Abstract
The β1-integrin cytoplasmic domain consists of a membrane proximal subdomain common to the four known isoforms (“common” region) and a distal subdomain specific for each isoform (“variable” region). To investigate in detail the role of these subdomains in integrin-dependent cellular functions, we used β1A and β1B isoforms as well as four mutants lacking the entire cytoplasmic domain (β1TR), the variable region (β1COM), or the common region (β1ΔCOM-B and β1ΔCOM-A). By expressing these constructs in Chinese hamster ovary and β1 integrin-deficient GD25 cells (Wennerberg et al., J Cell Biol 132, 227–238, 1996), we show that β1B, β1COM, β1ΔCOM-B, and β1ΔCOM-A molecules are unable to support efficient cell adhesion to matrix proteins. On exposure to Mn++ ions, however, β1B, but none of the mutants, can mediate cell adhesion, indicating specific functional properties of this isoform. Analysis of adhesive functions of transfected cells shows that β1B interferes in a dominant negative manner with β1A and β3/β5 integrins in cell spreading, focal adhesion formation, focal adhesion kinase tyrosine phosphorylation, and fibronectin matrix assembly. None of the β1 mutants tested shows this property, indicating that the dominant negative effect depends on the specific combination of common and B subdomains, rather than from the absence of the A subdomain in the β1B isoform.
INTRODUCTION
Integrins are α/β heterodimeric transmembrane cell surface receptors that mediate cell adhesion and migration and also the bidirectional transfer of information across the plasma membrane. These properties are essential in regulating several biological processes, including morphogenesis, immune response, cell growth, differentiation, and survival (Hynes, 1992; Ruoslahti and Reed, 1994).
The cytoplasmic domains of integrin subunits are required for these functions (Hibbs et al., 1991; Yamada and Miyamoto, 1995; Dedhar and Hannigan, 1996). In vitro, the isolated β1 cytoplasmic domain was shown to bind talin, α-actinin, and paxillin, three cytoskeletal proteins that mediate the anchorage of actin filaments to the plasma membrane (Chen et al., 1995; Otey et al., 1993; Schaller et al., 1995). Binding of the β1 cytoplasmic sequence to focal adhesion kinase (FAK), a tyrosine kinase specifically localized to focal adhesions, and to the serine/threonine kinase integrin-linked kinase has also been reported (Schaller et al., 1995; Hannigan et al., 1996). In vivo, the β1 cytoplasmic domain is sufficient for its localization to preformed focal adhesions (LaFlamme et al., 1992, Akiyama et al., 1994) and for the initiation of signaling to FAK (Lukashev et al., 1994). A model has been proposed in which the β1 subunit cytoplasmic domain contains a default signal for interaction with cytoskeletal molecules that is masked by the α subunit cytoplasmic domain (Briesewitz et al., 1993; Ylanne et al., 1993). In response to matrix ligands or to multivalent antibody binding, the inhibitory effect of the α subunit can be released, and integrins can interact with cytoskeletal and signaling molecules (Miyamoto et al., 1995a,b).
Integrins can activate different intracellular signals, including tyrosine phosphorylation of a number of cellular proteins, elevation of the intracellular calcium level, cytoplasmic alkalinization, alteration in phospholipid metabolism, and activation of MAP kinases (Clark and Brugge, 1995; Schaller et al., 1995). The most studied pathway is represented by the activation of the FAK tyrosine kinase (Guan et al., 1991; Burridge et al., 1992). Tyrosine-phosphorylated FAK can function as a docking site for both cytoskeletal proteins, such as paxillin and talin, and signaling molecules, such as Src family tyrosine kinases, Grb2, and phosphatidyl inositol-3 kinase (Schaller and Parson, 1994; Burridge and Chrzanowska-Wodnicka, 1996). These interactions are important for coordinating actin cytoskeleton organization and gene expression and, thus, for regulating cell migration, proliferation, differentiation, and survival (Schwartz et al., 1995).
In addition to outside-in signaling, integrins can also transduce signals from the inside to the outside of the cell. The inside-out signaling occurs mainly in the form of regulation of the ligand-binding affinity state (Ginsberg et al., 1992; O’ Toole et al., 1994). By acting on the cytoplasmic domain, intracellular signals can modify the conformation of the integrin extracellular domain and, therefore, modify ligand binding capacity (O’ Toole et al., 1994).
The amino acid sequences of the β cytoplasmic domain that are important for outside-in and inside-out signaling have been mapped in some detail. Three major sites in the β1 cytoplasmic domain, termed cyto-1, 2, and 3, are important for localization of the integrin heterodimer to focal adhesions (Reszka et al., 1992). The cyto-1 site is proximal to the lipid bilayer and partially overlaps with the putative binding sites for FAK, paxillin, and α-actinin (Otey et al., 1993; Schaller et al., 1995). In β3-integrin, a region N-terminal to cyto-1 interacts with the highly conserved sequence GFFKR of the α subunits and is involved in the regulation of ligand-binding affinity (Hughes et al., 1996). The cyto-2 and cyto-3 sites correspond to two NPXY motifs and are also important for regulating the affinity state of the ectodomain (O’Toole et al., 1995).
Interestingly, four isoforms of the β1-integrin exist in humans, differing in their cytoplasmic sequence as well as in their functional properties (Balzac et al., 1994; Fornaro et al., 1995; Belkin et al., 1996). Based on the structural properties of the four splicing variants, the β1 cytoplasmic domain can be divided in two subdomains that we refer to as “common” and “variable” subdomains, respectively. The common subdomain consists of the 25 amino acid residues proximal to the membrane coded for by exon 6 (Altruda et al., 1990; Baudoin et al., 1996) and is shared by all four variants. This region is highly conserved among different β subunits and contains the cyto-1 site (Reszka et al., 1992) and the putative binding sites for FAK, paxillin (Schaller et al., 1995), and α-actinin (Otey et al., 1993). The variable subdomain extending toward the C terminus is coded for by different exons (Baudoin et al., 1996) and characterizes the four different β1 isoforms: β1A, β1B, β1C, and β1D. The β1A variable subdomain contains cyto-2 and cyto-3 sites, whereas that of β1B is characterized by a unique amino acid sequence lacking both sites. We have shown that these structural features have important consequences for the functional properties of β1A and β1B. In fact, whereas β1A is capable of triggering FAK tyrosine phosphorylation and localizing to focal adhesions, β1B lacks both functions (Balzac et al., 1994). Moreover, β1B acts as a dominant negative inhibitor, when expressed in Chinese hamster ovary (CHO) cells, by interfering with cell spreading and migration promoted by endogenous β1A (Balzac et al., 1993, 1994).
To investigate the structural basis of the β1B dominant negative effect further, we prepared different mutants lacking either the β1 cytoplasmic domain variable or common region. These constructs, together with β1B, were expressed both in GD25 cells, which express αVβ3/5 and lack β1 as a consequence of gene knockout (Wennerberg et al., 1996), and in CHO cells, which express endogenous hamster β1A. In these cellular systems we show that β1B acts as dominant negative on adhesive and signaling function of both β1- and β3/5-integrins. Analysis of mutants molecules shows that this dominant negative action is attributable to the unique β1B cytoplasmic domain.
MATERIALS AND METHODS
Antibodies and Reagents
The following antibodies were used: the rat anti-human β1 mAb 13 (Akiyama et al., 1989) was a gift from K. Yamada (National Institutes of Health, Bethesda, MD); the activating mouse anti-human β1 mAb TS2/16 (Hemler et al., 1984) was obtained from American Type Culture Collection (Rockville, MD); the mouse anti-human β1 mAb 12G10 was characterized previously (Mould et al., 1995); the rat anti-mouse β1 mAb 9EG7, with human cross-reactivity (Lenter et al., 1993), was a gift from D. Vestweber (ZMBE Technologiehof, Muenster, Germany); the blocking mouse anti-human β1 mAb AIIB2 (Werb et al., 1989) was a gift from C. Damsky (Department of Stomatology, University of California, San Francisco, CA); the inhibitory PB1 mAb against hamster α5β1 heterodimer was obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA); the blocking anti-mouse αV H9.2B8 mAb (Moulder et al., 1991) was purchased from PharMingen (San Diego, CA); the rat anti-mouse α6 mAb GoH3 (Sonnenberg et al., 1988) was a gift from A. Sonnenberg (The Neederland Cancer Institute, Amsterdam, the Netherlands); the anti-talin mAb 8d4 was obtained from Sigma (St. Louis, MO); the anti α-actinin mAb 1682 was from Chemicon (Temecula, CA); rabbit polyclonal antisera to human fibronectin and to αV, α3, and α5 integrin cytoplasmic domains were produced in our laboratory (Tarone et al., 1984, Defilippi et al., 1992); the FAK 4 polyclonal antibody and the mAb FAK9.2 for FAK immunoprecipitation and Western blotting, respectively, were prepared in our laboratory as described previously (Defilippi et al., 1995); a mouse anti-paxillin mAb and the anti-phosphotyrosine mAb PY20 were purchased from Transduction Laboratories (Nottingham, UK); fluorescein-labeled phalloidin, fluorescein-labeled goat anti-mouse IgG, rhodamine-labeled goat anti-mouse IgG, and rhodamine-labeled goat anti-rabbit IgG were all from Sigma. Fibronectin was purified from human plasma by affinity chromatography on gelatin-Sepharose according to the method of Engvall and Ruoslahti (1977); mouse laminin-1 was obtained from GIBCO BRL (St. Louis, MO); the peptides GRGDSP and GRGESP were synthesized by Drs. L. Lozzi and P. Neri (University of Siena, Siena, Italy).
Integrin β1 Cytoplasmic Domain Mutagenesis and cDNA Vector Construction
Restriction enzymes, T4 DNA ligase, and a Klenow fragment of DNA polymerase were from New England Biolabs (Beverly, MA); Taq DNA polymerase was from Promega (Madison, WI). The SV40-based expression vector pECE (Ellis et al., 1986) containing the β1A and β1B cDNA fragments was described previously (Giancotti and Ruoslahti, 1990; Balzac et al., 1993). These plasmids were used to generate four cytoplasmic domain deletion mutants of human β1-integrin as follows. The HindIII cloning site of pECE was eliminated (pECE/H−), and the 417-bp HindIII cDNA fragment, containing the entire cytoplasmic domain and a part of the transmembrane domain of human β1A, was excised and substituted with either a HindIII synthetic oligonucleotide fragment constituting the transmembrane and the cytoplasmic domain up to isoleucine residue 762 or a HindIII PCR fragment extending to the end of the β1 cytoplasmic common subdomain (threonine residue 782), followed by two stop codons, to generate the plasmids pECE/H−-β1TR and pECE/H−-β1COM, respectively. The internal deletion of the common subdomain (from isoleucine 762 to threonine 782 in the amino acid sequence) of β1A and β1B cDNA fragments was obtained by recombinant PCR mutagenesis (Higuchi, 1990). The resulting PCR fragments were used to generate the plasmid pECE/H−-β1ΔCOM-A and pECE/H−-β1ΔCOM-B. Each mutant cDNA sequence was confirmed by dideoxy sequencing (T7 sequencing kit; Pharmacia Biotech, Upsala, Sweden).
Cells and Transfections
CHO cells expressing human β1A- or β1B-integrin isoforms or the relative deletion mutants β1TR, β1COM, β1ΔCOM-A, and β1ΔCOM-B were obtained by cotransfection with pSV2-neo using the calcium phosphate precipitation method as described (Balzac et al., 1993). The GD25 cell line, derived from β1-deficient embryonic stem cells (Wennerberg et al., 1996), was transfected with β1B, β1A, β1TR, and β1COM expression constructs, together with the plasmid pECV6-hyg (Belt et al., 1989), by electroporation. Resistant populations were selected in medium containing 300 μg/ml hygromycin β (Boehringer Mannheim, Mannheim, Germany).
After 10 d of selection, both CHO and GD25-positive cells were sorted for β1 expression by a modification of the panning method (Margolskee et al., 1993). Briefly, transfected cells were adsorbed to bacteriological dishes coated with 10 μg/ml sterile-filtered anti-human β1 mAb TS2/16 in PBS and allowed to attach for 5–30 min at 4°C. Dishes were then rinsed several times with PBS to remove unbound cells, and adherent cells were detached from the substrate by trypsin-EDTA treatment and transferred to tissue culture dishes. To select populations expressing high levels of all β1 forms used, five cycles of panning in stringent conditions (short time of cell adhesion) were performed.
Integrin Analysis by Flow Cytometry and Immunoprecipitation
The expression level of transfected β1 integrins and their conformational state was measured by FACS analysis. Cells were suspended in PBS [10 mM phosphate buffer (pH 7.3) and 150 mM NaCl] containing 1 mg/ml BSA. One millimolar MnCl2 was also added when indicated. The cells were then incubated for 1 h at 4°C with saturating concentrations of β1 monoclonal antibodies followed by fluorescein-labeled goat anti-mouse IgG. After washing, cells were analyzed (5000 per sample) in a FACScan (Becton Dickinson, Mountain View, CA) equipped with 5 W argon laser at 488 nm.
Integrins were immunoprecipitated from cells labeled with 125I as described previously (Rossino et al., 1990; Balzac et al., 1994).
Cell Adhesion and Spreading Assays
Cell adhesion and spreading assays to matrix-coated microtiter plates were performed as described previously (Balzac et al., 1994). To analyze the role of divalent cations in adhesion, cells were plated in “adhesion buffer” [20 mM Tris (pH 7.4), 135 mM NaCl, 5 mM KCl, 2 mM l-glutamine, 1.8 mM glucose, and 1% BSA] containing 1 mM MnCl2, MgCl2, or CaCl2. Where indicated, adhesion was blocked by addition of the β1 mAb AIIB2 (1:5 dilution of culture supernatant) or 0.5 mg/ml GRGDSP peptide; the GRGESP peptide was also used as control. Cell adhesion was evaluated by colorimetric assay for acid phosphatase activity as described (Defilippi et al. 1991).
Fibronectin Matrix Assembly Assay
Matrix assembly was evaluated by adding exogenous purified human plasma fibronectin (Engvall and Ruoslahti, 1977) to a final concentration of 200 nM for 2 d to confluent cell monolayers grown on glass coverslips in medium containing 1% serum. Where indicated, inhibitory mAb PB1 against hamster α5β1 or mAb H9.2B8 against mouse αV was used (10 μg/ml). The resulting fibronectin matrix was then visualized by immunofluorescence.
The same protocol was used to quantitate fibronectin assembly using 100 nM 125I-labeled fibronectin (specific activity, 0.08 mCi/nM). A deoxycholate-insoluble fraction was obtained from cell monolayers as described (McKeown-Longo and Mosher, 1985; Wu et al., 1993, 1995). 125I-labeled fibronectin incorporated into the deoxycholate-insoluble extracellular matrix was analyzed by reducing SDS-PAGE (6% running gel) and autoradiography.
Immunofluorescence Microscopy
For immunofluorescence cells were seeded on circular (1-cm-diameter) glass coverslips in 12-well plates and grown for the indicated time. Where indicated, coverslips were coated with 10 μg/ml human plasma fibronectin. Immunofluorescence staining of paraformaldehyde-fixed cells was performed using a standard protocol (Balzac et al., 1993). A 1:500 dilution of the polyclonal antibody to human fibronectin, a 0.5 μg/ml solution of paxillin mAb, a 1:200 dilution of the αV cytoplasmic domain polyclonal antibody, and a 10 μg/ml solution of mAb TS2/16 were used. Bound primary antibodies were then visualized by appropriate rhodamine-labeled secondary antibodies. In some experiments cells were double stained with fluorescein-conjugated phalloidin.
Measurement of Cellular Contractility
Silicone rubber substrata for assessing cellular contractility were made as described previously (Harris et al., 1980; Danowski, 1989). Films were produced by glow discharge polymerization (5 sec, 20 mA). Briefly, 0.5 ml of silicone rubber (dimethyl polysiloxane; viscosity, 10,000–60,000 centistokes; Sigma) was aliquoted into tissue culture dishes and allowed to spread for 24 h. The top of the silicone was then coated with a thin layer of gold-palladium using a cold sputter coater. The UV glow discharge that occurred during the gold-palladium coating polymerized the silicone rubber. Cells were plated for 1 day on the cross-linked rubber substrata in growth medium with 10% serum, and the presence or absence of wrinkles was examined using an inverted phase-contrast Leitz microscope.
Detection of Phosphotyrosine-containing Proteins
To specifically trigger tyrosine phosphorylation of intracellular proteins mediated by the transfected or endogenous integrins, cells were plated on plastic dishes coated with specific monoclonal antibodies as described (Balzac et al., 1994). Cells were lysed in the presence of phosphatase inhibitors, and FAK was immunoprecipitated as described (Retta et al., 1996). After SDS-PAGE, proteins were transferred to nitrocellulose and processed for Western blotting with the anti-phosphotyrosine mAb PY20 followed by peroxidase-conjugated anti-mouse IgG (Sigma). Bound antibodies were visualized by an ECL detection method (Amersham, Buckinghamshire, UK). After stripping with 2% SDS at 42°C for 1 h to remove bound antibodies, the filter was reprobed with the mAb FAK9.2 to visualize the level of FAK protein.
Coimmunoprecipitation of Proteins Interacting with the β1 Cytoplasmic Domain
Transfected CHO cells from confluent culture dishes were suspended by EDTA treatment and incubated with 10 μg of purified TS2/16 mAb to human β1 for 30 min at 4°C on a rotator. Cells were centrifuged (1000 rpm, 3 min), and the pellets were extracted for 3 min on ice with 50 mM PIPES buffer (pH 6.9) containing 0.5% digitonin, 1 mM MgCl2, 1 mM EGTA, 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 0.5 mM PMSF. Cell extracts were centrifuged (12,000 rpm, 30 min, 4°C), and the resulting supernatants were incubated at 4°C for 45 min with protein G-Sepharose beads. Immunoprecipitates were washed in the same buffer, boiled in SDS sample buffer, and separated by 8% SDS-PAGE. Proteins were transferred onto Immobilon membranes and processed for Western blotting with 8d4 mAb against talin, 1682 mAb against α-actinin, or FAK9.2 mAb against FAK.
RESULTS
Preparation of β1-Integrin Cytoplasmic Domain Variants and Expression in CHO and β1-Null Cells
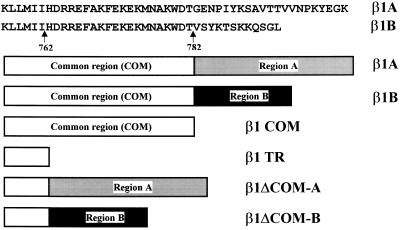
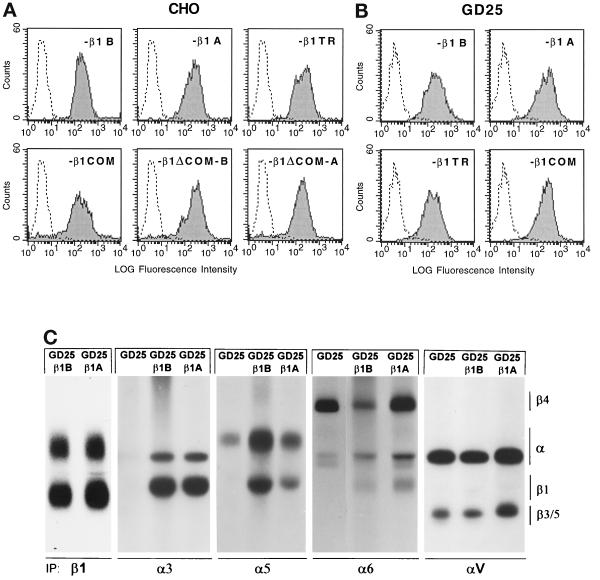
The cytoplasmic domain of β1-integrin consists of a membrane proximal subdomain, shared by all four β1 isoforms (common subdomain), and a distal subdomain toward the C terminus, unique for each isoform (variable subdomain) (Figure 1). To investigate the specific role of these two β1 cytoplasmic subdomains, we have generated the following mutants (Figure 1): β1TR, lacking the entire cytoplasmic domain; β1COM, containing only the common subdomain; and β1ΔCOM-B and β1ΔCOM-A, in which the common subdomain has been deleted and the variable B and A subdomains are directly linked to the transmembrane segment. These constructs and the natural β1B and β1A isoforms were expressed in CHO cells (Figure 2A) and in the mouse GD25 fibroblastic cell line, which lacks endogenous β1 as a consequence of gene inactivation (Fässler et al., 1995; Wennerberg et al., 1996) (Figure 2, B and C). Previous results showed that human β1A and β1B isoforms correctly associated with endogenous α subunits in CHO cells (Balzac et al., 1993, 1994). Identical results were obtained with the four β1 mutants described above (Retta, Balzac, Ferraris, Belkin, Fässler, Humphries, De Leo, Silengo, and Tarone, unpublished results). The major integrin complexes at the GD25 cell surface are α6β4-, αVβ3-, and αVβ5-integrins (Figure 2C; Wennerberg et al., 1996). Transfection of these cells with the above β1 constructs led to surface expression of β1 integrin heterodimers with the endogenous α3, α5, and α6 subunits but not with the αV subunit (Figure 2C). A significant amount of α5 was detected at the surface of untransfected GD25 cells, suggesting that a fraction of the molecule can still reach the cell surface in the absence of the β subunit.
Figure 1.
Schematic representation of β1A, β1B, and cytoplasmic domain deletion mutants prepared for these studies. The amino acid sequences of β1A and β1B cytoplasmic domain are indicated. The β1COM mutant was truncated at threonine 782 and contains only the subdomain common to the different human β1 isoforms; the β1TR mutant was truncated at isoleucine residue 762 and lacks the entire cytoplasmic domain; the β1ΔCOM-A and β1ΔCOM-B lack the common subdomain, so that the distal A and B subdomains, respectively, are directly linked to the transmembrane segment. These mutants were prepared by oligonucleotide-mediated and recombinant PCR mutagenesis as described in MATERIALS AND METHODS.
Figure 2.
Surface expression of transfected β1 variants in CHO and GD25 cells. CHO and GD25 cells were transfected with DNA constructs for human β1B or β1A or for the β1 cytoplasmic domain deletion mutants indicated, and positive cells were sorted by panning on β1 antibodies as described in MATERIALS AND METHODS. (A and B) To detect surface expression of transfected β1 variants, CHO (A) and GD25 (B) transfectants were stained with mAb TS2/16 to human β1, followed by fluorescein-labeled anti-mouse IgG, and analyzed by FACS. The level of β1 surface expression reported as fluorescence intensity is shown. Untransfected cells were included as a negative control. (C) Integrin heterodimers in 125I-surface-labeled GD25, GD25-β1B, and GD25-β1A cells as detected by nonreducing SDS-PAGE and autoradiography of immunoprecipitated integrins with antibodies specific for β1, α3, α5, α6, and αV subunits.
Adhesive Properties of β1-Integrin Cytoplasmic Variants
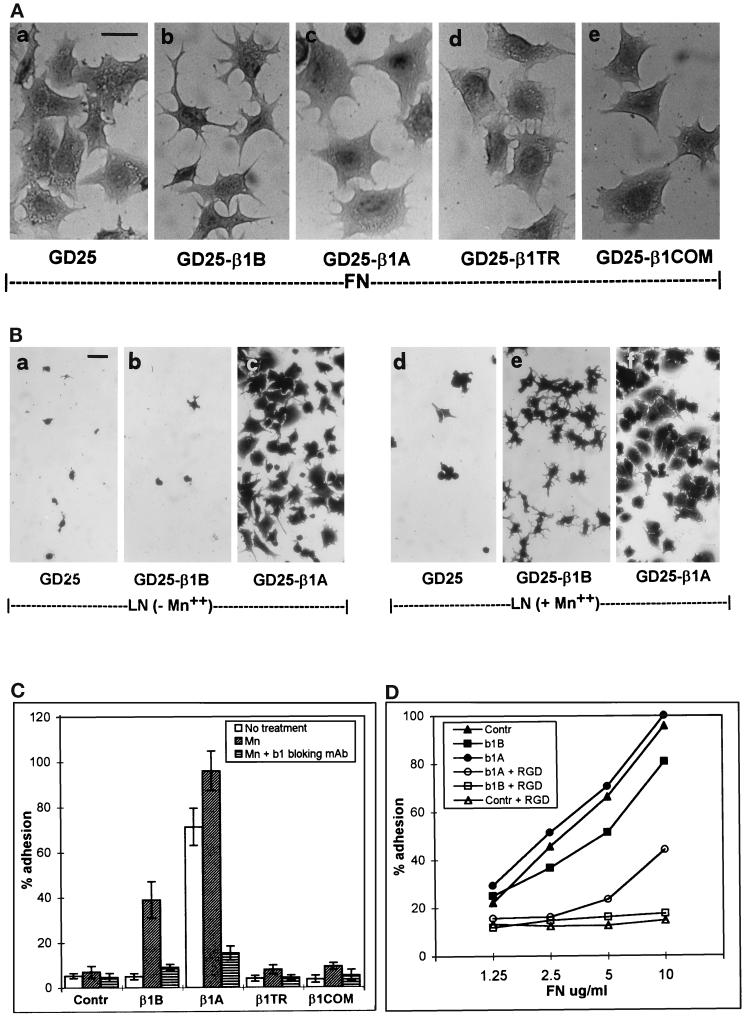
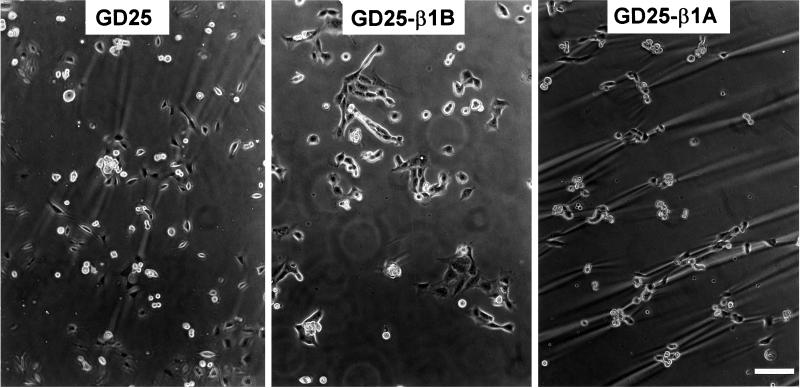
The functional properties of GD25 and CHO cells transfected with either the β1B or β1A isoform or the four β1 cytoplasmic domain mutants were evaluated by testing their adhesive properties toward fibronectin and laminin-1. A previous report showed that GD25 β1-null cells adhere to fibronectin via αVβ3 integrin (Wennerberg et al., 1996). Here we show that GD25-β1B cells have reduced ability to spread on fibronectin and retain a stellate shape with numerous cytoplasmic protrusions (Figure 3A, b). Both untransfected GD25 and GD25-β1A cells, on the other hand, showed normal spreading capacity (Figure 3A, a and c). Differences in spreading capacity between GD25-β1B and GD25 cells were maximal 1 h after plating on fibronectin in serum-free medium.
Figure 3.
Adhesion and spreading of β1 variant-transfected GD25 cells on fibronectin and laminin-1. Dishes were coated with either fibronectin or laminin-1, and cells were plated in serum-free medium for 1 h at 37°C. (A) The morphology of cells adherent to fibronectin (10 μg/ml coating) is shown for GD25 (a), GD25-β1B (b), GD25-β1A (c), GD25-β1TR (d), and GD25-β1COM (e). (B) The morphology of the cells plated on laminin-1 (10 μg/ml coating) in the absence (a–c) and presence of 1 mM Mn++ (d–f) is shown for GD25 (a and d), GD25-β1B (b and e), and GD25-β1A (c and f). Similar results were obtained using increasing doses of laminin-1 up to 50 μg/ml. Note the severe reduction of spreading in GD25-β1B cells both on fibronectin and laminin-1. Bars, 25 μm. (C) The histograms show attachment of cells to 10 μg/ml of laminin-1 in the absence (open bars), in the presence of 1 mM Mn++ (slashed bars), and in the presence of 1 mM Mn++ plus the β1 blocking mAb AIIB2 (striped bars). Results are plotted ± SE as percentage of input cells and are the mean of three experiments. (D) The graph shows cell attachment to increasing doses of fibronectin both in the absence (closed symbols) and in the presence of 500 μg/ml of GRGDSP peptide (open symbols). Adhesion is expressed as percentage of an equal number of input cells. Symbols used are as follows: GD25 (▴ and ▵), GD25-β1B (▪ and □), and GD25-β1A (• and ○).
This result in GD25 cells extends previous data obtained in CHO cells (Balzac et al., 1994) and indicates that β1B is capable of a trans-dominant negative effect toward αVβ3 integrin. Interestingly, neither β1TR nor β1COM caused reduced spreading when expressed both in GD25 (Figure 3A, d and e) and CHO cells (Retta, Balzac, Ferraris, Belkin, Fässler, Humphries, De Leo, Silengo, and Tarone, unpublished results). Moreover, also the β1ΔCOM-A and β1ΔCOM-B mutants did not interfere with cell spreading (Retta, Balzac, Ferraris, Belkin, Fässler, Humphries, De Leo, Silengo, and Tarone, unpublished results).
We then tested adhesion to laminin-1. GD25 cells do not adhere to laminin-1, allowing the direct evaluation of adhesive properties of β1 cytoplasmic domain variants in the absence of functional endogenous integrins. Although expression of β1A restored the ability of GD25 cells to adhere and spread on laminin-1, expression of the β1B isoforms did not (Figure 3B, a–c), indicating that α/β1B heterodimers do not bind efficiently to laminin-1. Lack of adhesion to laminin-1 was also observed in β1TR- and β1COM-transfected GD25 cells (Figure 3C).
To assay whether the adhesive capacity of GD25-β1B cells can be modified, we tested Mn++ ions, which are known to increase binding affinity of integrins for their ligands (Gailit and Ruoslahti, 1988). Plating in the presence of 1 mM Mn++ restored adhesive capacity to laminin-1 of GD25-β1B (Figure 3, B, b and e, and C) but not of GD25-β1TR and GD25-β1COM cells (Figure 3C), thus indicating that the β1B isoform has unique functional properties with respect to the artificial mutants. Blocking antibodies to human β1 (mAb AIIB2) prevented Mn++-dependent adhesion in GD25-β1B cells, indicating that Mn++ induces β1B to bind its ligand (Figure 3C). Mg++ and Ca++ were also tested and found to be ineffective in inducing cell adhesion to laminin-1 (Retta, Balzac, Ferraris, Belkin, Fässler, Humphries, De Leo, Silengo, and Tarone, unpublished results). Interestingly, adhesion of GD25-β1B cells to laminin-1 resulted in poor spreading with respect to GD25-β1A cells (Figure 3B, e and f).
To test whether lack of adhesion of β1B-transfected cells was restricted to laminin-1, we examined the attachment of these cells to fibronectin-coated dishes. Previous results showed that expression of β1A in GD25 cells leads to altered susceptibility to the Gly-Arg-Gly-Asp-Ser-Pro (GRGDSP) peptide (Wennerberg et al., 1996). In agreement with this, although adhesion of GD25 cells to fibronectin was completely inhibited by 500 μg/ml GRGDSP peptide, only 50% inhibition was observed in GD25-β1A cells (Figure 3D), indicating that transfected β1A modifies adhesive properties of GD25 cells to fibronectin. On the other hand 500 μg/ml GRGDSP peptide fully inhibited adhesion of GD25-β1B on fibronectin, as in the case of GD25 cells (Figure 3D), indicating that the β1B molecule does not significantly contribute to cell adhesion to fibronectin.
Cytoplasmic Domain Sequences Affect β1-Integrin Ectodomain Conformation
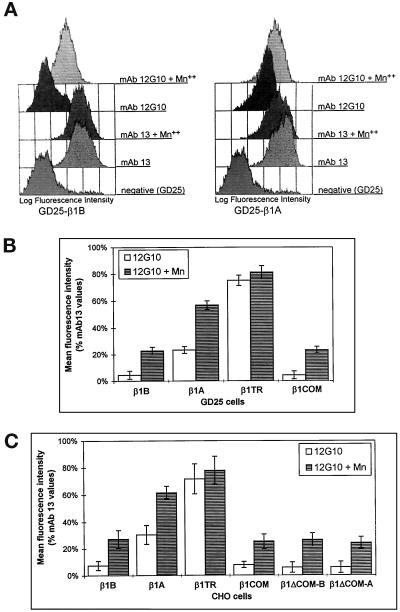
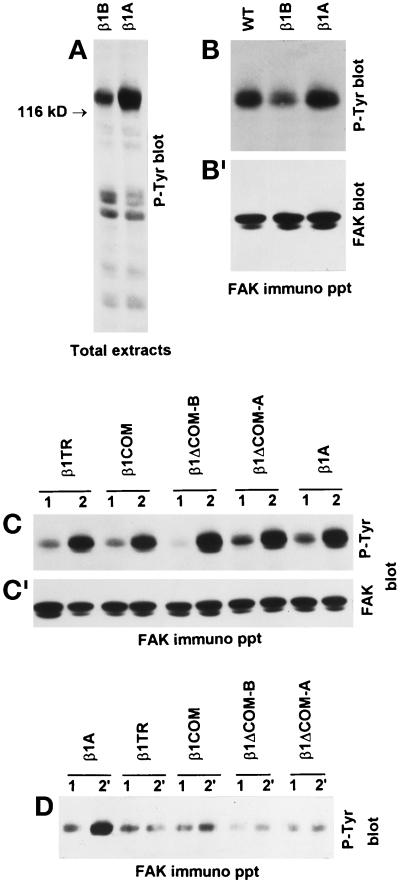
The data discussed above suggest that integrin heterodimers containing the β1B subunit are expressed at the cell surface in a conformation that does not allow efficient binding to matrix ligands. To investigate this possibility further, we used two antibodies, mAb 12G10 and mAb 9EG7, which recognize epitopes expressed only in the ligand-competent and ligand-occupied β1 (Lenter et al., 1993; Mould et al., 1995). Expression of these epitopes reflects changes in integrin ectodomain conformation, which can also be induced by exposing β1 to Mn++ (Bazzoni et al., 1995; Mould et al., 1995). The binding of both 12G10 and 9EG7 mAbs to the β1 extracellular domain was always compared with that obtained with mAb 13, which recognizes a constitutive epitope in human β1. When cells were probed with mAb 12G10, virtually no binding was detected on GD25-β1B and GD25-β1COM cells, unless Mn++ was present in the medium (Figure 4, A and B). On the contrary, a high level of mAb 12G10 binding was detected on GD25-β1A and GD25-β1TR cells, addition of Mn++ causing a further increase (Figure 4, A and B). By comparing the binding values of mAb 12G10 with those of mAb 13, we estimated that only 5–8% of the β1B at the cell surface expressed the 12G10 mAb epitope compared with 25–30% for β1A. Similar results were obtained with mAb 9EG7 (Retta, Balzac, Ferraris, Belkin, Fässler, Humphries, De Leo, Silengo, and Tarone, unpublished results).
Figure 4.
Effect of Mn++ on the conformation of β1B, β1A, and the four mutants as detected by 12G10 mAb. (A) GD25-β1B (left) and GD25-β1A (right) cells were analyzed by flow cytometry for surface expression of the 12G10 mAb epitope in the presence or absence of 1 mM Mn++. As a control, cells were also stained with mAb 13, which recognizes an Mn++-insensitive β1 epitope. GD25 cells were used as a negative control. (B and C) The histograms show the level of expression of the 12G10 mAb epitope in β1-transfected GD25 (B) and CHO (C) cells in the absence (open bars) or presence (striped bars) of 1 mM Mn++. Mean fluorescence intensities are expressed ± SE as percentage of mAb 13 values and represent the mean of five experiments.
Expression of the above β1 variants in CHO cells confirmed the data obtained with GD25 transfectans (Figure 4C). Moreover, in the CHO system we also found that β1ΔCOM-B and β1ΔCOM-A variants, like β1B and β1COM, do not express the mAb 12G10 epitope unless exposed to Mn++ ions (Figure 4C).
These data show that all of the β1 variants analyzed changed the ectodomain conformation after exposure to Mn++ ions. Despite this, after Mn++ activation only β1B, but none of the artificial β1 mutants, was able to support cell adhesion, thus indicating that both the ectodomain conformation and specific cytoplasmic subdomains are required for adhesive function. The adhesive function of Mn++-activated β1B, however, is not comparable to that of β1A, because GD25-β1B cells cannot reach full spreading (see Figure 3B, e).
β1B Inhibits Fibronectin Matrix Assembly
The assembly of fibronectin matrix in control and transfected cells was evaluated by immunofluorescence staining of cell monolayers incubated with purified human plasma fibronectin as described in MATERIALS AND METHODS.
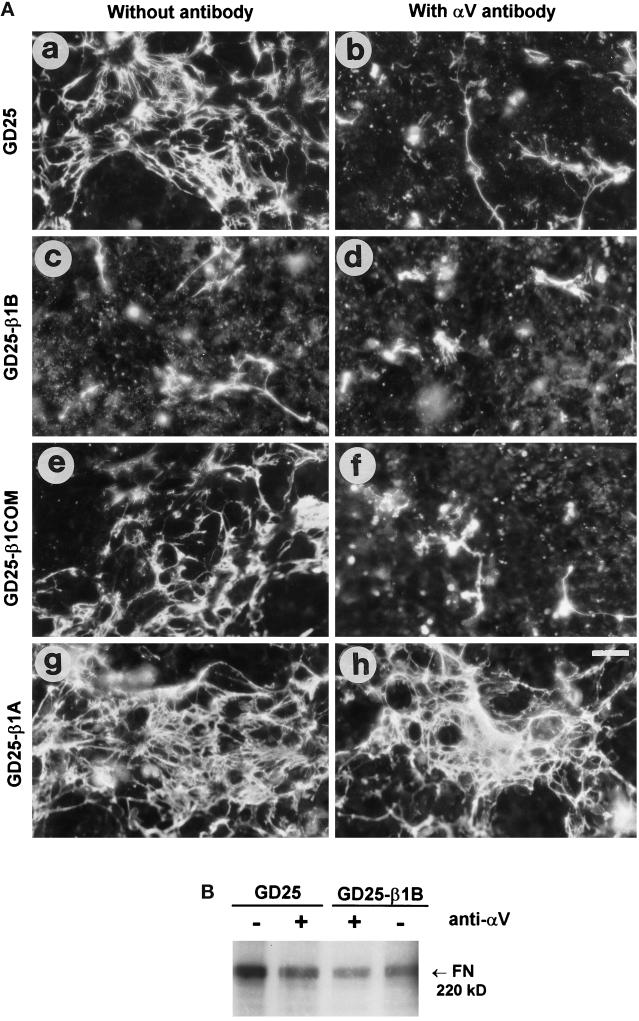
GD25 untransfected cells assemble thin fibronectin fibrils on the cell apical surface (Figure 5). This process was inhibited by an αV-blocking antibody (Figure 5, A, a and b, and B), indicating that αVβ3/5 integrins mediate matrix assembly in these cells as suggested previously (Wennerberg et al., 1996). Expression of β1B in GD25 cells resulted in dramatic inhibition of fibronectin matrix assembly (Figure 5A, c), thus indicating that β1B interferes with αV-containing endogenous integrins. Quantitative data, obtained by measuring 125I-labeled fibronectin incorporation in a detergent-insoluble matrix (Figure 5B), indicated a fivefold reduction in matrix assembly in GD25-β1B compared with parental cells. Interestingly, the expression of the two mutants β1COM (Figure 5A, e and f) and β1TR (Retta, Balzac, Ferraris, Belkin, Fässler, Humphries, De Leo, Silengo, and Tarone, unpublished results) differed from β1B and did not interfere with matrix assembly. Moreover, expression of β1A in GD25 cells resulted in increased assembly of fibronectin matrix compared with untransfected cells (Figure 5A, g and h).
Figure 5.
Exogenous fibronectin matrix assembly in control and transfected GD25 cells in the presence of an anti-αV-blocking antibody. (A) Confluent cell monolayers were cultured for 2 d with 200 nM human plasma fibronectin either in the absence (a, c, e, and g) or in the presence (b, d, f, and h) of the inhibitory mAb H9.2B8 against mouse αV, used to block the endogenous αV integrins. Cells were then fixed for 10 min in 3.7% (v/v) paraformaldehyde in PBS, and the fibronectin in the matrix was stained by a polyclonal antibody followed by a rhodamine-labeled anti-rabbit IgG. Bar, 50 μm. (B) Biochemical evaluation of fibronectin matrix assembly. 125I-labeled fibronectin incorporated into deoxycholate-insoluble matrix of cells either in the absence (−) or in the presence (+) of the αV-blocking H9.2B8 mAb was visualized by SDS-PAGE and autoradiography. The molecular weight of reduced fibronectin is shown.
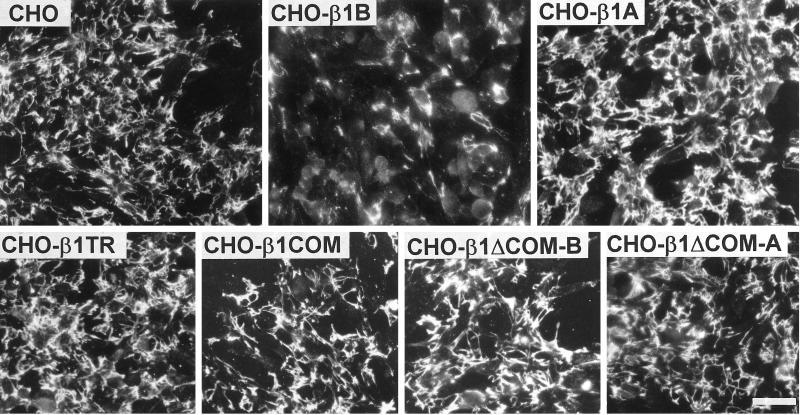
To further analyze the β1B dominant negative effect, we assayed the fibronectin matrix assembly ability of CHO cells expressing the β1B or β1A isoform as well as the four β1 mutants. As shown in Figure 6, also in CHO cells the expression of β1B resulted in a dominant negative effect; moreover, none of the other β1 constructs showed this ability. Exogenous fibronectin assembly in these cells is driven by the a5β1-integrin, because this function was blocked by the anti-hamster α5β1 mAb PB1 (Retta, Balzac, Ferraris, Belkin, Fässler, Humphries, De Leo, Silengo, and Tarone, unpublished results). This result indicates that in CHO cells β1B interferes with the endogenous a5β1 function.
Figure 6.
Fibronectin matrix assembly in control and transfected CHO cells. Confluent cell monolayers were cultured for 2 d with 200 nM human plasma fibronectin as described in MATERIALS AND METHODS. Cells were then fixed for 10 min in 3.7% (v/v) paraformaldehyde in PBS, and the fibronectin in the matrix was stained by a specific polyclonal antibody followed by a rhodamine-labeled anti-rabbit IgG. Note the dramatic reduction of fibronectin assembly in β1B-expressing cells. Bar, 80 μm.
Thus, the ability of β1B to block fibronectin matrix assembly in both GD25 and CHO cells indicates that this molecule is able to interfere with different classes of integrins involved in this process.
Expression of β1-Integrin Variants Specifically Affects Focal Adhesion and Stress Fiber Organization
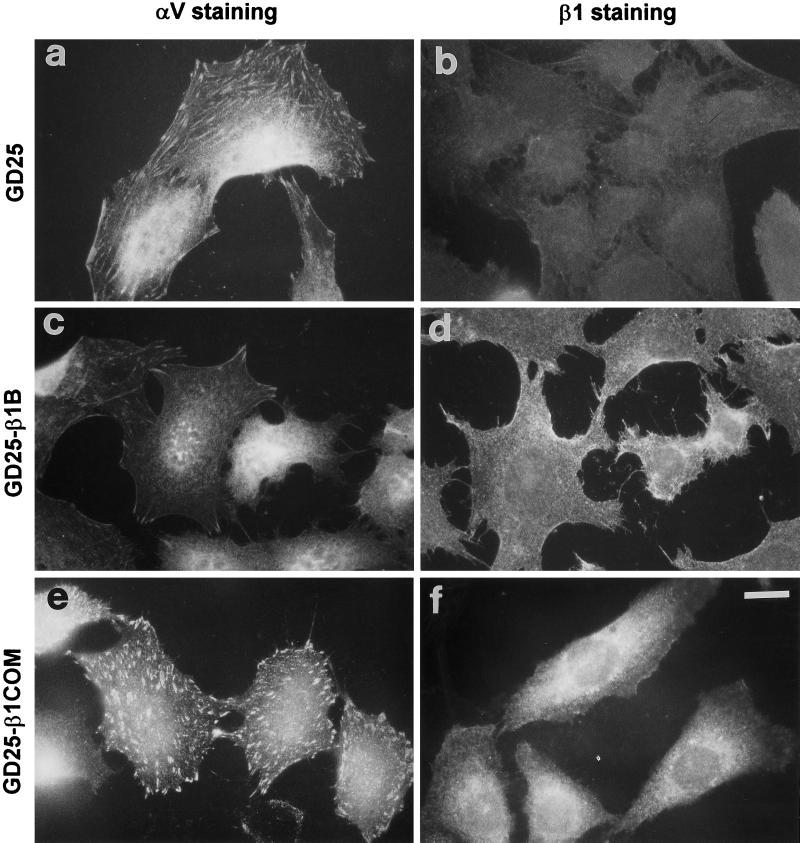
Given the fact that β1B impaired cell spreading and fibronectin matrix assembly, we also examined its ability to affect focal adhesion organization. As shown in Figure 7a, GD25 cells form αV-containing prominent focal adhesions when plated on fibronectin-coated dishes, consistent with the reported ability of αVβ3 to support attachment to fibronectin in these cells (Wennerberg et al., 1996). GD25-β1B cells plated on fibronectin showed a clear reduction of αV-containing focal adhesions when compared with GD25 cells (Figure 7, a and c). On the contrary, GD25-β1COM (Figure 7e) and GD25-β1TR (Retta, Balzac, Ferraris, Belkin, Fässler, Humphries, De Leo, Silengo, and Tarone, unpublished results) cells did not show a reduction in αV-containing focal adhesions.
Figure 7.
Localization of αV- and β1-integrin subunits to focal adhesion in control and transfected GD25 cells. Cells were plated on 10 μg/ml fibronectin-coated glass coverslips for 3 h in complete culture medium. Cells were then fixed for 10 min in 3.7% (v/v) paraformaldehyde in PBS, permeabilized with 0.5% Triton X-100 and 3.7% formaldehyde in PBS for 5 min, and stained for αV (A, C, E, G) or β1 (B, D, F, H) integrins by specific antibodies followed by rhodamine-labeled secondary antibodies. Note the reduction of αV-containing focal adhesion in β1B-transfected cells. Bar, 15 mm.
β1B, β1COM (Figure 7, d and f), and β1TR (Retta, Balzac, Ferraris, Belkin, Fässler, Humphries, De Leo, Silengo, and Tarone, unpublished results) molecules were uniformly distributed on the cell surface, as shown by immunofluorescence; thus β1B displaced αV heterodimers without localizing to focal adhesions.
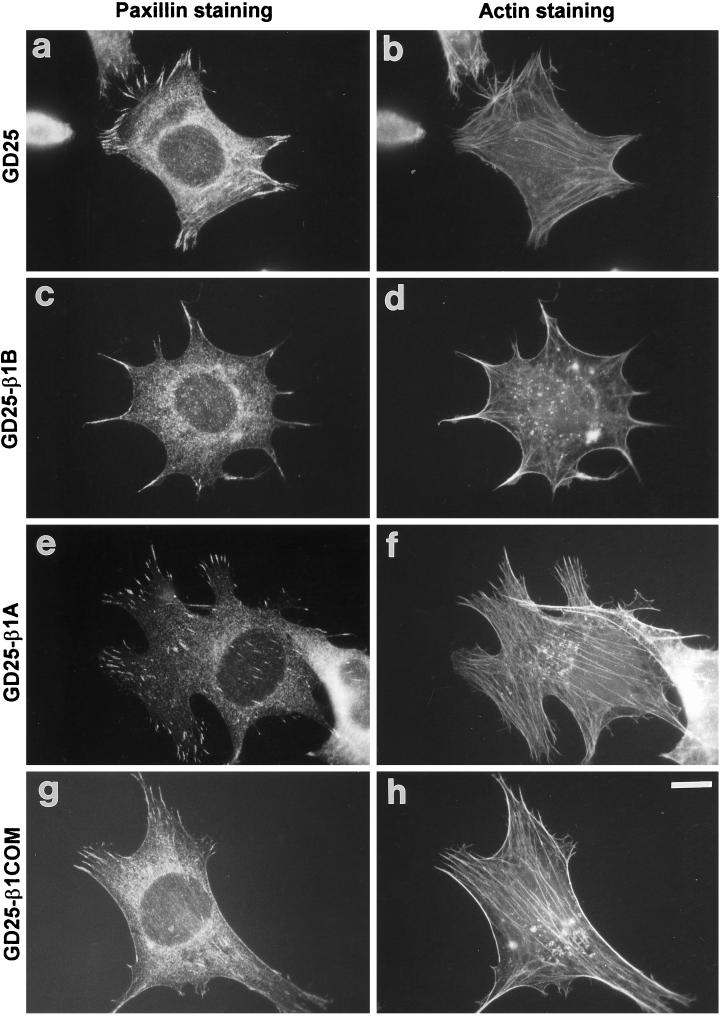
We then tested whether focal adhesion localization of cytoskeletal proteins, such as paxillin, and actin organization were also affected. As shown in Figure 8, β1B, but not β1A or β1COM, was able to reduce the number of paxillin-containing focal adhesions (Figure 8a–g). Similar results were obtained when we analyzed actin stress fibers. In fact, β1B-expressing cells showed only residual stress fibers in cell protrusions (Figure 8d), whereas cells expressing the other β1 constructs did not show significant changes compared with untransfected cells (Figure 8h). The interference of β1B on stress fiber organization was also supported by analysis of cell contractility by plating cells on silicon rubber films (Harris et al., 1980; Danowski, 1989). As shown in Figure 9, GD25 cells are able to pull on the substrate, inducing wrinkles in the silicon film. On the other hand, no wrinkles were observed in dishes seeded with GD25-β1B cells. Altogether, these data show a specific dominant negative effect of β1B on focal adhesion and stress fiber organization.
Figure 8.
Focal adhesion and stress fiber organization in control and transfected GD25 cells. Cells were plated on 10 μg/ml fibronectin-coated glass coverslips for 3 h in complete culture medium. Cells were then fixed for 10 min in 3.7% (v/v) paraformaldehyde in PBS, permeabilized with 0.5% Triton X-100 and 3.7% formaldehyde in PBS for 5 min, and double stained for paxillin (A, C, E, G) and F-actin (B, D, F, H) as described in MATERIALS AND METHODS. Note the reduction of stress fibers and paxillin-containing focal adhesions in GD25-β1B cells. Bar, 10 μm.
Figure 9.
Rubber substrate contractility assay. GD25, GD25-β1B, and GD25-β1A cells were plated for 1 d on silicone rubber substrata and photographed under phase contrast. Note the incapacity of GD25-β1B cells to wrinkle the silicon substrate. Bar, 50 μm.
β1B Has a Dominant Negative Effect on FAK Tyrosine Phosphorylation
By plating CHO cells on dishes coated with antibodies specific for the transfected human β1-integrin, we have previously shown that β1B is unable to trigger FAK tyrosine phosphorylation (Balzac et al., 1994). Given the dominant negative effect of β1B on cell adhesive functions, we tested the ability of this molecule to interfere with endogenous integrin signaling. CHO cells expressing different β1-integrin cytoplasmic variants were plated on dishes coated with anti-hamster β1, antibody and tyrosine phosphorylation of FAK was evaluated by Western blot. As shown in Figure 10, A and B, endogenous hamster β1A had a reduced capacity to induce FAK tyrosine phosphorylation in cells expressing β1B but not in cells expressing human β1A or the four β1 cytoplasmic domain mutants (Figure 10C). At the same time, the four β1 mutants, like β1B, were not capable of triggering FAK tyrosine phosphorylation to a significant extent (Figure 10D).
Figure 10.
Inhibition of β1A-induced FAK tyrosine phosphorylation by β1B expression. To specifically trigger tyrosine phosphorylation of intracellular proteins mediated by the the endogenous or transfected β1 integrins, cells were plated on plastic dishes coated with the anti-hamster β1 mAb 7E2 (A–C) or with the anti-human β1 mAb TS2/16 (D), respectively. (A and B) Tyrosine phosphorylation of total cellular proteins (A) and FAK (B) induced by endogenous hamster β1A was detected by Western blotting with anti-phosphotyrosine mAb PY20. Note reduced tyrosine phosphorylation of 116- to 130-kDa proteins (A) and FAK (B) in β1B-transfected CHO cells. (B′) The reprobing of the blot with FAK antibodies is shown. (C) The tyrosine phosphorylation of FAK via endogenous β1A was also evaluated in CHO cells expressing the different cytoplasmic domain mutants. Cells were plated on poly-l-lysine (lanes 1) for control and on 7E2 mAb to endogenous hamster β1 (lanes 2). (C′) The reprobing of the blot with FAK antibodies is shown. (D) The ability of the different mutants to trigger FAK tyrosine phosphorylation directly was evaluated by plating cells on mAb TS2/16 to the transfected human β1 (lanes 2′) or poly-l-lysine as control (lanes 1).
Association of Talin and α-Actinin with β1 Cytoplasmic Domain Variants
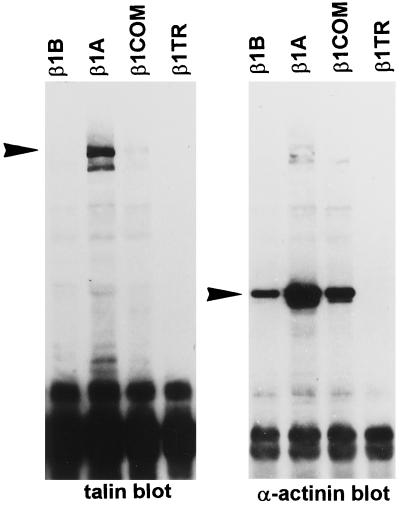
To evaluate the ability of different β1 cytoplasmic domain variants to interact with talin, α-actinin, and FAK, we performed coimmunoprecipitation studies by extracting cells under mild detergent conditions (see MATERIALS AND METHODS). As detected by Western blots, talin was coprecipitated with β1A but not with β1B, β1COM, or β1TR (Figure 11). On the other hand, α-actinin was coprecipitated with β1A, β1B, and β1COM but not with β1TR (Figure 11). The amount of α-actinin associated with β1B and β1COM immunoprecipitates, however, was reduced compared with β1A immunoprecipitate.
Figure 11.
Association of talin and α-actinin with β1 cytoplasmic domain variants. CHO cells transfected with β1B, β1A, β1COM, and β1TR integrin variants were incubated in suspension with TS2/16 mAb to human β1, and cells were lysated in digitonin-containing buffer. Immunocomplexes were recovered from cell extracts with protein G-Sepharose beads and analyzed by Western blotting with talin antibody. The blot was stripped and reprobed with α-actinin antibody. Arrowheads point to the talin and α-actinin bands.
FAK was not detected in the immunocomplexes associated with any of the four β1 molecules tested (Retta, Balzac, Ferraris, Belkin, Fässler, Humphries, De Leo, Silengo, and Tarone, unpublished results).
These data show that β1B is incapable of interacting with talin but retains the ability to bind α-actinin. This property is shared with β1COM variants.
DISCUSSION
We have previously shown that β1B, an integrin isoform with a distinct cytoplasmic domain, causes reduced cell spreading and migration when expressed in CHO cells (Balzac et al., 1994). In the present study we have examined the role of specific β1 cytoplasmic subdomains in determining this dominant negative function. To achieve this aim, we have constructed four deletion mutants (see Figure 1): β1TR, lacking the entire cytoplasmic domain; β1COM, lacking the cytoplasmic distal subdomain; and β1ΔCOM-B and β1ΔCOM-A, in which the distal B and A subdomains are linked to the transmembrane segment by deletion of the common region.
These molecules were expressed both in GD25 cells, which lack the endogenous β1 as a consequence of gene knockout (Wennerberg et al., 1996), and in CHO cells. Adhesion experiments in GD25 cells showed that β1B is unable to support cell adhesion. In fact, GD25-β1B cells, like untransfected GD25 cells, do not adhere to laminin-1, despite correct surface expression of α3β1B-, α5β1B-, and α6β1B-integrin heterodimers. Moreover, β1B-containing heterodimers are not functional in mediating adhesion to fibronectin. On the other hand, β1A supports adhesion of GD25 cells to laminin-1 and fibronectin. β1TR and β1COM behave as β1B, being unable to support cell adhesion to laminin-1.
The inability of β1B complexes to mediate cell adhesion can be rescued by treating cells with Mn++ ions, which are known to increase binding affinity of integrins for their ligands (Gailit and Ruoslahti, 1988). Mn++ treatment, however, did not restore the adhesive function of β1TR- and β1COM-transfected GD25 cells, indicating that the β1B cytoplasmic domain confers specific adhesive function.
Mn++ ions affect conformation of the β1 ectodomain. This was confirmed by using 12G10 and 9EG7 mAbs, which recognize conformation-specific epitopes in the β1 ectodomain. Both mAbs bind very poorly to β1B compared with β1A. Addition of Mn++ to the medium strongly increases 12G10 and 9EG7 mAb binding to β1B. Interestingly, β1TR, which does not support adhesion to laminin-1 even in the presence of Mn++ ions, constitutively expresses 12G10 and 9EG7 epitopes, indicating that ectodomain conformation is not sufficient to endow β1 with adhesive properties, but cytoplasmic sequences are also required. On the other hand, the β1COM variant, similarly to β1B, expresses 12G10 and 9EG7 epitopes in response to Mn++, but it does not support cell adhesion. Thus, the conserved common region of the β1 cytoplasmic domain, present in the β1COM mutant, is not sufficient to support cell adhesion but needs to be linked to either the A or B distal subdomain. Although the combination COM + A gives raise to a fully functional molecule, COM + B is only partially functional, because it requires Mn++ to support attachment and does not allow cell spreading.
The results presented show that β1B and the four β1 cytoplasmic domain mutants β1TR, β1COM, β1ΔCOM-A, and β1ΔCOM-B are incapable of inducing fibronectin matrix assembly. Interestingly, however, β1B shows a strong capacity to inhibit matrix assembly controlled by endogenous integrins, a property that is not shares by β1TR, β1COM, β1ΔCOM-A, and β1ΔCOM-B. Fibronectin matrix assembly involves different receptors in CHO and GD25 β1-null cells. Although α5β1 is the major CHO integrin receptor involved in this function, in GD25 cells this role is played by αV-containing integrin complexes. The ability of β1B to inhibit fibronectin matrix assembly in both cell types indicates a dominant negative effect on both α5β1 and αVβ3/β5 integrins. Work in progress indicates that β1B also inhibits fibronectin matrix assembly in epithelial cells and primary mouse fibroblasts, thus showing that this is a general property of β1B in several cell types.
We also found a strong effect of β1B on focal adhesion and actin stress fiber organization. In fact, in GD25 cells that adhere to fibronectin via αVβ3, β1B expression led to a reduced number of αV-containing focal adhesions. In these cells β1B is uniformly diffuse at the cell surface but does not associate with the αV subunit; thus reduction of αV localization to focal adhesion cannot be explained by competitive displacement. On the other hand, paxillin and F-actin staining showed an overall reduction of focal adhesions and stress fibers in GD25-β1B cells. This reduction could explain the GD25-β1B cells’ poor spreading on fibronectin as well as the reduced contractility on silicon rubber film. The interference with actin cytoskeleton organization is specific for the β1B isoform, because neither β1TR nor β1COM leads to this effect.
To investigate at a molecular level the dominant negative effects of β1B, we analyzed the signal transduction ability of endogenous integrins in cells expressing the β1 cytoplasmic domain variants. The results obtained showed that β1B clearly exerts a dominant negative effect on the ability of endogenous β1 to trigger FAK tyrosine phosphorylation, whereas neither β1A nor the four β1 mutants showed this effect.
Altogether, these data show that β1B interferes with adhesive functions of integrins by inhibiting FAK tyrosine phosphorylation, focal adhesion and stress fiber organization, cell spreading, and fibronectin matrix assembly. This dominant negative function is specific to β1B.
Both the ectoplasmic and cytoplasmic domains of β1B are functionally altered. We have shown, in fact, that the β1B extracellular domain is exposed at the cell surface in a conformation that cannot support cell adhesion; in addition, β1B is defective in two properties depending on the cytoplasmic domain, namely localization to focal adhesions and triggering of FAK tyrosine phosphorylation (Balzac et al., 1994). Functional analysis of the four β1 cytoplasmic domain mutants presented in this work allows us to conclude that the dominant negative effect does not depend on the altered functional properties of the β1B ectodomain. In fact, the β1COM mutant, which is exposed at the cell surface in a conformation that cannot support cell adhesion like β1B, does not act as a dominant negative molecule. Moreover, the β1TR mutant, lacking the entire cytoplasmic domain, does not show a dominant negative action. These data indicate that the β1B cytoplasmic domain is responsible for the dominant negative effect. In addition, the β1COM and β1ΔCOM-B mutants indicate that the membrane proximal common and the variable B subdomains alone are not sufficient for this function. We can conclude that the combination of common and B subdomains is required to confer the dominant negative effect.
Among the possible mechanisms at the basis of the dominant negative function, we can hypothesize that β1B may act by generating specific negative signals, or it may act by binding and sequestering cytoskeletal or signaling molecules crucial for integrin function. The latter possibility is less likely, as suggested by coprecipitation experiments; in fact, β1B did not bind talin or FAK. β1B was able to bind α-actinin, however, but this cannot account for the dominant negative effect, because similar binding was also observed in the case of β1COM, which does not act as dominant negative.
In conclusion, our data show that the specific linkage of the β1 cytoplasmic common subdomain with the variable A and B subdomains leads to specific functional properties, suggesting that splicing in the β1 cytoplasmic domain plays a key role in generating β1 isoforms that may serve to regulate adhesive functions specifically.
ACKNOWLEDGMENTS
The authors thank the following colleagues for generously providing important reagents : Caroline Damsky, Filippo Giancotti, Arnoud Sonnenberg, Kenneth Yamada, and Dieter Vestweber. Massimo Geuna is gratefully acknowledged for help in the flow cytometric analysis. The authors also thank Lucio Nitch, Corrado Garbi, and Gennaro Calì for helpful discussion and Massimo Ternullo for helping in some experiments. This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro, from the Consiglio Nazionale delle Ricerche Progetto Finalizzato Applicazioni Cliniche Ricerca Oncologica, and from Comitato Promotore Telethon.
REFERENCES
- Akiyama SK, Yamada SS, Chen WT, Yamada KM. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989;109:863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama SK, Yamada SS, Yamada KM, LaFlamme SE. Transmembrane signal transduction by integrin cytoplasmic domains expressed in single-subunit chimeras. J Biol Chem. 1994;269:15961–15964. [PubMed] [Google Scholar]
- Altruda F, Cervella P, Tarone G, Botta C, Balzac F, Stefanuto G, Silengo L. A human integrin β1 with a unique cytoplasmic domain generated by alternative mRNA processing. Gene. 1990;95:261–266. doi: 10.1016/0378-1119(90)90369-3. [DOI] [PubMed] [Google Scholar]
- Balzac F, Belkin A, Koteliansky V, Balabanow Y, Altruda F, Silengo L, Tarone G. Expression and functional analysis of a cytoplasmic domain variant of the β1 integrin subunit. J Cell Biol. 1993;211:171–178. doi: 10.1083/jcb.121.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzac F, Retta SF, Albini A, Melchiorri A, Koteliansky V, Geuna M, Silengo L, Tarone G. Expression of β1B integrin isoform in CHO cells results in a dominant negative effect on cell adhesion and motility. J Cell Biol. 1994;127:557–565. doi: 10.1083/jcb.127.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudoin C, Van der Flier A, Borradori L, Sonnenberg A. Genomic organization of the mouse beta 1 gene: conservation of the beta1D but not of the beta 1B and beta 1C integrin splice variants. Cell Adhes Commun. 1996;4:1–11. doi: 10.3109/15419069609010759. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel β1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–25577. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- Belkin AM, Zhidkova NI, Balzac F, Altruda F, Tomatis D, Maier A, Tarone G, Koteliansky VE, Burridge K. β1D integrin displaced the β1A isoform in striated muscles: localization at junctional structures and signaling potential in nonmuscle cells. J Cell Biol. 1996;132:211–226. doi: 10.1083/jcb.132.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belt PB, Groeneveld H, Teubel WJ, Putte vdP, Backendorf C. Construction and properties of an Epstein-Barr-virus-derived cDNA expression vector for human cells. Gene. 1989;84:407–417. doi: 10.1016/0378-1119(89)90515-5. [DOI] [PubMed] [Google Scholar]
- Briesewitz R, Kern A, Marcantonio EE. Ligand-dependent and -independent integrin focal contact localization: the role of the α chain cytoplasmic domain. Mol Biol Cell. 1993;4:593–604. doi: 10.1091/mbc.4.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and p125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–904. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Appeddu PA, Parsons JT, Hildebrand JD, Schaller MD, Guan JL. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995;270:16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Danowski BA. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci. 1989;93:255–266. doi: 10.1242/jcs.93.2.255. [DOI] [PubMed] [Google Scholar]
- Dedhar S, Hannigan GE. Integrin cytoplasmic interactions and bidirectional transmembrane signaling. Curr Opin Cell Biol. 1996;8:657–669. doi: 10.1016/s0955-0674(96)80107-4. [DOI] [PubMed] [Google Scholar]
- Defilippi P, Retta SF, Olivo C, Palmieri M, Venturino M, Silengo L, Tarone G. p125FAK tyrosine phosphorylation and focal adhesion assembly: studies with phosphotyrosine phosphatase inhibitors. Exp Cell Res. 1995;221:141–152. doi: 10.1006/excr.1995.1361. [DOI] [PubMed] [Google Scholar]
- Defilippi P, Silengo L, Tarone G. α6/β1 integrin (laminin-receptor) is down-regulated by tumor necrosis factor β1 and interleukin-1β in human endothelial cells. J Biol Chem. 1992;267:18303–18307. [PubMed] [Google Scholar]
- Defilippi P, van Hinsbergh V, Bertolotto A, Rossino P, Silengo L, Tarone G. Differential distribution and modulation of expression of a1β1 integrin on human endothelial cells. J Cell Biol. 1991;114:855–863. doi: 10.1083/jcb.114.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L, Clauser E, Morgan DO, Edery M, Roth RA, Rutter WJ. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromise insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986;45:721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Engvall E, Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977;20:1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Fässler R, Pfaff M, Murphy J, Noegel AA, Johansson S, Timpl R, Albrecht R. Lack of beta 1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J Cell Biol. 1995;128:979–988. doi: 10.1083/jcb.128.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M, Zheng DQ, Languino LR. The novel structural motif Gln795-Gln802 in the integrin β1C cytoplasmic domain regulates cell proliferation. J Biol Chem. 1995;270:24666–24669. doi: 10.1074/jbc.270.42.24666. [DOI] [PubMed] [Google Scholar]
- Gailit J, Ruoslahti E. Regulation of the fibronectin receptor affinity by divalent cations. J Biol Chem. 1988;263:12927–12932. [PubMed] [Google Scholar]
- Giancotti F, Ruoslahti E. Elevated levels of alpha5/beta1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990;60:849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Ginsberg MH, Du X, Plow EF. Inside-out signaling. Curr Opin Cell Biol. 1992;4:766–771. doi: 10.1016/0955-0674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- Guan JL, Trevethick JE, Hynes RO. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120 kD protein. Cell Regul. 1991;2:951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208:177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- Hemler ME, Sanchez-Madrid F, Flotte TJ, Krensky AM, Burakoff SJ, Bhan AK, Springer TA, Strominger JL. Glycoproteins of 210,000 and 130,000 m. w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol. 1984;132:3011–3018. [PubMed] [Google Scholar]
- Hibbs ML, Xu Stacker HSA, Springer TA. Regulation of adhesion to ICAM-1 by the cytoplasmic domain of LFA-1 β-subunit. Science. 1991;251:1611–1613. doi: 10.1126/science.1672776. [DOI] [PubMed] [Google Scholar]
- Higuchi R. Recombinant PCR. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: A Guide to Methods and Applications. San Diego: Academic Press; 1990. pp. 177–183. [Google Scholar]
- Hughes PE, Diaz-Gonzalez F, Leong L, Wu C, McDonald JA, Shattil SJ, Ginsberg MH. Breaking the integrin hinge. J Biol Chem. 1996;271:6571–6574. doi: 10.1074/jbc.271.12.6571. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- LaFlamme SE, Akiyama SK, Yamada KM. Regulation of fibronectin receptor distribution. J Cell Biol. 1992;117:437–447. doi: 10.1083/jcb.117.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc Natl Acad Sci USA. 1993;90:9051–9055. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashev ME, Sheppard D, Pytela R. Disruption of integrin function and induction of tyrosine phosphorylation by the autonomously expressed beta 1 integrin cytoplasmic domain. J Biol Chem. 1994;269:18311–18314. [PubMed] [Google Scholar]
- Margolskee RF, McHendry-Rinde B, Horn R. Panning transfected cells for electrophysiological studies. BioTechniques. 1993;15:906–911. [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J Cell Biol. 1985;100:364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Akiyama SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995a;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995b;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould AP, Garrat AN, Askari JA, Akiyama SK, Humphries MJ. Identification of a novel anti-integrin monoclonal antibody that recognizes a ligand-induced binding site epitope on β1 subunit. FEBS Lett. 1995;363:118–122. doi: 10.1016/0014-5793(95)00301-o. [DOI] [PubMed] [Google Scholar]
- Moulder K, Roberts K, Shevach EM, Coligan JE. The mouse vitronectin receptor is a T cell activation antigen. J Exp Med. 1991;173:343–347. doi: 10.1084/jem.173.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole T, Katagiri Y, Faull R, Peter K, Tamura R, Quaranta V, Loftus JC, Shattie SJ, Ginsberg MH. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole TE, Ylanne J, Culley BM. Regulation of integrin affinity states through an NPXY motif in the β subunit cytoplasmic domain. J Biol Chem. 1995;270:8553–8558. doi: 10.1074/jbc.270.15.8553. [DOI] [PubMed] [Google Scholar]
- Otey CA, Vasquez GB, Burridge K, Erickson BW. Mapping of the α-actinin binding site within the β1 integrin cytoplasmic domain. J Biol Chem. 1993;268:21193–21197. [PubMed] [Google Scholar]
- Reszka AA, Hayashi RA, Horwitz AF. Identification of amino acid sequences in the integrin β1 cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992;117:1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retta SF, Barry ST, Critchley DR, Defilippi P, Silengo L, Tarone G. Focal adhesion and stress fiber formation is regulated by tyrosine phosphatase activity. Exp Cell Res. 1996;229:307–317. doi: 10.1006/excr.1996.0376. [DOI] [PubMed] [Google Scholar]
- Rossino P, Gavazzi I, Timpl R, Aumailley M, Abbadini M, Giancotti F, Silengo L, Marchisio PC, Tarone G. Nerve growth factor induces increased expression of a laminin-binding integrin in rat pheochromocytoma PC12 cells. Exp Cell Res. 1990;189:100–108. doi: 10.1016/0014-4827(90)90262-9. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Reed JC. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Inegrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Otey CA, Hildebrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Parson JT. Focal adhesion kinase and associated proteins. Curr Opin Cell Biol. 1994;6:705–710. doi: 10.1016/0955-0674(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A, Hogervorst F, Osterop A, Veltman FEM. Identification and characterization of a novel antigen complex on mouse mammary tumor cells using a monoclonal antibody against platelet glycoprotein Ic. J Biol Chem. 1988;263:14030–14038. [PubMed] [Google Scholar]
- Tarone G, Ferracini R, Galetto G, Comoglio PM. A cell surface, integral, membrane glycoprotein of 85,000 D (GP85) associated With Triton X100-insoluble cell skeleton. J Cell Biol. 1984;99:512–519. doi: 10.1083/jcb.99.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg K, Lohikangas L, Gullberg D, Pfaff M, Johansson S, Fässler R. β1 integrin-dependent and -independent polymerization of fibronectin. J Cell Biol. 1996;132:227–238. doi: 10.1083/jcb.132.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z, Tremble PM, Behrendsen O, Crowley E, Damsky CD. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;109:877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Bauer JS, Juliano RL, McDonald JA. The alpha 5 beta 1 integrin fibronectin receptor, but not the alpha 5 cytoplasmic domain, functions in an early and essential step in fibronectin matrix assembly. J Biol Chem. 1993;268:21883–21888. [PubMed] [Google Scholar]
- Wu C, Keivens VM, O’Toole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995;83:715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Miyamoto S. Integrin transmembrane signaling and cytoskeletal control. Curr Opin Cell Biol. 1995;7:681–689. doi: 10.1016/0955-0674(95)80110-3. [DOI] [PubMed] [Google Scholar]
- Ylanne J, Chen Y, O’ Toole TE, Loftus JC, Takada Y, Ginsberg MH. Distinct functions of integrin α and β subunit cytoplasmic domains in cell spreading and formation of focal adhesions. J Cell Biol. 1993;122:223–233. doi: 10.1083/jcb.122.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]