Abstract
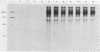
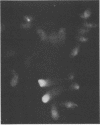
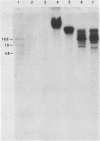
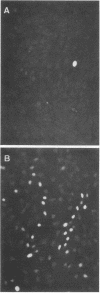
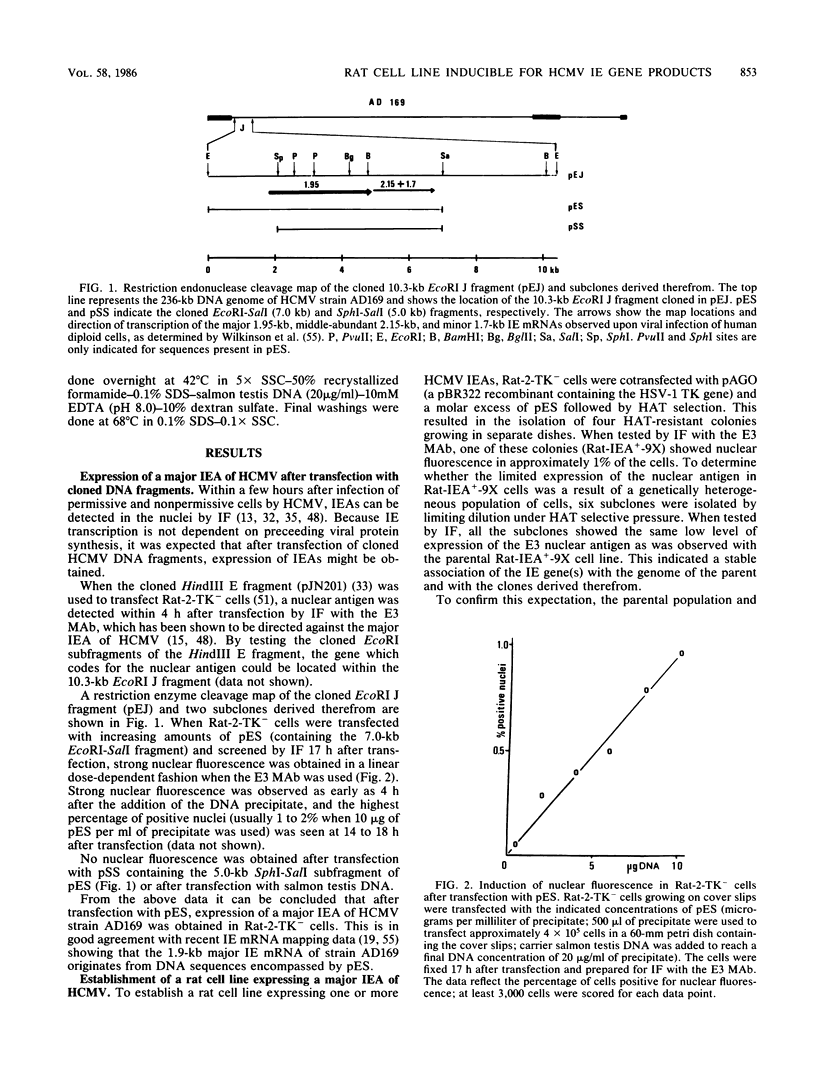
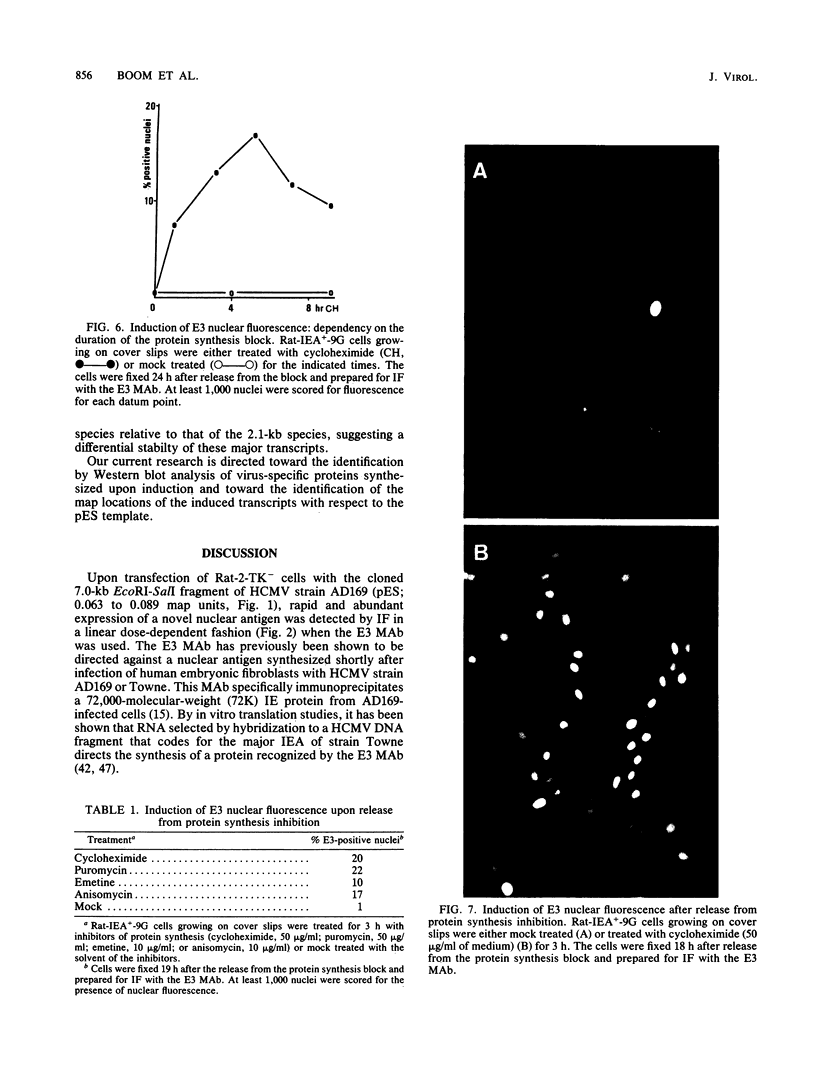
Upon transfection of Rat-2-TK- cells with plasmid pES, containing the cloned 7.0-kilobase (kb) EcoRI-SalI fragment (0.063 to 0.089 map units) of the human cytomegalovirus genome, major immediate-early antigen expression was obtained in 1 to 2% of the nuclei of the transfected cells, as determined by immunofluorescence with the E3 monoclonal antibody. Cotransfection of pES with the cloned herpes simplex virus type 1 thymidine kinase gene resulted in the establishment of a hypoxanthine-aminopterin-thymidine-resistant cell line which expressed a major immediate-early antigen in approximately 1% of the cells at early passages, with expression gradually declining to less than 0.1% upon subculturing. Southern blot analysis of DNA extracted from this cell line revealed the presence of multiple integration events of pES DNA sequences into cellular DNA, including a head-to-tail tandem array of approximately 10 copies of pES. The integration pattern was stable for at least 80 passages. Metaphase chromosomes prepared from this cell line showed, upon in situ hybridization, a strong hybridization signal in both sister chromatids of a large submetacentric chromosome which is considered to have harbored the tandemly integrated pES molecules. Whereas in most cells of the population, immediate-early expression seemed to be repressed, this repression could be overcome by protein synthesis inhibition, resulting in a massive induction of human-cytomegalovirus-specific transcripts of 2.1 and 1.9 kb and a minor species of 2.9 kb. After release from protein synthesis inhibition, approximately 20% of the cells showed nuclear fluorescence when the E3 monoclonal antibody was used.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaij C., Borst P. The gel electrophoresis of DNA. Biochim Biophys Acta. 1972 May 10;269(2):192–200. doi: 10.1016/0005-2787(72)90426-1. [DOI] [PubMed] [Google Scholar]
- Akrigg A., Wilkinson G. W., Oram J. D. The structure of the major immediate early gene of human cytomegalovirus strain AD169. Virus Res. 1985 Mar;2(2):107–121. doi: 10.1016/0168-1702(85)90242-4. [DOI] [PubMed] [Google Scholar]
- Blanton R. A., Tevethia M. J. Immunoprecipitation of virus-specific immediate-early and early polypeptides from cells lytically infected with human cytomegalovirus strain AD 169. Virology. 1981 Jul 15;112(1):262–273. doi: 10.1016/0042-6822(81)90631-0. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Cameron J. M., Preston C. M. Comparison of the immediate early polypeptides of human cytomegalovirus isolates. J Gen Virol. 1981 Jun;54(Pt 2):421–424. doi: 10.1099/0022-1317-54-2-421. [DOI] [PubMed] [Google Scholar]
- Colbere-Garapin F., Chousterman S., Horodniceanu F., Kourilsky P., Garapin A. C. Cloning of the active thymidine kinase gene of herpes simplex virus type 1 in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3755–3759. doi: 10.1073/pnas.76.8.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Darnell J. E., Jr Cycloheximide stimulates early adenovirus transcription if early gene expression is allowed before treatment. J Virol. 1983 Feb;45(2):683–692. doi: 10.1128/jvi.45.2.683-692.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani C., Blanchard J. M., Piechaczyk M., El Sabouty S., Marty L., Jeanteur P. Extreme instability of myc mRNA in normal and transformed human cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7046–7050. doi: 10.1073/pnas.81.22.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarchi J. M. Post-transcriptional control of human cytomegalovirus gene expression. Virology. 1983 Jan 30;124(2):390–402. doi: 10.1016/0042-6822(83)90355-0. [DOI] [PubMed] [Google Scholar]
- Demarchi J. M. Human cytomegalovirus DNA: restriction enzyme cleavage maps and map locations for immediate-early, early, and late RNAs. Virology. 1981 Oct 15;114(1):23–38. doi: 10.1016/0042-6822(81)90249-x. [DOI] [PubMed] [Google Scholar]
- Elder P. K., Schmidt L. J., Ono T., Getz M. J. Specific stimulation of actin gene transcription by epidermal growth factor and cycloheximide. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7476–7480. doi: 10.1073/pnas.81.23.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier M. L., Mars W., Florine D. L., Montagna R. A., Saunders G. F. Efficient extraction of RNA from mammalian tissue. Mol Cell Biochem. 1983;56(2):113–122. doi: 10.1007/BF00227211. [DOI] [PubMed] [Google Scholar]
- Geder L. Evidence for early nuclear antigens in cytomegalovirus-infected cells. J Gen Virol. 1976 Aug;32(2):315–319. doi: 10.1099/0022-1317-32-2-315. [DOI] [PubMed] [Google Scholar]
- Gibson W. Immediate-early proteins of human cytomegalovirus strains AD 169, Davis, and Towne differ in electrophoretic mobility. Virology. 1981 Jul 15;112(1):350–354. doi: 10.1016/0042-6822(81)90641-3. [DOI] [PubMed] [Google Scholar]
- Goldstein L. C., McDougall J., Hackman R., Meyers J. D., Thomas E. D., Nowinski R. C. Monoclonal antibodies to cytomegalovirus: rapid identification of clinical isolates and preliminary use in diagnosis of cytomegalovirus pneumonia. Infect Immun. 1982 Oct;38(1):273–281. doi: 10.1128/iai.38.1.273-281.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn G., Knust E., Schmolla H., Sarre T., Nelson J. A., McDougall J. K., Fleckenstein B. Predominant immediate-early transcripts of human cytomegalovirus AD 169. J Virol. 1984 Feb;49(2):363–370. doi: 10.1128/jvi.49.2.363-370.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Kamata T., Tanaka S., Watanabe Y. Characterization of the human cytomegalovirus-induced chromatin factor responsible for activation of host cell chromatin template. Virology. 1979 Aug;97(1):224–228. doi: 10.1016/0042-6822(79)90393-3. [DOI] [PubMed] [Google Scholar]
- Kamata T., Tanaka S., Watanabe Y. Human cytomegalovirus-induced chromatin factors responsible for changes in template activity and structure of infected cell chromatin. Virology. 1978 Oct 15;90(2):197–208. doi: 10.1016/0042-6822(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum A. L., Huang E. S. Chromatin pattern consisting of repeating bipartite structures in WI-38 cells infected with human cytomegalovirus. J Virol. 1978 Nov;28(2):661–664. doi: 10.1128/jvi.28.2.661-664.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Landegent J. E., Jansen in de Wal N., Ploem J. S., Van der Ploeg M. Sensitive detection of hybridocytochemical results by means of reflection-contrast microscopy. J Histochem Cytochem. 1985 Dec;33(12):1241–1246. doi: 10.1177/33.12.2415575. [DOI] [PubMed] [Google Scholar]
- Landegent J. E., Jansen in de Wal N., van Ommen G. J., Baas F., de Vijlder J. J., van Duijn P., Van der Ploeg M. Chromosomal localization of a unique gene by non-autoradiographic in situ hybridization. Nature. 1985 Sep 12;317(6033):175–177. doi: 10.1038/317175a0. [DOI] [PubMed] [Google Scholar]
- Landegent J. E., Jasen in de Wal N., Baan R. A., Hoeijmakers J. H., Van der Ploeg M. 2-Acetylaminofluorene-modified probes for the indirect hybridocytochemical detection of specific nucleic acid sequences. Exp Cell Res. 1984 Jul;153(1):61–72. doi: 10.1016/0014-4827(84)90448-8. [DOI] [PubMed] [Google Scholar]
- Makino R., Hayashi K., Sugimura T. C-myc transcript is induced in rat liver at a very early stage of regeneration or by cycloheximide treatment. Nature. 1984 Aug 23;310(5979):697–698. doi: 10.1038/310697a0. [DOI] [PubMed] [Google Scholar]
- McDonough S. H., Spector D. H. Transcription in human fibroblasts permissively infected by human cytomegalovirus strain AD169. Virology. 1983 Feb;125(1):31–46. doi: 10.1016/0042-6822(83)90061-2. [DOI] [PubMed] [Google Scholar]
- Michelson-Fiske S., Horodniceanu F., Guillon J. C. Immediate early antigens in human cytomegalovirus infected cells. Nature. 1977 Dec 15;270(5638):615–617. doi: 10.1038/270615a0. [DOI] [PubMed] [Google Scholar]
- Michelson S., Horodniceanu F., Kress M., Tardy-Panit M. Human cytomegalovirus-induced immediate early antigens: analysis in sodium dodecyl sulfate-polyacrylamide gel electrophoresis after immunoprecipitation. J Virol. 1979 Oct;32(1):259–267. doi: 10.1128/jvi.32.1.259-267.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. A., Fleckenstein B., Galloway D. A., McDougall J. K. Transformation of NIH 3T3 cells with cloned fragments of human cytomegalovirus strain AD169. J Virol. 1982 Jul;43(1):83–91. doi: 10.1128/jvi.43.1.83-91.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Reynolds D. W. Development of early nuclear antigen in cytomegalovirus infected cells in the presence of RNA and protein synthesis inhibitors. J Gen Virol. 1978 Aug;40(2):475–480. doi: 10.1099/0022-1317-40-2-475. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Dieckmann B., Vannice J. L., Trahey M., McCormick F. Inhibition of protein synthesis stimulates the transcription of human beta-interferon genes in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3964–3968. doi: 10.1073/pnas.81.13.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive H. L., Heintz N., Roeder R. G. Regulation of human histone gene expression during the HeLa cell cycle requires protein synthesis. Mol Cell Biol. 1984 Dec;4(12):2723–2734. doi: 10.1128/mcb.4.12.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare J., Summers W. C. Structure and function of herpesvirus genomes. II. EcoRl, Sbal, and HindIII endonuclease cleavage sites on herpes simplex virus. Virology. 1977 Feb;76(2):581–595. doi: 10.1016/0042-6822(77)90240-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Hock L., Tamashiro J. C. Cleavage maps for human cytomegalovirus DNA strain AD169 for restriction endonucleases EcoRI, BglII, and HindIII. J Virol. 1982 May;42(2):558–582. doi: 10.1128/jvi.42.2.558-582.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Thomsen D. R., Stinski M. F. Structural analysis of the major immediate early gene of human cytomegalovirus. J Virol. 1984 Jan;49(1):190–199. doi: 10.1128/jvi.49.1.190-199.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimac E., Groppi V. E., Jr, Coffino P. Inhibition of protein synthesis stabilizes histone mRNA. Mol Cell Biol. 1984 Oct;4(10):2082–2090. doi: 10.1128/mcb.4.10.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Roehr T. J. Activation of the major immediate early gene of human cytomegalovirus by cis-acting elements in the promoter-regulatory sequence and by virus-specific trans-acting components. J Virol. 1985 Aug;55(2):431–441. doi: 10.1128/jvi.55.2.431-441.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F. Sequence of protein synthesis in cells infected by human cytomegalovirus: early and late virus-induced polypeptides. J Virol. 1978 Jun;26(3):686–701. doi: 10.1128/jvi.26.3.686-701.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F. Synthesis of proteins and glycoproteins in cells infected with human cytomegalovirus. J Virol. 1977 Sep;23(3):751–767. doi: 10.1128/jvi.23.3.751-767.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Thomsen D. R., Stenberg R. M., Goldstein L. C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983 Apr;46(1):1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Otsuka M., Ihara S., Maeda F., Watanabe Y. Induction of pre-early nuclear antigen(s) in HEL cells infected with human cytomegalovirus. Microbiol Immunol. 1979;23(4):263–271. doi: 10.1111/j.1348-0421.1979.tb00462.x. [DOI] [PubMed] [Google Scholar]
- The T. H., Klein G., Langenhuysen M. M. Antibody reactions to virus-specific early antigens (EA) in patients with cytomegalovirus (CMV) infection. Clin Exp Immunol. 1974 Jan;16(1):1-7,9-12. [PMC free article] [PubMed] [Google Scholar]
- Thomsen D. R., Stenberg R. M., Goins W. F., Stinski M. F. Promoter-regulatory region of the major immediate early gene of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Feb;81(3):659–663. doi: 10.1073/pnas.81.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp W. C. Normal rat cell lines deficient in nuclear thymidine kinase. Virology. 1981 Aug;113(1):408–411. doi: 10.1016/0042-6822(81)90168-9. [DOI] [PubMed] [Google Scholar]
- VENEMA G., PRITCHARD R. H., VENEMA-SCHROEDER T. FATE OF TRANSFORMING DEOXYRIBONUCLEIC ACID IN BACILLUS SUBTILIS. J Bacteriol. 1965 May;89:1250–1255. doi: 10.1128/jb.89.5.1250-1255.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Stinski M. F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982 Feb;41(2):462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Thomsen D. R., Stinski M. F. Temporal regulation of human cytomegalovirus transcription at immediate early and early times after infection. J Virol. 1981 May;38(2):446–459. doi: 10.1128/jvi.38.2.446-459.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G. W., Akrigg A., Greenaway P. J. Transcription of the immediate early genes of human cytomegalovirus strain AD169. Virus Res. 1984;1(2):101–106. doi: 10.1016/0168-1702(84)90067-4. [DOI] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]