and Frazier, 1995). The antibodies used were PECAM 1.3 (a Mab against hPECAM-1 at 0.5 μg/ml, a gift from Dr. P.J. Newman, Blood Research Institute, Milwaukee, WI) and a polyclonal anti-mouse PECAM-1 antibody at 1 μg/ml (a gift from Dr. B.A. Imhof, Basel Institute for Immunology, Basel, Switzerland).
Three-Dimensional Culture
Matrigel (Collaborative Research, Bedford, MA) was diluted to 10 mg/ml with serum-free medium, and 0.5 ml was added per well of 24-well tissue culture plates and allowed to gel at 37°C for at least 30 min. Cells were removed by trypsin-EDTA, washed with growth medium, resuspended at 1 × 105 cells/ml, and 0.5 ml was gently added to each of duplicate wells. The plates were monitored for 6 to 24 h and photographed with a Nikon microscope. Each experiment was repeated at least twice with identical results.
Tumorigenesis Assay
The tumorigenesis assays were performed as described previously (Sheibani and Frazier, 1995). Briefly, cells were removed by trypsin-EDTA, washed with growth medium, resuspended at 2 × 107 cells/ml in growth medium, and 0.25 ml of cell suspension was injected subcutaneously on each side into the rear flanks of 6-wk-old male nude mice [Harlan Sprague Dawley, Indianapolis, IN (two sites per mouse and three mice per cell line)]. The mice were maintained in a sterile environment and examined daily. The care of the mice was provided by the Washington University veterinary staff and was performed according to institutional guidelines.
Cell Adhesion Assays
The cell adhesion assays were performed as described previously (Gao et al., 1996a). Briefly, 96-well Nunc plates were coated with different concentrations of vitronectin in TBS with Ca2+ and Mg2+ (20 mM Tris-HCl, pH 7.6, 150 mM NaCl with 2 mM each CaCl2 and MgCl2) at 4°C. The next day, plates were washed with TBS containing Ca2+/Mg2+ and blocked with 1% BSA in the same buffer for at least 1 h. Cells were lifted with EDTA (0.04% EDTA with 0.05% BSA prepared in PBS), washed once with TBS plus Ca2+/Mg2+, and resuspended at about 5 × 105 cells/ml in HBS buffer (20 mM HEPES, pH 7.4, 150 mM NaCl) containing CaCl2 and MgCl2 at 2 mM each. Fifty microliters of the cell suspension were added to each well of the coated plate and incubated for 1 h at 37°C. After incubation, plates were washed with TBS plus Ca2+/Mg2+ to remove nonadherent cells. Adherent cells were quantified with endogenous phosphatase activity as described. Triplicate wells were used for each concentration of vitronectin and the experiments were repeated at least three times with identical results.
RESULTS
Expression of Antisense PECAM-1 in bEND.3 Cells
bEND.3 cells proliferate rapidly in culture, express little or no TS1, and are capable of forming hemangiomas in mice within 48 h. This is accomplished by rapid recruitment of host ECs (Williams et al., 1989). PECAM-1, an EC adhesion molecule, may be involved in mediating interactions between bEND.3 cells and host ECs. This is supported by the fact that TS1 expression in bEND.3 cells suppresses PECAM-1 expression concomitant with a block in the ability of these cells to form hemangiomas (Sheibani and Frazier, 1995). To further investigate the role of PECAM-1 in endothelial cell–cell interactions during angiogenesis and tumorigenesis, bEND.3 cells were transfected with PECAM-1 antisense constructs (pREP8/BAMAS or pMEP4/BAMAS). After transfection, cells were selected in the presence of the appropriate selection reagent and resistant colonies were isolated, expanded, and screened by Western blot analysis. The population of cells transfected with pREP8/BAMAS (designated with the suffix “POP”) were also enriched by magnetic bead selection for cells that lacked PECAM-1 expression, and several clones were isolated.
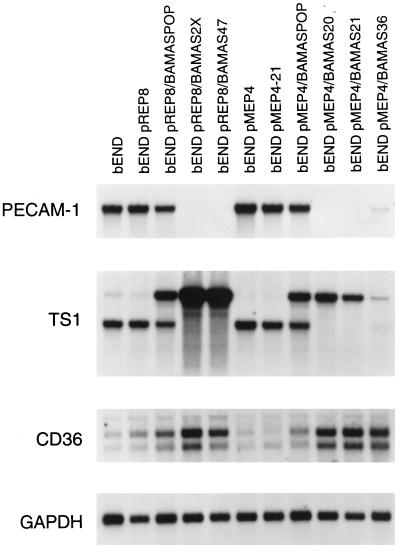
We isolated at least 60 clones from transfections with each of the expression vectors representing a wide range of residual PECAM-1 expression levels. Figure 1 illustrates the expression levels of PECAM-1 mRNA in populations and several representative clones of antisense PECAM-1-transfected bEND.3. We observed very effective down-regulation of PECAM-1 levels in the transfected cells with both expression vectors. We obtained several representative clones of antisense-transfected cells that expressed intermediate levels of PECAM-1 (pMEP4/BAMAS36) or no PECAM-1 (pREP8/BAMAS47, pMEP4/BAMAS20, and pMEP4/BAMAS21) that were used for additional studies. Fluorescence-activated cell sorter (FACS) analysis using an anti-mouse PECAM-1 monoclonal antibody indicates a good correlation between PECAM-1 mRNA levels and the levels of surface expression of PECAM-1 (see Figures 1 and 4).
Figure 1.
Analysis of steady-state PECAM-1, TS1, and CD36 mRNA levels in antisense-transfected cells. Poly(A+) RNA was prepared from logarithmically growing cells, size fractionated on 1.2% agarose-formaldehyde gel, and transferred to ζ-probe membrane. The mRNAs for PECAM-1, TS1, and CD36 were detected by using specific cDNA probes for PECAM-1, TS1, and CD36. This blot was also probed with the cDNA for GAPDH to control for loading. The bEND are parental cells; the bEND pREP8 and bEND pMEP4 are vector-transfected controls; the bEND pREP8/BAMASPOP and bEND pMEP4/BAMASPOP are the population of transfected cells; the bEND pREP8/BAMASPOP2× are the population of transfected cells enriched twice for cells that lacked PECAM-1; and the bEND pREP8/BAMAS47, pMEP4/BAMAS20, -21, and -36 are clones of antisense-transfected cells.
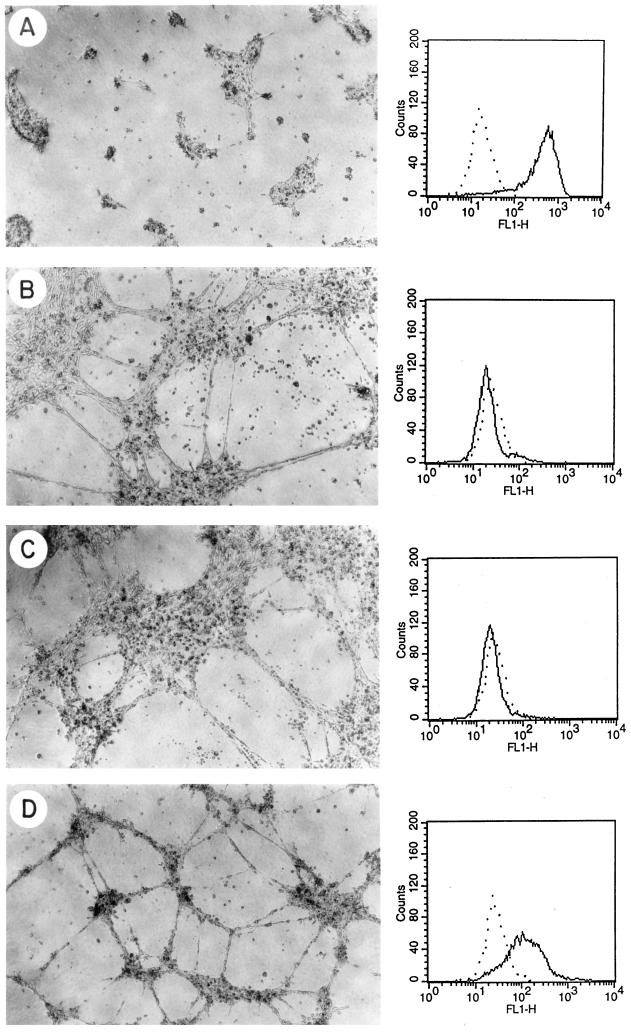
Figure 4.
Differentiation of antisense-transfected bEND.3 cells that express different levels of PECAM-1 in three-dimensional Matrigel cultures after 24 h. The left panels illustrate the phase micrograph and the right panels display the FACS analysis of the same cells: A, pMEP4 (control); B, pMEP4/BAMAS20; C, pMEP4/BAMAS21; and D, pMEP4/BAMAS36. These results are representative of more than a dozen similar experiments performed with vector or antisense PECAM-1-transfected clones of bEND.3 cells.
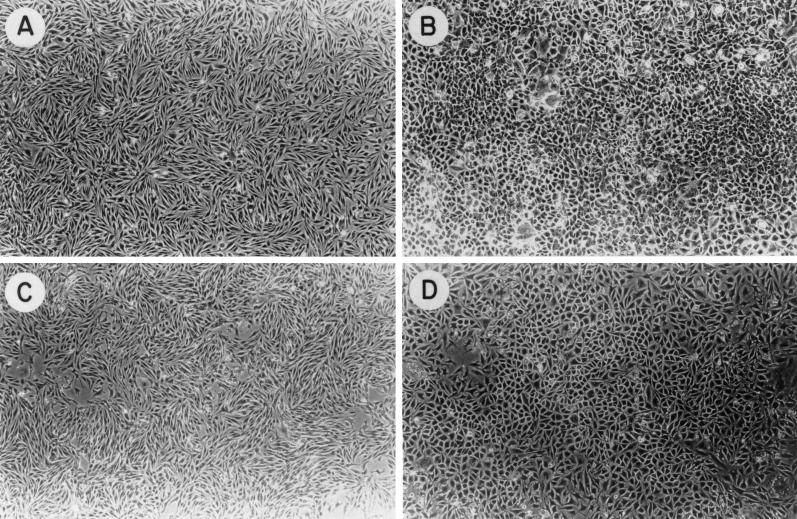
The morphology of transfected bEND.3 cells, vector controls (population), and antisense (clones) are shown in Figure 2. Complete down-regulation of PECAM-1 in bEND.3 cells had a dramatic effect on their morphology compared with vector-transfected or parental bEND.3 cells. The cells that completely lacked PECAM-1 exhibited a more normal cobblestone-like morphology (Figure 2, B and D) as compared with the spindle-shaped morphology of vector-transfected or parental bEND.3 cells (Figure 2, A and C). The cells lacking PECAM-1 also grew at a much slower rate when compared with control cells (our unpublished data). This is consistent with our previous observation that the TS1-transfected bEND.3 cells that lacked PECAM-1 expression grew at a much slower rate than did control cells (Sheibani and Frazier, 1995). We consistently observed a large number of floating cells, which failed to exclude trypan blue, in the culture of antisense-transfected bEND.3 cells that completely lacked PECAM-1 expression. Clones expressing intermediate levels of PECAM-1 grew at rates intermediate between parental bEND.3 cells and clones lacking PECAM-1, with far fewer floating dead cells.
Figure 2.
Phase micrograph of bEND.3 parental cells transfected with (A) pREP8, (B) pREP8/BAMAS5, (C) pMEP4, and (D) pMEP4/BAMAS21.
Up-Regulation of TS1 and CD36 Expression in Antisense-transfected bEND.3 Cells
We had previously shown that bEND.3 cells rapidly proliferate in culture and that addition of exogenous human TS1 (RayChaudhury et al., 1994) or expression of human TS1 (Sheibani and Frazier, 1995) in these cells has a dramatic effect on their proliferation and morphology. We next examined the expression of the endogenous mouse TS1 gene in the antisense-transfected bEND.3 cells (Figure 1). The expression of TS1 mRNA was increased in every one of the dozen or more clones in which PECAM-1 expression was down-regulated. The bEND.3 cells or vector-transfected cells expressed little or no full-length TS1 mRNA (∼6 kb). However, a smaller, presumably polyadenylated, TS1 transcript (∼4.0 kb) was present in these cells, but was not translated (Sheibani and Frazier, unpublished data). In contrast, the antisense-transfected cells that completely lacked PECAM-1 expressed high levels of full-length TS1 mRNA concomitant with loss of the shorter transcript. This observation suggests that the mechanism of TS1 suppression in bEND.3 cells may involve altered processing of TS1 mRNA rather than transcriptional regulation.
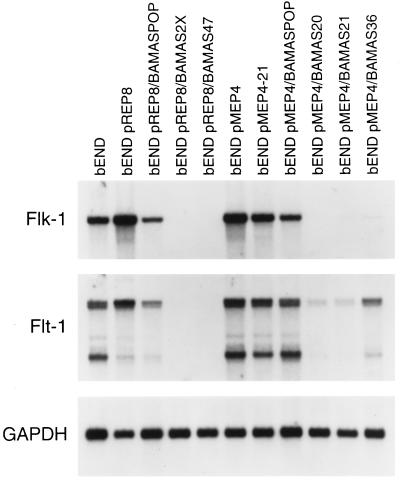
We have recently shown that the inhibitory effects of TS1 on ECs in vitro are mediated through CD36, a known cell surface receptor for TS1 that is normally expressed on microvascular ECs (Dawson et al., 1997). The expression of CD36 mRNA was rarely detected in parental bEND.3 cells and was dramatically increased in antisense-transfected bEND.3 cells (Figure 1). Thus, enhanced expression of TS1 and its inhibitory receptor may contribute to the dramatic effects of antisense PECAM-1 expression on the morphology and proliferation of bEND.3 cells. Figure 3 illustrates the expression of mRNA for two EC-specific growth factor receptors that mediate mitogenic and angiogenic signals in EC (Mustonen and Alitalo, 1995), namely, flk-1 and flt-1. The bEND.3 and vector-transfected cells expressed high levels of mRNA for these receptors, whereas their expression was dramatically down-regulated in parallel with PECAM-1 expression in antisense-transfected bEND.3 cells. This observation is consistent with the reduced rate of proliferation observed in the antisense PECAM-1-transfected bEND.3 cells.
Figure 3.
Analysis of the steady-state flk-1 and flt-1 mRNA levels in antisense-transfected cells. The Northern blot described in Figure 2 was striped and probed with specific cDNAs for murine flk-1, flt-1, and GAPDH to control for loading.
PECAM-1 Expression and EC Morphogenesis
To investigate the role of PECAM-1 in EC morphogenesis, we determined the ability of the antisense-transfected cells that express different levels of PECAM-1 to differentiate on Matrigel. We observed that parental bEND.3 or vector-transfected cells, which express high levels of PECAM-1, lacked the ability to organize on Matrigel and formed only clumps of cells, which over several days grew and formed hemangioma-like structures (Sheibani and Frazier, 1995). The cell clumps that formed after 24 h in Matrigel are shown in Figure 4A. The PECAM-1 protein expression level determined by FACS analysis correlates well with the PECAM-1 mRNA levels and is shown in the right panels of Figure 4. It should be noted that PECAM-1 expression levels on parental bEND.3 cells and the vector-transfected control cells (see Figure 4A) are much higher than those observed on any other EC in culture. For example, the mean fluorescence staining for Py4–1 cells (a murine EC line) is 270, comparable to the levels of PECAM-1 in HUVEC (mean fluorescence of 240) and human dermal microvascular EC (mean fluorescence of 250), and is much less than PECAM-1 levels in bEND.3 cells (mean fluorescence of 490, Table 1).
Table 1.
Hemangioma formation by cells expressing different levels of PECAM-1
| Cell line | PECAM-1 level (mean fluorescence intensity)a | Cell no. | Incubation time (d) | Tumor incidence |
|---|---|---|---|---|
| bEND | 490 | 5 × 106 | 2 | 3/3 |
| bEND pMEP4 | 450 | 5 × 106 | 2 | 3/3 |
| bEND pMEP4/BAMAS20 | 24 | 5 × 106 | 42 | 0/3 |
| bEND pMEP4/BAMAS21 | 22 | 5 × 106 | 42 | 0/3 |
| bEND pMEP4/BAMAS36 | 144 | 5 × 106 | 42 | 0/3 |
The mean (log) fluorescence intensity for each cell line was determined by FACS using the Mab390 as described in MATERIALS AND METHODS. The values are averages of at least three independent experiments. The mean fluorescence intensity for control IgC was 22. SDs were less than 10%.
Cells that completely lacked PECAM-1 expression, due to antisense transfection, formed large islands of cells with only a few of the cells migrating out to form cords (Figure 4, B and C, and corresponding FACS data). However, cells that expressed moderate levels of PECAM-1 exhibited an enhanced ability to form extensive networks of cords on Matrigel (Figure 4D and corresponding FACS data). These results are consistent with our previous observations that the TS1-transfected bEND.3 cells, which express no PECAM-1, form large islands of cells with relatively few cords (Sheibani and Frazier, 1995; Sheibani et al., 1997), whereas expression of murine or human PECAM-1 isoforms in these cells (mean fluorescence ranging from 70 to 140) enhanced their ability to organize and form extensive networks of cords on Matrigel (Sheibani et al., 1997). However, higher levels of PECAM-1 appeared to have no stimulatory effect on EC morphogenesis (mean fluorescence ranging from 250 to 390). The levels of PECAM-1 expression referred to here as “intermediate” are very similar to those obtained by transfection of bEND/TS cells with PECAM-1 cDNA expression constructs (mean fluorescence ranging from 70 to 140) that exhibited enhanced ability to organize on Matrigel (Sheibani et al., 1997). Therefore, these results reveal that there is an optimal level of PECAM-1 expression for EC interactions.
Tumorigenesis Assay of Antisense-transfected Cells
Expression of TS1 in bEND.3 cells completely suppresses their ability to form hemangiomas in mice (Sheibani and Frazier, 1995). This is, in part, attributed to down-regulation of PECAM-1 expression in TS1-transfected cells because bEND.3 cells must recruit host EC to form the hemangiomas so rapidly within 48 h (Montesano et al., 1990). To examine the role of PECAM-1 in hemangioma formation, we tested the ability to form in mice hemangiomas of several antisense-transfected bEND.3 clones that express different levels of PECAM-1. Parental cells and vector-control cells that express very high levels of PECAM-1, pMEP4/BAMAS20 and pMEP4/BAMAS21 cells that express no PECAM-1, and pMEP4/BAMAS36 cells that express moderate levels of PECAM-1 (Figures 1 and 4) were injected into nude mice as described previously (Sheibani and Frazier, 1995). The parental or the vector-control cells rapidly formed hemangiomas in 48 h with 100% incidence (Table 1, and Sheibani and Frazier, 1995). The antisense-transfected cells that expressed no PECAM-1 or even moderate levels of PECAM-1 did not form hemangiomas even after 6 wk (Table 1).
Adhesion Characteristics of Antisense-transfected bEND.3 Cells
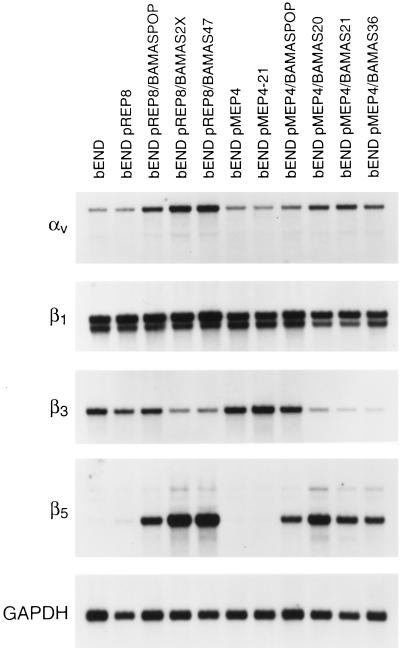
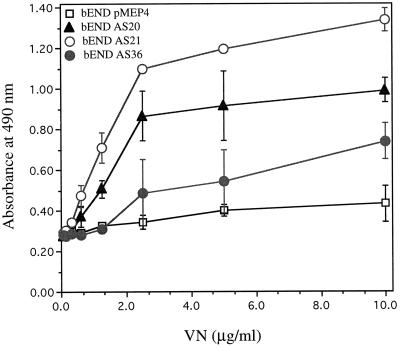
As indicated above, we observed a dramatic change in the morphology of antisense-transfected bEND.3 cells in culture. To determine whether these changes in cell morphology might be due to changes in the adhesion characteristics of the cells, we examined the expression of several integrins. Figure 5 shows the Northern blot analysis of poly(A+) RNA isolated from control and antisense PECAM-1-transfected bEND.3 cells that was probed for expression of several integrin subunits with important roles in angiogenesis (Drake et al., 1992; Brooks et al., 1994a,b). These included αv, β3, β5, and β1. We observed an increase in the expression of αv and β5 integrin mRNAs in the antisense-transfected cells, whereas the mRNA level for β3 was dramatically diminished and the mRNA level for β1 remained unchanged. These changes in the expression of integrins may be manifested in changes in adhesion characteristics of the cells, resulting in a differentiated phenotype. Thus, we examined the adhesion of these cells to vitronectin using a quantitative assay. Figure 6 shows that the antisense-transfected cells that completely lack PECAM-1 and express β5 integrin adhere much better to vitronectin than vector-transfected cells that express high levels of PECAM-1 or antisense-transfected cells that express intermediate levels of PECAM-1. This observation is consistent with the enhanced expression of αv and β5 integrins in antisense-transfected bEND.3 cells (Figure 5) because αvβ5 is a better cell-adhesion receptor for vitronectin than is αvβ3 (Gao et al., 1996a).
Figure 5.
Analysis of the steady-state αv, β1, β3, and β5 integrins mRNA levels in antisense-transfected cells. Poly(A+) RNA was prepared from antisense-transfected bEND.3 cells as described in Figure 2 and analyzed by Northern blot. The blot was probed with specific cDNA probes for αv, β1, β3, β5, and GAPDH to control for loading.
Figure 6.
Cell adhesion of antisense-transfected bEND.3 cells to vitronectin. Cells were prepared as described in MATERIALS AND METHODS and examined for their ability to adhere to different concentrations of vitronectin. These experiments were repeated at least twice with several clones of antisense-transfected cells with identical results.
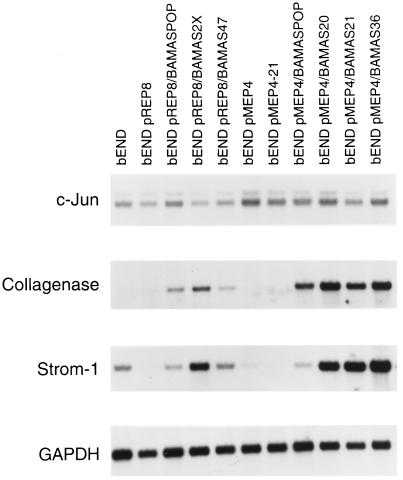
Changes in the expression of metalloproteinases can also influence cell adhesion as well as regulation of angiogenesis. We observed a dramatic coordinate increase in the expression of collagenase and stromelysin-1 mRNA in antisense-transfected cells (Figure 7). Expression of the proto-oncogene c-jun contributes to formation of active AP1 transcription factor complexes that are involved in induction of expression of these metalloproteinases in other cells (Matrisian, 1992). However, the up-regulation of collagenase and stromelysin-1 expression appeared to be independent of changes in c-jun expression (Figure 7) in the bEND.3 cells. The expression of collagenase and stromelysin-1 is also coordinately up-regulated in bEND/TS cells that lack PECAM-1 expression (Sheibani and Frazier, unpublished results).
Figure 7.
Analysis of the steady-state c-jun, collagenase, and stromelysin-1 mRNA levels in antisense-transfected cells. The poly(A+) RNA prepared from antisense-transfected cells was analyzed by Northern blot. Blots were probed with specific cDNAs for c-jun, collagenase, stromelysin-1, and GAPDH to control for loading.
DISCUSSION
bEND.3 cells express very high levels of PECAM-1, rapidly proliferate in culture, form hemangiomas in mice within 48 h, organize poorly in Matrigel, and express little or no TS1. We now demonstrate that down-regulation of PECAM-1 in bEND.3 cells by antisense expression affects their proliferation, morphogenesis, and hemangioma formation. Perhaps most interestingly, the down-regulation of PECAM-1 results in enhanced expression of TS1 and its antiangiogenic receptor, CD36. These changes in EC phenotype brought about by decreased PECAM-1 expression are very similar to those observed in TS1-transfected bEND.3 cells (Sheibani and Frazier, 1995; Sheibani et al., 1997). Therefore, it appears that a reciprocal relationship exists between TS1 and PECAM-1 expression in bEND.3 cells. This finding is consistent with the expression patterns of these genes during vascular development. PECAM-1 is expressed very early during vascular development (embryonic day 7.5; DeLisser et al., 1994b), but TS1 expression is detected only in blood vessels of later fetal stages and in the adult (Reed et al., 1995). These observations are also supported by the proangiogenic and antiangiogenic roles established for PECAM-1 (DeLisser et al., 1994b, 1997; Sheibani et al., 1997) and TS1 (Sheibani and Frazier, 1995; Dawson et al., 1997), respectively. The expression of TS1 and down-regulation of PECAM-1 in bEND.3 cells share a number of downstream effects including changes in cell proliferation, morphology, morphogenesis, hemangioma formation, and expression of integrins and metalloproteinases. These data suggest the idea that there is a complex two-state program that switches between two phenotypes of ECs. One is the quiescent, differentiated vessel phenotype and the other is the invasive, proliferative, and hence angiogenic phenotype (Hanahan and Folkman, 1996). It is not presently known whether the effects of TS1 or PECAM-1 expression on this reciprocal relationship are direct or are the consequence of a common downstream signaling event. It is clear, however, that a change in expression of either TS1 or PECAM-1 causes a reciprocal change in the expression of the other. A close examination of expression of these molecules during vascular development may provide some insights on the regulatory pathways that suppress TS1 expression at early times but induce its expression at later times.
The clones of antisense-transfected bEND.3 cells that completely lacked PECAM-1 exhibited a more normal EC morphology (Figure 2, B and D) when compared with parental or vector-transfected cells (Figure 2, A and C). The bEND/TS cells, in which PECAM-1 is also completely down-regulated, have a morphology very similar to antisense PECAM-1-transfected bEND.3 cells. The antisense-transfected bEND.3 cells, like bEND/TS cells, exhibited a much reduced growth rate. This is at least partially due to changes in the survival of antisense-transfected cells. Therefore, expression of PECAM-1 may provide a survival signal and/or protect these cells from death as has been reported for EC integrins, e.g., αvβ3 (Brooks et al., 1994b), which is also down-regulated in parallel with PECAM-1 (Figure 5). Another contributor to decreased cell proliferation and survival is likely to be the down-regulation of vascular endothelial growth factor receptors, flk-1 and flt-1 (Figure 3), which are essential for transmitting mitogenic and survival signals in ECs. Most interesting in terms of its implications for a regulatory switch or program in ECs was the enhanced expression of TS1 in antisense-transfected cells (Figure 1). This is consistent with our previous observation that expression of TS1 in bEND.3 (Sheibani and Frazier, 1995) and the addition of exogenous TS1 (RayChaudhury et al., 1994) affect the morphology and proliferation of these cells. The bEND.3 or vector-transfected cells expressed a short TS1 transcript (∼4.0 kb) which was not translated (Sheibani and Frazier, unpublished data). This mRNA is perhaps generated by alternative splicing and/or use of an alternative polyadenylation signal in the large 3′ untranslated region of TS1 transcript. Studies are in progress to determine the precise identity of this transcript.
The enhanced expression of TS1 and its antiangiogenic receptor CD36 (Figure 1) is another major signaling pathway that contributes to the changes observed in proliferation and survival of antisense-transfected bEND.3 cells. We have shown that expression of CD36 in HUVEC, which normally lack CD36, has a dramatic effect on their proliferation, migration, and morphogenesis and CD36 mediates the inhibitory effects of TS1 on EC in vitro (Dawson et al., 1997). However, the contribution of CD36 to EC survival requires more investigation. TS1 and heparin-binding type I repeat peptides of TS1 can specifically induce apoptosis in ECs concomitant with changes in cell morphology and inhibition of cell proliferation (Guo et al., 1997). However, these peptides do not interact directly with CD36. Thus, the effects of TS1 in antisense-transfected cells may involve interactions with several cell surface receptors initiating signaling pathways that are integrated to favor a differentiated, nonproliferative state of endothelium.
The antisense PECAM-1-transfected bEND.3 cells exhibited a difference in their ability to undergo morphogenesis in three-dimensional cultures. The cells that expressed high levels of PECAM-1 lacked the ability to organize on Matrigel (Figure 4A), whereas the cells that completely lacked PECAM-1 formed large islands of cells with only a few cells migrating out to form cords (Figure 4, B and C). The cells that expressed intermediate levels of PECAM-1 exhibited an enhanced ability to organize a much more extensive network of cords on Matrigel (Figure 4D). These observations are consistent with our previous studies, which showed that TS1-transfected bEND.3 cells that lacked PECAM-1 expression formed large islands of cells with a few cells migrating out to form cords (Sheibani and Frazier, 1995; Sheibani et al., 1997). Furthermore, the ability of the bEND/TS cells to form cords was greatly enhanced after transfection with PECAM-1 cDNA, resulting in expression levels similar to those of the “intermediate” PECAM-1 antisense clones reported here (Sheibani et al., 1997). Together these data suggest that an optimum level may exist for expression of PECAM-1. Very high levels of PECAM-1 may provide such strong cell–cell interactions that they interfere with the ability of the cells to migrate during EC morphogenesis. However, some expression of PECAM-1 appears to be essential for EC morphogenesis (DeLisser et al., 1997; Sheibani et al., 1997). In fact, the levels of PECAM-1 in normal ECs are comparable to the intermediate levels of PECAM-1 observed in antisense clones with enhanced morphogenic ability. In addition, the ability of antisense-transfected cells to form hemangiomas in mice also correlated with PECAM-1 expression levels. The parental bEND.3 cells and the vector-control cells that express inordinately high levels of PECAM-1 rapidly formed hemangiomas in nude mice as we had seen previously (Table 1; Sheibani and Frazier, 1995). However, the antisense-transfected bEND.3 cells failed to form tumors even after 6 wk. These data suggest that PECAM-1 plays a major role in interactions with host EC and/or their recruitment in rapid formation of hemangiomas.
TS1 can directly modulate the activity of αvβ3 integrin via its C-terminal receptor, integrin-associated protein, or CD47 (Gao et al., 1996a,b). Therefore, TS1 may regulate EC adhesion by modulating function and/or expression of various integrins and perhaps their ligands, thus establishing the proper environment for differentiated endothelium. The expression of αv and β5 integrin subunits was up-regulated, whereas that of β3 was dramatically down-regulated in antisense-transfected bEND.3 cells (Figure 5). However, the expression of β1 integrin remained unchanged. PECAM-1 has been reported to be a ligand for αvβ3 (Piali et al., 1995; Buckley et al., 1996); thus, the concomitant down-regulation of PECAM-1 and αvβ3 may be another example of coordinate regulation of a ligand-receptor pair. These changes in the expression of integrins were consistent with changes observed in the adhesion characteristics of antisense-transfected cells. The antisense-transfected cells exhibited an enhanced ability to adhere to vitronectin consistent with enhanced expression of αvβ5 in these cells (Figure 6). It was recently shown that endothelial cell–cell and cell–matrix interactions can regulate endothelial adhesiveness through PECAM-1 (Litwin et al., 1997), further reinforcing the notion that the integration of intercellular and intracellular signaling pathways may be essential in regulation of EC phenotype.
Cell–matrix interactions are essential for cell proliferation, migration, differentiation, and survival. The expression and ligation of αvβ3 integrin is essential during angiogenesis and survival of at least some ECs (Brooks et al., 1994a,b). Therefore, the down-regulation of β3 integrin may contribute to changes observed in the survival of antisense-transfected bEND.3 cells. These changes are complemented with the regulated expression of metalloproteinases, and perhaps their inhibitors [tissue inhibitor of metalloproteinases (TIMPS)] during angiogenesis. Changes in the expression of integrins and their ligands can, in part, modulate expression of metalloproteinases. The ligation of fibronectin receptors by antibodies to the receptor or polypeptides containing the Arg-Gly-Asp sequences of fibronectin induce coordinate expression of the secreted extracellular matrix-degrading metalloproteinases collagenase and stromelysin in rabbit synovial fibroblasts (Werb et al., 1989). In addition, collagenase activity is essential during morphogenesis of ECs in collagen gels (Fisher et al., 1994). We observed a coordinate increase in the expression of collagenase and stromelysin-1 in antisense-transfected bEND.3 cells (Figure 7). The expression of collagenase and stromelysin-1, as well as TIMP-1 and TIMP-2, are also coordinately up-regulated in bEND/TS cells that lack PECAM-1 expression (Sheibani and Frazier, unpublished results), suggesting a more controlled proteolytic activity (Matrisian, 1992) in these cells. The coordinate expression of collagenase and stromelysin genes is also observed in phorbol 12-myristate 13-acetate-treated rabbit brain ECs, synovial fibroblasts, and alveolar macrophages (Frisch et al., 1987). The lack of changes in the expression of c-jun mRNA in antisense-transfected cells (Figure 7) suggests that AP-1 activity is not directly responsible for enhanced expression of these proteases. However, other members of the jun family may be involved.
Down-regulation of PECAM-1 in bEND.3 cells has a dramatic effect on their phenotype. This is perhaps mediated by up-regulation of the expression of TS1 and its antiangiogenic receptor CD36, as occurs in TS1-transfected bEND.3 cells. The reciprocal relationship between TS1 and PECAM-1 expression suggests that they are components of a switch mechanism for coordinated regulation of EC behavior. The interaction of TS1 with its antiangiogenic receptor CD36, and perhaps other TS1 receptors such as integrin-associated protein, on the surface of ECs initiates a series of complex signaling pathways that ultimately result in altered expression of several genes in concert. We believe that it is the proper integration of these changes that can lead to an angioinhibitory response and maintenance of a differentiated endothelium. Our data indicate that PECAM-1 may provide an alternative target for modulation of TS1 expression and regulation of angiogenesis. Future studies will focus on determining how the reciprocal relationship between TS1 and PECAM-1, which appears to be critical during vasculogenesis and angiogenesis, is regulated.
ACKNOWLEDGMENTS
We thank Drs. B.A. Imhof (Basel Institute for Immunology, Basel, Switzerland), S.A. Bogen (Boston University, Boston, MA), and S.M. Albelda (University of Pennsylvania, Philadelphia, PA) for kind gifts of murine PECAM-1 antibodies and murine PECAM-1 cDNA (lacking exon 15). We thank Dr. C.M. Sorenson for critical reading of the article, and P. Chand at the FACS facility for FACS analysis and cell sorting. This work was supported by grant CA-65872.
Footnotes
Abbreviations used: EC, endothelial cell; HUVEC, human umbilical vein endothelial cell; MBEC, mouse brain endothelial cell; PECAM-1, platelet endothelial cell adhesion molecule-1; TS1, thrombospondin-1.
REFERENCES
- Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell–cell adhesion molecule. J Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalon O, Sabanai H, Lampugnani M-G, Dejana E, Geiger B. Spatial and temporal relationships between cadherins and PECAM-1 in cell–cell junctions of human endothelial cells. J Cell Biol. 1994;126:247–258. doi: 10.1083/jcb.126.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin HS, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- Berman ME, Muller WA. Ligation of platelet/endothelial cell adhesion molecule 1 (PECAM-1/CD31) on monocytes and neutrophils increases binding capacity of leukocyte CR3 (CD11b/CD18) J Immunol. 1995;154:299–307. [PubMed] [Google Scholar]
- Bischoff J. Approaches to studying cell adhesion molecules in angiogenesis. Trends Cell Biol. 1995;5:69–74. doi: 10.1016/s0962-8924(00)88949-7. [DOI] [PubMed] [Google Scholar]
- Bogen SA, Baldwin HS, Watkins SC, Albelda SM, Abbas AK. Association of murine CD31 with transmigrating lymphocytes following antigenic stimulation. Am J Pathol. 1992;141:843–854. [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Clark RAF, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994a;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AMP, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994b;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Buckley CD, Doyonnas R, Newton JP, Blystone SD, Brown EJ, Watt SM, Simmon S. Identification of αvβ3 as a heterotypic ligand for CD31/PECAM-1. J Cell Sci. 1996;109:437–445. doi: 10.1242/jcs.109.2.437. [DOI] [PubMed] [Google Scholar]
- Cooper D, Lindberg FP, Gamble JR, Brown EJ, Vadas MA. Transendothelial migration of neutrophils involves integrin-associated protein (CD47) Proc Natl Acad Sci USA. 1995;92:3978–3982. doi: 10.1073/pnas.92.9.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DW, Pearce SFA, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E, Corada M, Lampugnani MG. Endothelial cell-to-cell junctions. FASEB J. 1995;9:910–918. [PubMed] [Google Scholar]
- DeLisser HM, Chilkotowsky J, Yan HC, Daise ML, Buck CA, Albelda SM. Deletions in the cytoplasmic domain of platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) result in changes in ligand binding properties. J Cell Biol. 1994a;124:195–203. doi: 10.1083/jcb.124.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisser HM, et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
- DeLisser HM, Newman PJ, Albelda SM. Molecular and functional aspects of PECAM-1/CD31. Immunol Today. 1994b;15:490–495. doi: 10.1016/0167-5699(94)90195-3. [DOI] [PubMed] [Google Scholar]
- DeLisser HM, Yan HC, Newman PJ, Muller WA, Buck CA, Albelda SM. Platelet/endothelial cell adhesion molecule-1 (CD31)-mediated cellular aggregation involves cell surface glycosaminoglycans. J Biol Chem. 1993;268:16037–16046. [PubMed] [Google Scholar]
- Drake CJ, Davis LA, Little CD. Antibodies to β1-integrins cause alterations of aortic vasculogenesis in vivo. Dev Dyn. 1992;193:83–91. doi: 10.1002/aja.1001930111. [DOI] [PubMed] [Google Scholar]
- Famiglietti J, Sun J, DeLisser HM, Albelda SM. Tyrosine residue in exon 14 of the cytoplasmic domain of platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) regulates ligand binding specificity. J Cell Biol. 1997;138:1425–1435. doi: 10.1083/jcb.138.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher C, Gilbertson-Beadling S, Powers EA, Petzold G, Poorman R, Mitchell MA. Interstitial collagenase is required for angiogenesis in vitro. Dev Biol. 1994;162:499–510. doi: 10.1006/dbio.1994.1104. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Clark EJ, Werb Z. Coordinate regulation of stromelysin and collagenase genes determined with cDNA probes. Proc Natl Acad Sci USA. 1987;84:2600–2604. doi: 10.1073/pnas.84.9.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao AG, Lindberg FP, Dimitry JM, Brown EJ, Frazier W. Thrombospondin modulates αvβ3 function through integrin-associated protein. J Cell Biol. 1996a;135:533–544. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem. 1996b;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- Guo NH, Krutzsch HC, Inman JK, Roberts DD. Thrombospondin 1 and type 1 repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res. 1997;57:1735–1742. [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Horak ER, Leek R, Klenk N, LeJeune S, Smith K, Stuart N, Greenall M, Stepniewska K, Harris AL. Angiogenesis, assessed by platelet/endothelial cell adhesion molecule antibodies, as indicator of node metastases and survival in breast cancer. Lancet. 1992;340:1120–1124. doi: 10.1016/0140-6736(92)93150-l. [DOI] [PubMed] [Google Scholar]
- Jackson DE, Kupcho KR, Newman PJ. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of platelet/endothelial cell adhesion molecule-1 (PECAM-1) that are required for the cellular association and activation of the protein-tyrosine phosphatase, SHP-2. J Biol Chem. 1997a;272:24868–24875. doi: 10.1074/jbc.272.40.24868. [DOI] [PubMed] [Google Scholar]
- Jackson DE, Ward CM, Wang R, Newman PJ. The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation. J Biol Chem. 1997b;272:6986–6993. doi: 10.1074/jbc.272.11.6986. [DOI] [PubMed] [Google Scholar]
- Kirschbaum NE, Gumina RJ, Newman PJ. Organization of the gene for human platelet/endothelial cell adhesion molecule-1 shows alternatively spliced isoforms and a functionally complex cytoplasmic domain. Blood. 1994;84:4028–4037. [PubMed] [Google Scholar]
- Litwin M, Clark K, Noack L, Furze J, Berndt M, Albelda S, Vadas M, Gamble J. Novel cytokine-independent induction of endothelial adhesion molecules regulated by platelet/endothelial cell adhesion molecule (CD31) J Cell Biol. 1997;139:219–228. doi: 10.1083/jcb.139.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TL, Barreuther M, Davis S, Madri JA. Platelet endothelial cell adhesion molecule-1 is phosphorylatable by c-Src, binds Src-Src homology 2 domain, and exhibits immunoreceptor tyrosine-based activation motif-like properties. J Biol Chem. 1997;272:14442–14446. doi: 10.1074/jbc.272.22.14442. [DOI] [PubMed] [Google Scholar]
- Lu TL, Yan L, Madri J. Integrin engagement mediates tyrosine dephosphorylation of platelet endothelial cell adhesion molecule-1. Proc Natl Acad Sci USA. 1996;93:11808–11813. doi: 10.1073/pnas.93.21.11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Osawa M, Shigematsu H, Harada N, Fujiwara K. Platelet endothelial cell adhesion molecule-1 is a major SH-PTP2 binding protein in vascular endothelial cells. FEBS Lett. 1997;408:331–336. doi: 10.1016/s0014-5793(97)00457-2. [DOI] [PubMed] [Google Scholar]
- Matrisian LM. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Montesano R, Pepper MS, Mohle-Steinlein U, Risau W, Wagner EF, Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990;62:435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- Muller WA. The role of PECAM-1 (CD31) in leukocyte emigration: studies in vitro and in vivo. J Leukoc Biol. 1995;57:523–528. doi: 10.1002/jlb.57.4.523. [DOI] [PubMed] [Google Scholar]
- Muller WA, Berman ME, Newman PJ, DeLisser HM, Albelda SM. A heterophilic adhesion mechanism for platelet/endothelial cell adhesion molecule 1 (CD31) J Exp Med. 1992;175:1401–1404. doi: 10.1084/jem.175.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustonen T, Alitalo K. Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol. 1995;129:895–898. doi: 10.1083/jcb.129.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ. The biology of PECAM-1. J Clin Invest. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ, Berndt MC, Gorski J, White II GC, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Osawa M, Masuda M, Harada N, Lopes RB, Fujiwara K. Tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in mechanically stimulated vascular endothelial cells. Eur J Cell Biol. 1997;72:229–237. [PubMed] [Google Scholar]
- Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA. CD31/PECAM-1 is a ligand for αvβ3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter E, Barreuther M, Lu T, Imhof BA, Madri JA. Platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) tyrosine phosphorylation state changes during vasculogenesis in the murine conceptus. Am J Pathol. 1997;150:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- RayChaudhury A, Frazier WA, D’Amore PA. Comparison of normal and tumorigenic endothelial cells: differences in thrombospondin production and responses to transforming growth factor-beta. J Cell Sci. 1994;107:39–46. doi: 10.1242/jcs.107.1.39. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Iruela-Arispe L, O’Brien ER, Truong T, LaBell T, Bornstein P, Sage EH. Expression of thrombospondins by endothelial cells. Am J Pathol. 1995;147:1068–1080. [PMC free article] [PubMed] [Google Scholar]
- Romer LH, McLean NV, Yan HC, Daise M, Sun J, DeLisser HM. INF-γ and TNF-α induce redistribution of PECAM-1 (CD31) on human endothelial cells. J Immunol. 1995;154:6582–6592. [PubMed] [Google Scholar]
- Sheibani N, Frazier WA. Thrombospondin 1 expression in transformed endothelial cells restores a normal phenotype and suppresses their tumorigenesis. Proc Natl Acad Sci USA. 1995;92:6788–6792. doi: 10.1073/pnas.92.15.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheibani N, Newman PJ, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, regulates platelet-endothelial cell adhesion molecule-1 expression and endothelial cell morphogenesis. Mol Biol Cell. 1997;8:1329–1341. doi: 10.1091/mbc.8.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheibani N, Rhim JS, Allen-Hoffmann BL. Malignant human papillomavirus type 16-transformed human keratinocytes exhibit altered expression of extracellular matrix glycoproteins. Cancer Res. 1991;51:5967–5975. [PubMed] [Google Scholar]
- Sun QH, DeLisser HM, Zukowski MM, Paddock C, Albelda SM, Newman PJ. Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity. J Biol Chem. 1996;271:11090–11098. doi: 10.1074/jbc.271.19.11090. [DOI] [PubMed] [Google Scholar]
- Werb Z, Tremble PM, Behrendsten O, Crowley E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;109:877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RL, Risau W, Zerwes HG, Drexler H, Aguzzie A, Wagner EF. Endothelioma cells expressing the polyoma middle T oncogene induce hemangiomas by host cell recruitment. Cell. 1989;57:1053–1063. doi: 10.1016/0092-8674(89)90343-7. [DOI] [PubMed] [Google Scholar]
- Xie Y, Muller WA. Molecular cloning and adhesive properties of murine platelet/endothelial cell adhesion molecule 1. Proc Natl Acad Sci USA. 1993;90:5569–5573. doi: 10.1073/pnas.90.12.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HC, Baldwin HS, Sun J, Buck CA, Albelda SM, DeLisser HM. Alternative splicing of a specific cytoplasmic exon alters the binding characteristics of murine platelet/endothelial cell adhesion molecule-1 (PECAM-1) J Biol Chem. 1995;270:23672–23680. doi: 10.1074/jbc.270.40.23672. [DOI] [PubMed] [Google Scholar]