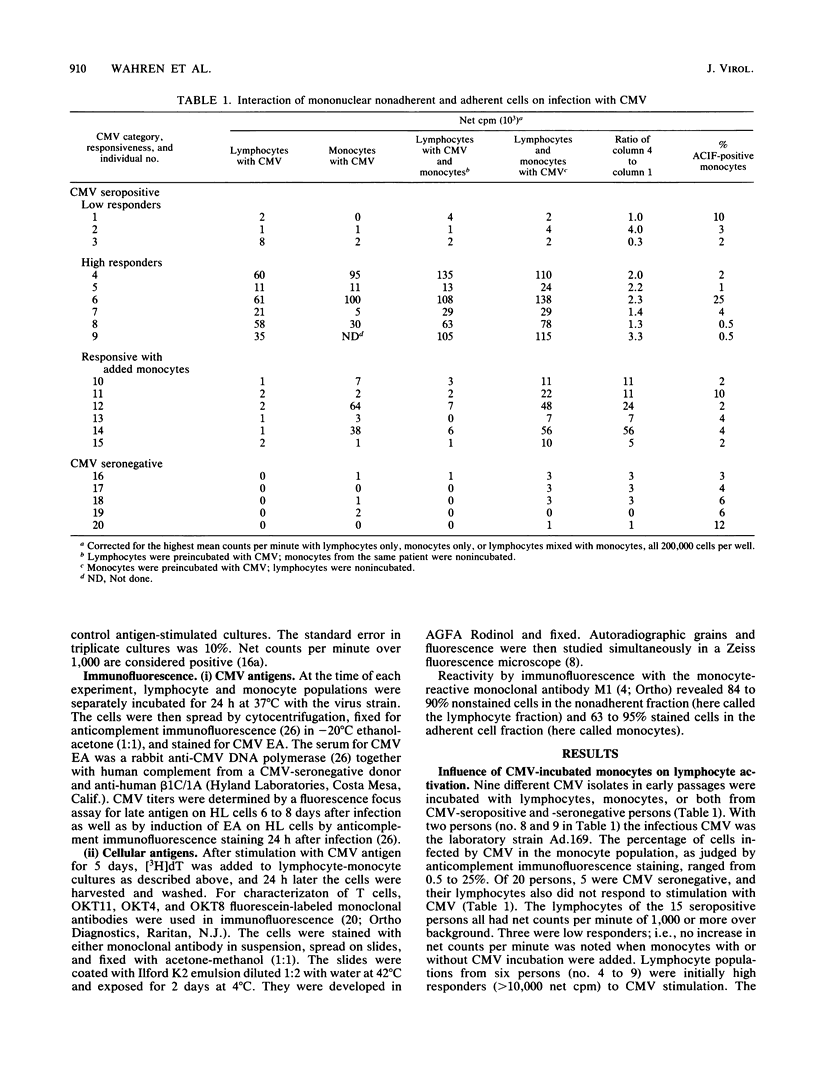
Abstract
Virus-specific lymphocyte proliferation in the presence of cytomegalovirus (CMV) without and with monocytes was studied in healthy persons. Three categories of lymphocyte response could be distinguished: seropositive low responders, naturally high responders, and lymphocyte populations responding well to CMV antigen in the presence of added CMV-incubated autologous monocytes. This latter category could be identified by preincubating autologous monocytes with CMV. CMV-seronegative persons were nonresponders. Early CMV antigens were produced in monocytes but not in lymphocytes by all CMV isolates. Infection of monocytes as detected by antibody to early viral protein did not appear to abort the antigen-presenting ability. The virus-specific responding lymphocytes were mainly of the T4+ phenotype. In contrast, addition of CMV to polyclonal mitogens significantly suppressed total lymphocyte DNA synthesis. CMV thus may have an enhanced virus-specific stimulatory effect on lymphocytes together with monocytes but a suppressive effect on the total lymphocyte population.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berle E. J., Jr, Thorsby E. The proliferative T cell response to herpes simplex virus (HSV) antigen is restricted by self HLA-D. Clin Exp Immunol. 1980 Mar;39(3):668–675. [PMC free article] [PubMed] [Google Scholar]
- Borysiewicz L. K., Morris S., Page J. D., Sissons J. G. Human cytomegalovirus-specific cytotoxic T lymphocytes: requirements for in vitro generation and specificity. Eur J Immunol. 1983 Oct;13(10):804–809. doi: 10.1002/eji.1830131005. [DOI] [PubMed] [Google Scholar]
- Boyum A. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4(4):269–274. [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Carney W. P., Rubin R. H., Hoffman R. A., Hansen W. P., Healey K., Hirsch M. S. Analysis of T lymphocyte subsets in cytomegalovirus mononucleosis. J Immunol. 1981 Jun;126(6):2114–2116. [PubMed] [Google Scholar]
- Chesnut R. W., Colon S. M., Grey H. M. Antigen presentation by normal B cells, B cell tumors, and macrophages: functional and biochemical comparison. J Immunol. 1982 Apr;128(4):1764–1768. [PubMed] [Google Scholar]
- Eckels D. D., Lake P., Lamb J. R., Johnson A. H., Shaw S., Woody J. N., Hartzman R. J. SB-restricted presentation of influenza and herpes simplex virus antigens to human T-lymphocyte clones. Nature. 1983 Feb 24;301(5902):716–718. doi: 10.1038/301716a0. [DOI] [PubMed] [Google Scholar]
- Einhorn L., Ernberg I. Induction of EBNA precedes the first cellular S-phase after EBV-infection of human lymphocytes. Int J Cancer. 1978 Feb 15;21(2):157–160. doi: 10.1002/ijc.2910210205. [DOI] [PubMed] [Google Scholar]
- Einhorn L., Gadler H., Wahren B. Adsorption of purified human cytomegalovirus and induction of early antigens in different cells. J Med Virol. 1982;10(4):225–234. doi: 10.1002/jmv.1890100402. [DOI] [PubMed] [Google Scholar]
- Einhorn L., Ost A. Cytomegalovirus infection of human blood cells. J Infect Dis. 1984 Feb;149(2):207–214. doi: 10.1093/infdis/149.2.207. [DOI] [PubMed] [Google Scholar]
- Gärtner L., Wilhelm J. A., Czieschnek R. In vitro stimulation of lymphocytes by different strains of cytomegalovirus. Med Microbiol Immunol. 1982;171(1):53–57. doi: 10.1007/BF02122707. [DOI] [PubMed] [Google Scholar]
- Hirai K., Watanabe Y. Induction of alpha type DNA polymerases in human cytomegalovirus-infected WI-38 cells. Biochim Biophys Acta. 1976 Oct 18;447(3):328–339. doi: 10.1016/0005-2787(76)90056-3. [DOI] [PubMed] [Google Scholar]
- Hutt-Fletcher L. M., Balachandran N., Elkins M. H. B cell activation by cytomegalovirus. J Exp Med. 1983 Dec 1;158(6):2171–2176. doi: 10.1084/jem.158.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeor S. C., Albrecht T. B., Funk F. D., Rapp F. Stimulation of cellular DNA synthesis by human cytomegalovirus. J Virol. 1974 Feb;13(2):353–362. doi: 10.1128/jvi.13.2.353-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide Y., Yoshida T. O. Restriction molecules involved in the interaction of human T cell clones with antigen-presenting cells. Immunol Lett. 1984;7(4):209–213. doi: 10.1016/0165-2478(84)90045-2. [DOI] [PubMed] [Google Scholar]
- Kreeb G., Zinkernagel R. M. Virus-specific proliferative T-cell responses: parameters and specificity. Cell Immunol. 1980 Aug 1;53(2):267–284. doi: 10.1016/0008-8749(80)90328-7. [DOI] [PubMed] [Google Scholar]
- Larsen H. S., Feng M. F., Horohov D. W., Moore R. N., Rouse B. T. Role of T-lymphocyte subsets in recovery from herpes simplex virus infection. J Virol. 1984 Apr;50(1):56–59. doi: 10.1128/jvi.50.1.56-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman P., Lönnqvist B., Wahren B., Ringdén O., Gahrton G. Lymphocyte responses after cytomegalovirus infection in bone marrow transplant recipients--a one-year follow-up. Transplantation. 1985 Nov;40(5):515–520. doi: 10.1097/00007890-198511000-00009. [DOI] [PubMed] [Google Scholar]
- Ljungman P., Wahren B., Sundqvist V. A. Lymphocyte proliferation and IgG production with herpesvirus antigens in solid phase. J Virol Methods. 1985 Dec;12(3-4):199–208. doi: 10.1016/0166-0934(85)90130-2. [DOI] [PubMed] [Google Scholar]
- Paulin T., Ringdén O., Lönnqvist B. Faster immunological recovery after bone marrow transplantation in patients without cytomegalovirus infection. Transplantation. 1985 Apr;39(4):377–384. doi: 10.1097/00007890-198504000-00008. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Ennis F. A. Cell-mediated immunity in cytomegalovirus infections--a review. Comp Immunol Microbiol Infect Dis. 1980;3(3):283–290. doi: 10.1016/0147-9571(80)90004-1. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Esber E., Saral R., Manischewitz J. F., Rogers J. L., Rook A. H., Santos G. W., Burns W. H. HLA-restricted cytotoxic T lymphocyte and nonthymic cytotoxic lymphocyte responses to cytomegalovirus infection of bone marrow transplant recipients. J Immunol. 1981 May;126(5):2036–2041. [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Rook A. H., Manischewitz J. F., Jackson L., Moreschi G., Santos G. W., Saral R., Burns W. H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982 Jul 1;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- Rice G. P., Schrier R. D., Oldstone M. B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist V. A., Wahren B. An interchangeable ELISA for cytomegalovirus antigen and antibody. J Virol Methods. 1981 Apr;2(5):301–312. doi: 10.1016/0166-0934(81)90029-x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Wahren B., Einhorn L., Gadler H. Early interactions between human cytomegalovirus and cells. Arch Virol. 1984;79(1-2):55–65. doi: 10.1007/BF01314303. [DOI] [PubMed] [Google Scholar]
- Wahren B., Oberg B. Inhibition of cytomegalovirus late antigens by phosphonoformate. Intervirology. 1980;12(6):335–339. doi: 10.1159/000149093. [DOI] [PubMed] [Google Scholar]
- Wahren B., Robèrt K. H., Nordlund S. Conditions for cytomegalovirus stimulation of lymphocytes. Scand J Immunol. 1981;13(6):581–586. doi: 10.1111/j.1365-3083.1981.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]


