Abstract
We have examined the behavior of demembranated sperm heads when injected into the germinal vesicle (GV) of amphibian oocytes. Xenopus sperm heads injected into Xenopus GVs swelled immediately and within hours began to stain with an antibody against RNA polymerase II (Pol II). Over time each sperm head became a loose mass of chromosome-like threads, which by 24–48 h resolved into individually recognizable lampbrush chromosomes (LBCs). Although LBCs derived from sperm are unreplicated single chromatids, their morphology and immunofluorescent staining properties were strikingly similar to those of the endogenous lampbrush bivalents. They displayed typical transcriptionally active loops extending from an axis of condensed chromomeres, as well as locus-specific “landmarks.” Experiments with [3H]GTP and actinomycin D demonstrated that transcription was not necessary for the initial swelling of the sperm heads and acquisition of Pol II but was required for maintenance of the lampbrush loops. Splicing was not required at any stage during formation of sperm LBCs. When Xenopus sperm heads were injected into GVs of the newt Notophthalmus, the resulting sperm LBCs displayed very long loops with pronounced Pol II axes, like those of the endogenous newt LBCs; as expected, they stained with antibodies against newt-specific proteins. Other heterologous injections, including sperm heads of the frog Rana pipiens and the zebrafish Danio rerio in Xenopus GVs, confirm that LBCs can be derived from taxonomically distant organisms. The GV system should help identify both cis- and trans-acting factors needed to convert condensed chromatin into transcriptionally active LBCs. It may also be useful in producing cytologically analyzable chromosomes from organisms whose oocytes do not go through a typical lampbrush phase or cannot be manipulated by current techniques.
INTRODUCTION
More than 100 years ago Flemming (1882) described giant chromosomes in the oocyte nucleus or germinal vesicle (GV) of the salamander Ambystoma mexicanum. His initial brief observations were soon followed by detailed investigations that established the existence of giant chromosomes in GVs of many animals, both vertebrate and invertebrate (Rückert, 1892; Carnoy and Lebrun, 1897; Maréchal, 1907). Rückert named them lampbrush chromosomes (LBCs) because of their fancied resemblance to the brushes used for cleaning kerosene lamp chimneys. Their overall organization and functional significance have been the subject of extensive experimentation and speculation (reviewed in Callan, 1986; Davidson, 1986). Because LBCs occur in oocytes during the growth period, they are in an arrested diplotene phase of meiosis I with homologous chromosomes held together at one or more chiasmata. Each homologue has a 4′,6-diamidino-2-phenylindole (DAPI)-positive axis of chromomeres, which correspond to transcriptionally inactive regions where sisters are closely associated. Numerous pairs of loops extend laterally from the chromomere axis; these consist of transcriptionally active regions where sisters are completely independent of one another. At the light optical level loops are not of uniform thickness but consist of one or more thin-to-thick regions, which correspond to transcription units. The electron microscope shows that the thin-to-thick morphology reflects the increasing length of nascent ribonucleoprotein (RNP) fibrils along the transcription unit in the direction of transcription.
Despite a wealth of detailed morphological and molecular information on LBCs, fundamental questions remain about their structure and especially about the significance of their highly active transcription. At one time their structure was regarded as unusual, but the discovery of looped chromatin domains in somatic nuclei (Paulson and Laemmli, 1977; Saitoh and Laemmli, 1993; Yokota et al., 1995) suggests that the lampbrush condition may be a good model for chromosomes in general. What factors cause a condensed chromosome to assume the lampbrush condition remain obscure, however. Questions about LBC structure and activity would be easier to address in a system in which the lampbrush state could be experimentally induced and altered. Recently, we have discovered that sperm chromatin injected into an amphibian GV gives rise within hours to typical transcriptionally active LBCs. This system should be useful in assessing the relative importance of cis and trans factors in establishing the morphological and molecular attributes of LBCs. It may also permit analysis of transcriptionally active chromosomes from organisms whose oocytes cannot be handled by current techniques or do not go through a typical lampbrush phase.
MATERIALS AND METHODS
Oocytes
A female Xenopus laevis was anesthetized in 0.1% methanesulfonate salt of 3-aminobenzoic acid ethyl ether (tricaine methanesulfonate or MS222; product A5040, Sigma, St. Louis, MO). A sample of ovary was removed surgically and held at 18–20°C in a small Petri dish of OR2 saline (Wallace et al., 1973). In some experiments the ovary sample was treated with crude collagenase from Clostridium histolyticum, which removes the outer layers of follicle cells and causes individual oocytes to come apart. Treatment was for 2 to 6 h at room temperature in 0.2% collagenase (type II, Sigma, product C6885) made up in Ca2+-free OR2. In other cases, individual oocytes or small clumps of oocytes with their follicle layers intact were separated with jeweler’s forceps.
GV Spreads
Detailed instructions for studying LBCs of Xenopus were published earlier (Gall et al., 1991), but a few critical modifications now dramatically improve the quality of GV spreads (Gall, 1998). Injected oocytes were transferred one at a time to isolation medium (83 mM KCl, 17 mM NaCl, 6.5 mM Na2HPO4, 3.5 mM KH2PO4, 1 mM MgCl2, 1 mM dithiothreitol, pH 7.0). The GV was removed with forceps and transferred within 20 s to spreading medium (21 mM KCl, 4 mM NaCl, 1.6 mM Na2HPO4, 0.9 mM KH2PO4, 1 mM MgCl2, 1 mM dithiothreitol, 0.1% paraformaldehyde, pH 7.0). The nuclear envelope was removed with jeweler’s forceps, and the nuclear gel was transferred with a pipette to a spreading chamber. If the procedure was carried out quickly, dispersal of the nuclear gel occurred within minutes and centrifugation could begin almost immediately. Slides were centrifuged in a special holder in the Sorvall HS4 rotor (DuPont, Wilmington, DE) at 5000 rpm (4800 × g), 4°C for 30 min. GV spreads were fixed with 2% paraformaldehyde in phosphate-buffered saline for 1 h or longer.
Immunofluorescence
GV spreads were blocked with 10% horse serum in phosphate-buffered saline for 15–30 min and then incubated with primary antibody for 1 h. After a brief rinse in 3% horse serum, they were treated with a secondary antibody for another hour. Rabbit serum was diluted 1:200 in 10% horse serum, whereas monoclonal antibodies (mAbs) were used as undiluted culture supernatant. Secondary antibodies were Cy3- or fluorescein-conjugated goat anti-mouse or goat anti-rabbit sera (Cappel/Organon Teknika, Durham, NC). Preparations were mounted in 50% glycerol containing 1 mg/ml phenylenediamine, pH 9, and 1 μg/ml DAPI.
Sperm Heads
Sperm heads were demembranated with lysolecithin as originally described by Gurdon (1976). Sperm heads of X. laevis and Silurana (Xenopus) tropicalis were prepared from testes as described (Newmeyer and Wilson, 1991). Sperm of the mouse Mus musculus and the zebrafish Danio rerio were prepared in the same way; mature sperm from the mouse were obtained from the epididymis, whereas mature sperm from the zebrafish were squeezed from adult males. Sperm of the cricket Acheta domesticus were obtained by homogenizing six to eight pairs of testes from adult insects in distilled water, which effectively destroys most cells but leaves mature and nearly mature sperm intact. The sperm were concentrated by centrifugation and then treated with lysolecithin as for the other species. In each case sperm counts were made with a hemacytometer.
Oocyte Injections
Some oocytes were partially defolliculated with collagenase before injection, whereas others were used without enzyme treatment. Defolliculated oocytes are easier to penetrate with the injection needle, but this advantage is offset by the time required for defolliculation and the somewhat greater fragility of treated oocytes. Before injection, oocytes were centrifuged at 600 × g for 20 to 30 min to bring the GV to the surface, where its position can be detected as a depigmented area. Glass needles were made from capillary tubing (0.5 mm i.d. and 1.2 mm o.d.) by using a Vertical Pipette Puller (David Kopf Instruments, Tujunga, CA). Before use, the needle was siliconized and the tip was broken off with forceps to give an internal diameter of 10–20 μm. The needle was filled from the back with 4–5 μl of sperm suspension and then with mineral oil. Injections of 4.8 nl or 9.6 nl were made under a dissecting microscope with a Drummond Nanoject microinjection apparatus (Drummond Scientific, Broomall, PA), which employs a plunger to displace liquid in the needle. We found it advantageous to make injections soon after filling the needle with the sperm suspension. Test spottings onto a microscope slide showed that the number of ejected sperm decreased with time after filling, presumably due to settling of sperm heads and adherence to the side of the needle.
Transcription and Splicing
Transcription was monitored by injecting [3H]GTP into the cytoplasm of oocytes. [3H]GTP (250 μCi; 5.7 Ci/mmol; Amersham, Arlington Heights, IL) in 50% ethanol was evaporated to dryness and redissolved in 1.25 μl of H2O. Approximately 1 μCi (5 nl) was injected into each oocyte. At various times after injection, GV spreads were made and fixed in 2% paraformaldehyde. They were dehydrated in an ethanol series and air dried from acetone. Unincorporated label was removed with 5% trichloroacetic acid at 4°C for 5 min. After a second dehydration and drying from acetone, the slides were dipped in NTB2 autoradiographic emulsion (Eastman Kodak, Rochester, NY). Autoradiographic exposure was for 1.5 d, followed by development in D19 developer for 2 min. GV contents were stained through the emulsion with Coomassie blue (Gall et al., 1991). To inhibit splicing, 10 ng of an oligodeoxynucleotide complementary to nucleotides 28–42 of Xenopus U2 small nuclear RNA (snRNA; 5′-CAGATACTACACTTG-3′) was injected with sperm into the GV (Pan and Prives, 1988; Tsvetkov et al., 1992). The oligonucleotide hybridizes with U2 snRNA in the GV, which is then destroyed by endogenous RNase H activity within 1–4 h. Preparations of LBCs made 24 h after injection show unusually large loops that lack all detectable U2 snRNA (Tsvetkov et al., 1992).
RESULTS
Injection of Sperm Heads
Initial experiments involved injection of demembranated X. laevis sperm heads into Xenopus GVs of stage IV–V oocytes (1.0–1.2 mm in diameter). In various experiments, the injected volume was 5–10 nl, nominally containing 1–5 sperm heads/nl, but the number of injected heads was often lower than calculated, probably due to adherence of the heads to the inside of the needle. At various times after injection, GVs were manually dissected from the oocytes, their contents were allowed to disperse in specially prepared well slides, and the slides were centrifuged to insure attachment of the GV contents to the glass substrate. We have now carried out several dozen injection experiments, each involving 20–60 oocytes. In a typical experiment, 90–95% of the injected oocytes appear normal after 24 h, and of these, 60–90% received sperm heads in the GV, the remaining ones presumably representing cases where the needle missed the GV or sperm heads were not ejected from the needle.
Initial Swelling of Sperm Heads
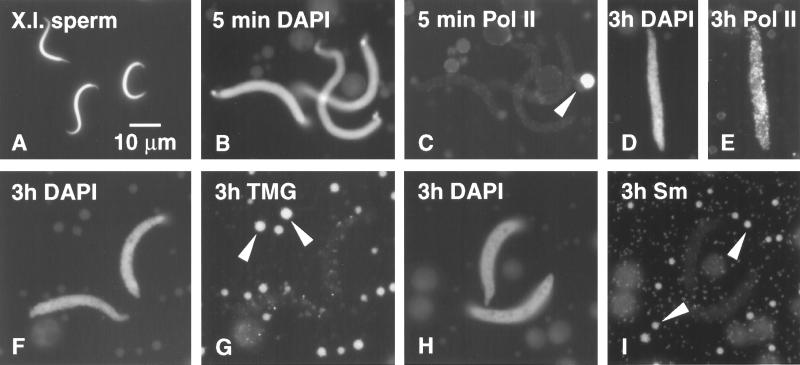
Demembranated Xenopus sperm heads as used for injection are worm-like structures roughly 20 μm in length, tapered toward both ends with a maximal diameter near the middle of about 1 μm (Figure 1A). After injection into the GV, they were easily recognized by their morphology and their intense staining with the DNA-specific dye DAPI. The first sign of change in the sperm heads was swelling (Figure 1, B and C), which took place without formation of a membrane or envelope detectable by phase contrast or differential interference (DIC) microscopy (Figure 2B). The timing of subsequent events was variable from experiment to experiment, possibly related to differences in oocytes from different females. In many cases the swollen sperm heads remained more or less unchanged during the first 3–6 h (Figure 1, D–I), whereas in other experiments there was marked decondensation and evidence of LBC structure (Figure 2F).
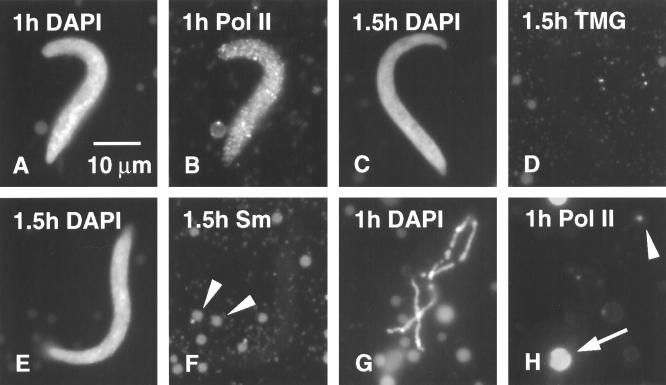
Figure 1.
(A) Demembranated sperm heads of X. laevis as injected (DAPI stain for DNA). (B) Swollen sperm heads 5 min after injection into a Xenopus GV (DAPI stain). (C) Same field stained with mAb H14 against RNA Pol II; sperm heads are negative, whereas a coiled body (sphere) stains brightly (arrowhead). (D) Sperm head 3 h after injection into a GV (DAPI stain). (E) Same sperm head shows strong staining with mAb H14 against RNA Pol II. (F) Sperm heads 3 h after injection into a GV (DAPI stain). (G) Same field stained with mAb K121 against the trimethylguanosine (TMG) cap of snRNAs. Sperm heads are negative; B-snurposomes are stained (arrowheads). (H) Sperm heads 3 h after injection into a GV (DAPI stain). (I) Same field stained with mAb Y12 against the Sm epitope of snRNPs. Sperm heads are negative; B-snurposomes are stained (arrowheads).
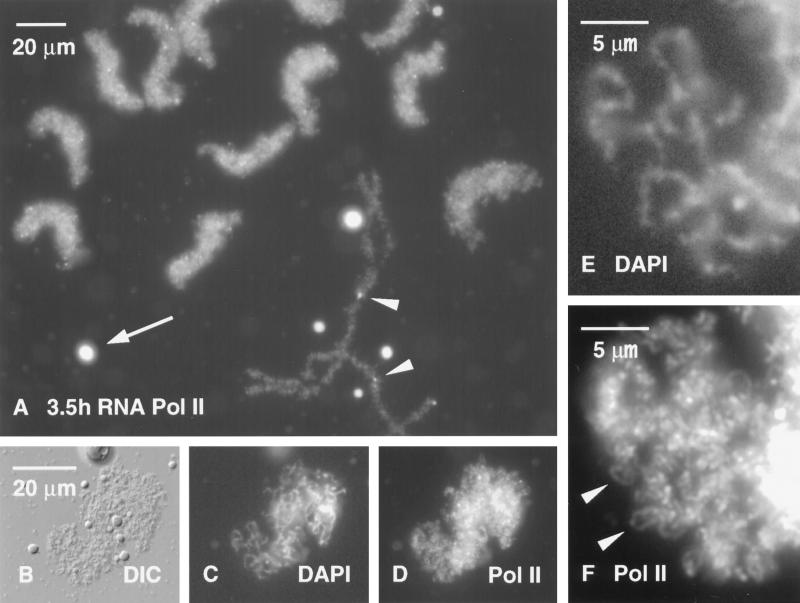
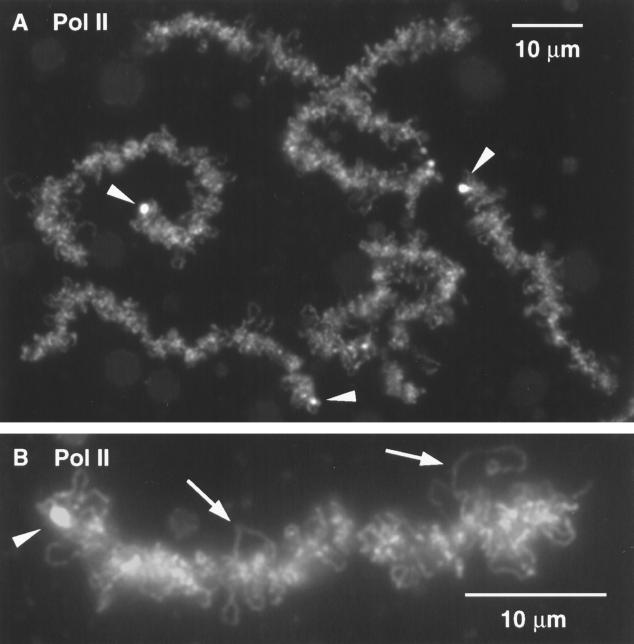
Figure 2.
(A) Group of Xenopus sperm heads 3.5 h after injection into a Xenopus GV have swollen extensively and stain strongly with mAb H14 against RNA Pol II. An endogenous lampbrush chromosome is fused at its terminal granules to two other chromosomes (arrowheads). Several brightly staining coiled bodies (spheres) are also present (arrow). (B) DIC image of a single sperm head from the same GV. (C) DAPI stain reveals individual chromatids within the sperm head. (D) Staining with mAb H14 against RNA Pol II gives a strong reaction in the sperm head. (E) Higher magnification of C showing DAPI-positive chromatids. (F) Individual Pol II-positive loops (arrowheads) can be seen in this enlargement of the same region from D.
For monitoring changes in the composition of sperm heads, we used three antibodies that detect transcription and splicing components of the nucleus: mAb H14 against RNA polymerase II (Pol II; Bregman et al., 1995), mAb Y12 against the Sm proteins associated with splicing small nuclear RNPs (snRNPs) (Lerner et al., 1981), and mAb K121 against the TMG cap of snRNAs (Krainer, 1988). mAb H14 is particularly useful for the study of LBCs because it stains only the axis of the lateral loops, leaving the RNP matrix unstained. In this way one can follow the course of individual loops even in regions where there is considerable overlap. Among the extrachromosomal organelles in the GV, mAb H14 is highly specific for the coiled bodies (spheres), leaving essentially everything else unstained (Figures 1C and 2A). It is not known whether the staining of coiled bodies is due to Pol II or to a cross-reacting epitope. Antibodies Y12 and K121 allow the identification of snRNPs; both stain the matrix of the loops (Figure 3) and the snRNP-containing bodies in the GV referred to as B-snurposomes (Wu et al., 1991; Figure 1, G and I).
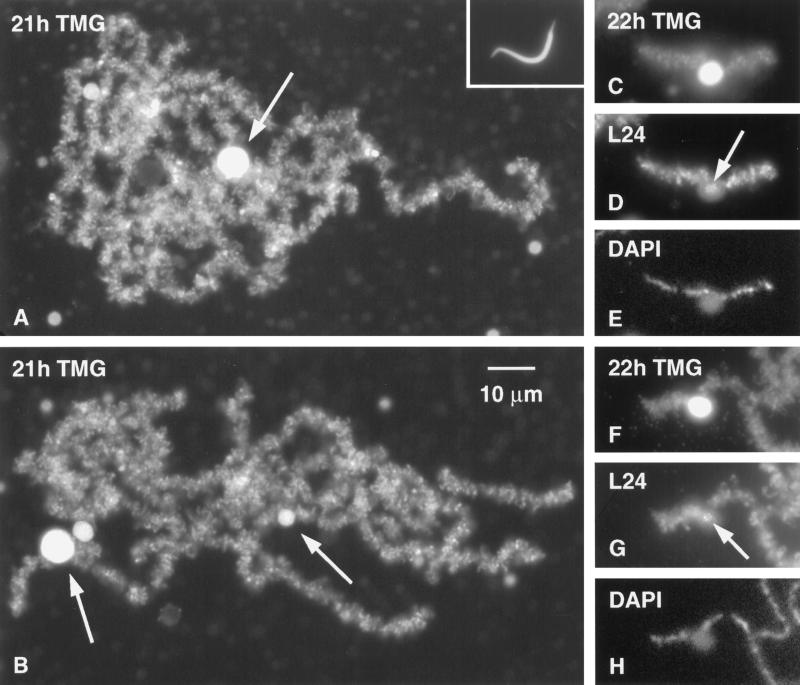
Figure 3.
(A and B) Two Xenopus sperm heads 21 h after injection into a Xenopus GV. Each has resolved into a loose cluster of chromosomes with most of the features of the endogenous LBCs. Stained with mAb K121 against the TMG cap of the splicing snRNAs. The loops of the sperm LBCs stain strongly for snRNAs. Each cluster of chromosomes also displays one very large K121 (+) mass and several smaller masses (arrows). These almost certainly correspond to the similar masses that occur on chromosomes 6, 8, and 14. Inset in A shows sperm head at same magnification. (C) Single sperm LBC with a large K121 (+) mass attached near its center. The position of the mass and the relative size of this chromosome identify it as chromosome 14. (D) Same chromosome was double stained with serum L24 against protein xnf7 (Reddy et al., 1991). This antibody stains small inclusions in the mass (arrow) and most of the lampbrush loops, as it does on endogenous LBCs. (E) DAPI staining of the same chromosome to show the axis of condensed chromomeres. (F–H) K121, L24, and DAPI staining of another example of what is probably sperm LBC 14, attached at one end to another chromosome. Arrow in G points to L24 (+) granules inside the mass.
Sperm heads begin to stain with mAb H14 within the first few hours after injection (Figure 1, D and E), presumably due to uptake of Pol II from stored reserves in the GV. As we discuss below, this uptake is not accompanied by transcription and occurs even when transcription is inhibited by actinomycin D. Splicing factors are not taken up initially, as demonstrated by the absence of Y12 and K121 staining (Figure 1, F–I). As the sperm heads continue to expand, they stain more and more intensely for Pol II (Figure 2A). DAPI staining for DNA provides the first indication of individual chromatids within these swollen heads (Figure 2, C and E). Even at this early stage, careful examination reveals the presence of Pol II (+) loops extending from the DNA (Figure 2F, arrowheads). These loops signal the onset of RNA synthesis and the accumulation of nascent transcripts.
Appearance of Definitive Lampbrush Structure
The sperm heads eventually resolved into loose clusters of fuzzy threads that closely resembled the endogenous LBCs (Figure 3). Over time these clusters fell apart into individual chromosomes or small groups of chromosomes, which we refer to as “sperm LBCs.” The sperm LBCs were easily recognizable, because they were unpaired and slightly shorter than the 18 endogenous bivalents in the same GV (Figure 4). Sperm LBCs did not disperse evenly throughout the volume of the GV but remained together, presumably moving only slowly from the site of injection. In various experiments, from 1 to about 20 sperm heads were injected into a single GV. Within these limits, the system was not saturable, in the sense that sperm LBCs appeared similar in morphology and staining properties whether derived from one or many sperm heads.
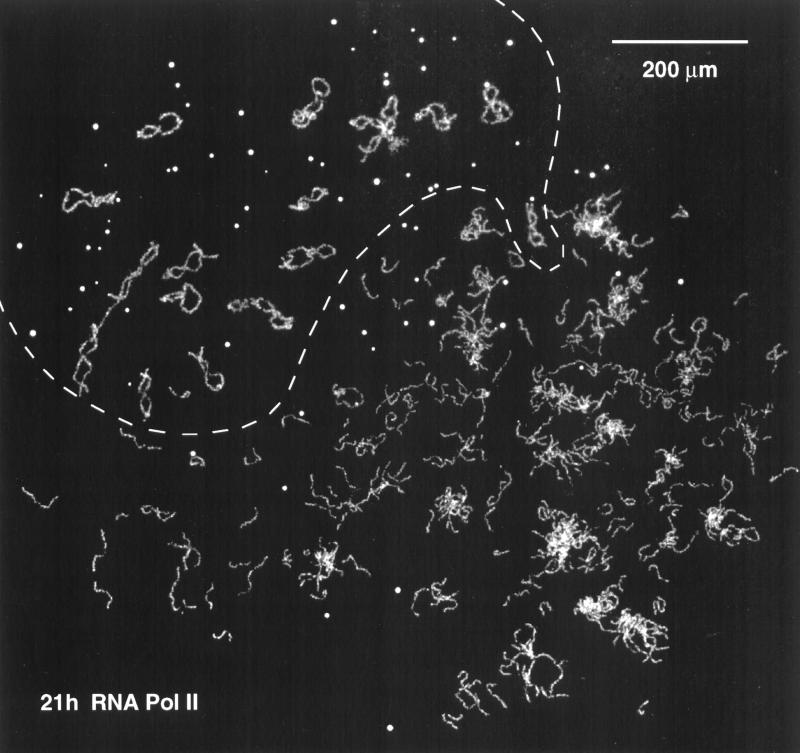
Figure 4.
Overview of the contents of a single Xenopus GV 21 h after injection of Xenopus sperm heads. The sperm heads have been converted into loose clusters of chromatids or single chromatids that have the characteristic features of LBCs. This GV probably received 15–20 sperm heads. The 18 endogenous LBCs and most of the coiled bodies (spheres) occupy the area above the dashed line. This nucleus was stained with mAb H14 against RNA Pol II.
In overall structure sperm LBCs closely resemble the endogenous chromosomes in the same nucleus. They each have a DAPI-positive chromomere axis (Figure 3, E and H), which is less intensely stained than that of the endogenous LBCs, presumably reflecting the difference between a single chromatid and two closely paired sister chromatids. Loops on the sperm LBCs stained with antibodies against various proteins associated with nascent transcripts, such as serum L24 against xnf7 (Reddy et al., 1991; Figure 3, D and G), and they also contained splicing factors, as demonstrated by staining with mAbs Y12 and K121 (Figure 3, A–C and F). The axis of each loop was well-delineated with mAb H14 against Pol II (Figure 5B).
Figure 5.
(A) Higher magnification of several sperm LBCs from the GV shown in Figure 4, stained with mAb H14 against RNA Pol II. The overall fuzzy appearance of the chromosomes is due to staining of the axes of the lateral loops. In addition, mAb H14 stains the terminal granules (arrowheads) found at the end of the long arm of 15 of the 18 Xenopus LBCs. (B) Single sperm LBC at still higher magnification, stained with mAb H14. The arrowhead points to the terminal granule, and arrows point to loop axes (DNA axes covered with Pol II).
Sperm LBCs Are Complete Chromosomes
The longest of the 18 lampbrush bivalents of Xenopus is just twice the length of the shortest, and the remaining chromosomes form a closely graded series (Müller, 1974; Callan et al., 1987). The sperm LBCs are similarly graded in size with no particularly long or short members (Figures 4 and 5). Superficially, therefore, they look as if they might be derived from undefined fragments of sperm chromatin. However, closer inspection and comparison with the endogenous bivalents show that most of them are whole unbroken chromosomes.
Of the 18 bivalents, 15 possess a conspicuous terminal granule at the end of the long arm, referred to in earlier articles as a telomere (Callan et al., 1987). This granule contains the genes coding for 5S rRNA (Callan et al., 1988) and stains intensely with mAb H14, although there is no direct proof that it contains RNA Pol II. These granules tend to fuse with one another in various combinations. Nearly every GV has at least one bivalent in which the terminal granules of the two homologues are fused into a single granule (Figure 6A), and fusions between the termini of nonhomologous chromosomes are equally frequent (Figure 2A, arrowheads). Casual inspection of sperm LBCs shows that many also have one end capped by a terminal granule that stains with mAb H14 (Figure 5, A and B). Nonhomologous fusions between sperm LBCs are especially common, giving rise to multiarmed configurations that look like branched chromosomes (Figure 6C).
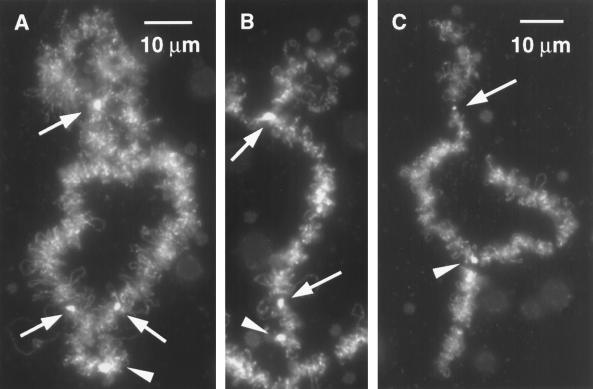
Figure 6.
(A) Bivalent 11 is identifiable by a terminal granule at the end of its long arm (arrowhead) and axial granules at positions 0.16 and 0.80 (arrows). In this example there is a single fused granule at position 0.80. The centromere lies immediately beyond the fused granule but is not visible with this stain. The short arm has a characteristic diffuse structure. Sample is stained with mAb H14 against RNA Pol II. (B) Chromosome 11 derived from a sperm. The terminal granule (arrowhead), two axial granules (arrows), and the diffuse short arm are recognizable. (C) Another example of chromosome 11 derived from a sperm. The axial granule at 0.16 is not evident in this image, but the chromosome is reliably identified by the axial granule at 0.80 (arrow), the diffuse short arm, and the terminal granule (arrowhead), which is fused to the terminal granules of two other chromosomes to give a triradiate figure.
The strongest proof that sperm LBCs are whole chromosomes comes from identification of specific chromosomes. Among the easiest LBCs to identify is chromosome 14. Near the middle of this chromosome is a short region to which are attached oval or spherical masses that stain intensely with mAbs Y12 and K121. Such a mass is a conspicuous feature in each cluster of sperm LBCs (Figure 3, A and B), and in many cases chromosome 14 can be individually recognized (Figure 3, C–H). Chromosome 11 is also easy to identify. In addition to a prominent terminal granule, it has two axial granules that stain brightly with mAb H14 (Figure 6A). The axial granule nearest the end of the long arm is the locus of the U2 snRNA genes and is often associated with a prominent pair of loops; the second axial granule lies immediately adjacent to the centromere. Highly characteristic of chromosome 11 is the short arm, in which sister chromatids are not paired, giving the whole region a loose and unstructured appearance. Figure 6, B and C, shows two examples of sperm LBCs that display the characteristics of chromosome 11. In Figure 6B all of the features just mentioned are recognizable; in Figure 6C the axial granule at the U2 locus is not obvious, but the chromosome is otherwise identifiable as chromosome 11.
Sperm LBCs Contain a Single Unreplicated Chromatid
Each sperm contains a haploid number of unreplicated chromatids, 18 in the case of Xenopus. Consequently, each sperm LBC should consist of an unreplicated chromatid, unless DNA synthesis occurs after the sperm is injected. When sperm heads are incubated in Xenopus egg extract, they enter the S phase and carry out DNA synthesis, as shown originally by Lohka and Masui (1983). However, the S phase of an oocyte occurs before the onset of meiosis, and there is no evidence for DNA synthesis in GVs at the lampbrush stage. Nevertheless, some caution is necessary in evaluating the DNA synthetic capacity of the GV, because an unusual amplification of rDNA does take place at pachytene in amphibian oocytes (Brown and Dawid, 1968; Gall, 1968).
The existence of two chromatids in each homologue of a lampbrush bivalent was originally inferred from the paired nature of the lateral loops (discussed in Callan, 1986). If sperm LBCs consist of unreplicated chromatids, they should have unpaired loops. Although the majority of sperm LBC loops are too short and similar in appearance for evaluation, their unpaired condition is revealed under several circumstances. First, when a specific loop is recognizable because of size or unusual morphology, it is always single, never paired, as it would be in a typical bivalent (Figure 7, A–D). Second, some loops can be stained specifically with antibodies. Again, these loops are single on sperm LBCs (Figure 8, E and F). Third, when conventional LBCs are stretched, they display a phenomenon known as “double-loop bridges” (Callan, 1955; Callan and Lloyd, 1960). A double-loop bridge occurs when the bases of a pair of loops give way under tension; what were originally paired sister loops end up as parallel strands stretched out along the axis of the chromosome. Such double bridges have not been seen in sperm LBCs, but instead “single-loop bridges” are relatively common (Figure 8, G and H). These bridges confirm that a sperm LBC consists of a single chromatid.
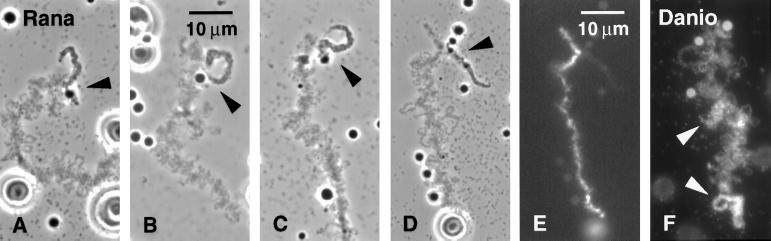
Figure 7.
(A–D) Phase-contrast images of four examples of the same sperm LBC derived from R. pipiens sperm heads injected 2 d previously into a Xenopus GV. Near one end of this chromosome is an unusually large loop with a prominent granule at its base (arrowheads). The loop is single, as expected for an unreplicated chromatid. (E) Same field as D, DAPI stain to show the prominent DNA axis. (F) Sperm LBC 2 d after injection of zebrafish D. rerio sperm heads into a Xenopus GV. Sample is stained with mAb K121 against TMG. Arrowheads point to two loops with unusually heavy matrix.
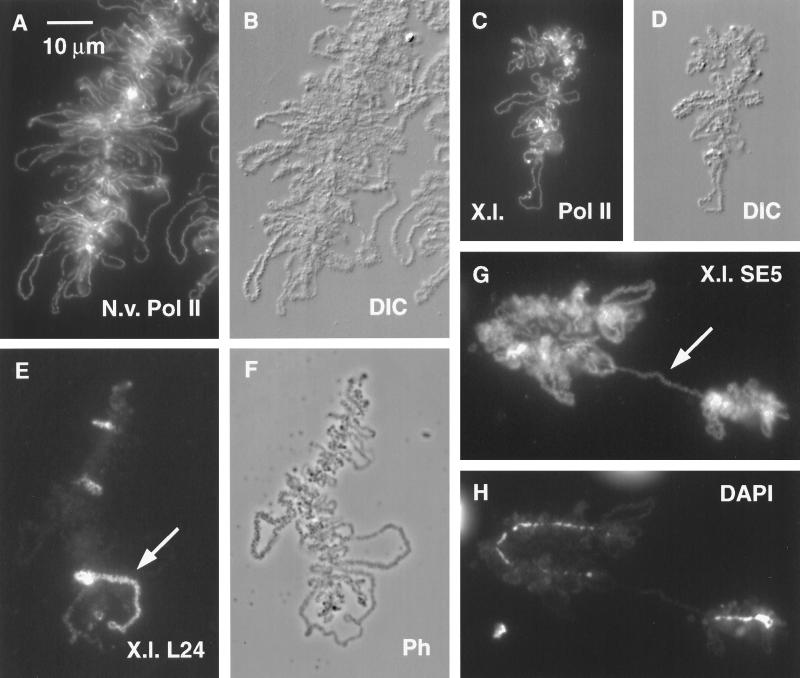
Figure 8.
(A) Small portion of a typical LBC from a GV of the newt N. viridescens, stained with mAb H14 against RNA Pol II. (B) Same field by DIC. Compared with a Xenopus LBC, the newt LBC has longer loops, more heavily textured loop matrix, and unusually distinct Pol II (+) loop axes. In addition, newt LBCs are longer than Xenopus LBCs. (C) LBC derived from a Xenopus sperm head that had been injected 2 d previously into a newt GV. Sample was stained for Pol II. (D) Same field by DIC. Note how strongly this Xenopus LBC resembles a newt LBC except for length. (E) Xenopus sperm LBC 2 d after injection of Xenopus sperm into a newt GV. Sample was stained with serum L24 against xnf7 (Reddy et al., 1991). Only a few loops react strongly with this antibody. The large loop (arrow) near the chromosome end is single, not paired like the loops of an oocyte LBC. This loop consists of two tandem transcription units, only one of which stains. (F) Same field by phase contrast. (G) Xenopus sperm LBC 2 d after injection into a newt GV. Sample was stained with mAb SE5 (Roth and Gall, 1987), which normally stains newt but not Xenopus LBC loops. Staining demonstrates that newt proteins were used to assemble the Xenopus LBC. Arrow indicates a single-loop bridge described in the text. (H) Same field (DAPI stain) showing that the loop bridge spans a gap in the chromomere axis.
Transcription and Splicing
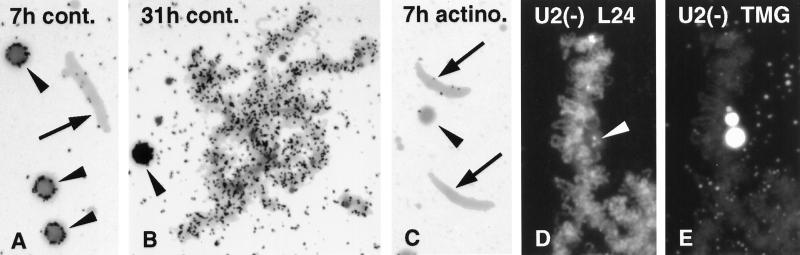
It has been demonstrated many times that lampbrush loops are actively engaged in RNA synthesis and that inhibition of transcription with actinomycin D or α-amanitin leads to loss of loop RNP matrix and retraction of the loops (Izawa et al., 1963; Schultz et al., 1981; Callan, 1986; Figure 9G). To examine a possible relationship between transcription and early events in the formation of sperm LBCs, we injected [3H]GTP into the cytoplasm of oocytes, followed immediately by injection of sperm into the GV. Some oocytes were preincubated in actinomycin D for about 1 h before and were returned to actinomycin D after injection. In both sets of oocytes, sperm heads underwent initial swelling and by 1 h stained intensely with mAb H14 against RNA Pol II (Figure 9, A and B). At this early time splicing components were not demonstrable in the sperm heads (Figure 9, C–F). No further changes in the sperm heads were seen after longer incubation in actinomycin, whereas typical sperm LBCs eventually appeared in oocytes held in OR2 saline. In oocytes held in OR2 saline for 7 h after injection of sperm and [3H]GTP, nucleoli and endogenous chromosomes were strongly labeled, whereas the swollen sperm heads were unlabeled (Figure 10A). Oocytes examined a day later displayed well-formed sperm LBCs, which by this time were actively transcribing (Figure 10B). [3H]GTP was not incorporated into any nuclear structures in actinomycin-treated oocytes (Figure 10C). Thus the initial stage of sperm enlargement and accumulation of RNA Pol II take place in the absence of transcription.
Figure 9.
(A) Xenopus sperm head 1 h after injection into a Xenopus GV. The oocyte was incubated in actinomycin D (20 μg/ml) for 1 h before injection and was returned to the drug after injection (DAPI stain). (B) Same sperm head stained with mAb H14, showing uptake of Pol II by the sperm even though transcription was inhibited. (C) Sperm head 1.5 h after injection into an actinomycin-treated oocyte (DAPI stain). (D) Same sperm head stained with mAb K121 against TMG, showing the absence of splicing snRNAs. (E) Sperm head 1.5 h after injection into an actinomycin-treated oocyte (DAPI stain). (F) Same sperm head stained with mAb Y12, showing absence of Sm proteins. Arrowheads point to Y12 (+) B-snurposomes. (G) Highly contracted chromosome from the same GV that contained the sperm head shown in A and B (DAPI stain). (H) Same area showing absence of chromosomal stain with mAb H14, except for the terminal granule (arrowhead). A coiled body (sphere) shows typical staining (arrow).
Figure 10.
(A) [3H]GTP was injected into the cytoplasm of a Xenopus oocyte and 1 h later Xenopus sperm heads were injected into the GV. An autoradiograph of the GV contents made 7 h later shows no label in a sperm head (arrow) but strong label in three nucleoli (arrowheads), indicative of rRNA transcription (1.5-d exposure). (B) Autoradiograph of labeled sperm LBCs 31 h after injection of [3H]GTP and sperm heads as in A. Active transcription takes place on the loops of the sperm LBCs. A nucleolus (arrowhead) is blackened by silver grains (1.5-d exposure). (C) Oocytes were preincubated for 1 h in actinomycin D (20 μg/ml), injected with [3H]GTP and sperm heads as in A, and then returned to the drug. An autoradiograph of GV contents made 7 h later shows no label in the sperm heads (arrows) or in the endogenous nucleoli (arrowhead; 1.5-d exposure). (D) Sperm LBC from an oocyte depleted for U2 snRNA and stained with serum L24 against xnf7 (Reddy et al., 1991). Loops are well stained, as are granules inside the large “mass” attached to the chromosome (arrowhead). (E) Same chromosome stained with mAb K121 against TMG. The loops show markedly reduced staining relative to untreated oocytes (compare Figure 3), but the K121 (+) masses are not affected.
Earlier experiments with antisense oligodeoxynucleotides showed that transcription on LBCs can take place in the absence of U2 snRNA and thus does not require concomitant splicing (Tsvetkov et al., 1992). We carried out a similar experiment that demonstrates assembly of sperm LBCs in oocytes in which the splicing machinery was disrupted. Figure 10, D and E, shows a sperm LBC from an oocyte injected 2 d earlier with sperm heads and with an oligodeoxyribonucleotide against U2 snRNA. This sperm LBC displays two features characteristic of U2-depleted GVs: the loops are unusually prominent and they show reduced staining with mAb K121. Because the targeted U2 snRNA was destroyed within the first 4 h (or less) after injection (Tsvetkov et al., 1992), this experiment demonstrates that sperm LBCs can form in the absence of splicing.
In summary, the initial stage of sperm enlargement and accumulation of RNA Pol II normally occur without transcription and can take place even when transcription is inhibited by actinomycin D. Whether transcription is necessary for the emergence of cytologically recognizable chromosomes from the sperm head has not been tested, but the maintenance of typical lampbrush loops does depend on transcription. Neither formation nor maintenance of sperm LBCs requires concomitant splicing.
Heterologous Injections
We have carried out several experiments in which demembranated sperm heads of one species were injected into the GV of another. Such experiments can begin to distinguish cis from trans factors in the assembly of LBCs; that is, to assess the relative contributions of sperm chromatin versus proteins and other factors provided by the host GV.
Sperm of Silurana (Xenopus) tropicalis were injected into X. laevis GVs. Sperm LBCs derived from S. tropicalis were similar to those from homologous Xenopus injections. Because S. tropicalis has only 10 chromosomes in its haploid set, specific chromosomes were easier to identify, providing additional evidence that sperm LBCs are whole chromosomes. Sperm of the leopard frog Rana pipiens were also injected into Xenopus GVs. Again, the resulting sperm LBCs closely resembled the endogenous LBCs in both length and general morphology, and specific chromosomes could be recognized (Figure 7, A–D). When examined by DAPI staining (Figure 7E), the chromomere axes of the sperm LBCs were more prominent than those of the endogenous Xenopus bivalents, even though the comparison is between single and paired chromatids. The strong DAPI stain probably reflects the fact that R. pipiens has fewer chromosomes than Xenopus (n = 13 vs. n = 18) yet has a larger genome (6.8 × 109 bp vs. 2.8 × 109 bp [Dawid, 1965]), so that the “average” chromatid of R. pipiens contains about three times as much DNA as a chromatid from Xenopus. Perhaps less expected was the similarity of most loops on the R. pipiens sperm LBCs to those on the endogenous bivalents. LBCs of R. pipiens within their own oocytes display larger loops, and we were prepared to see more prominent loops on the sperm LBCs; that is, we had expected the origin of the sperm chromatin to have a greater effect on the morphology of the resulting LBCs. We have not yet carried out the reciprocal experiment of Xenopus sperm in R. pipiens GVs.
When sperm of the zebrafish D. rerio were injected into Xenopus GVs, the resulting LBCs resembled the endogenous Xenopus bivalents in general morphology, although they were much shorter. The Danio chromosome in Figure 7F was the longest one seen. Because Danio has a haploid number of 25 and a genomic DNA content of 1.7 × 109 bp (Postlethwait and Talbot, 1997), the “average” fish chromatid has about 0.44 times as much DNA as a Xenopus chromatid.
Unexpected results were obtained when we injected Xenopus sperm into GVs of the newt Notophthalmus viridescens. The difference in morphology between Xenopus and newt LBCs is considerable, the lampbrush loops of the newt being among the largest known (Figure 8, A and B). We were surprised to find that Xenopus sperm LBCs in GVs of N. viridescens resembled the endogenous newt chromosomes to a remarkable degree, except for their shorter overall length. The resemblance involved three features of the loops: length, morphology of the RNP matrix, and prominence of the Pol II axis. The most striking feature was the length of the loops, which was greater than that normally seen on Xenopus chromosomes (compare Figure 8, C–H, with Figures 5 and 6). Many loops were 30–40 μm in length, although on average they were not so long as those on the endogenous newt chromosomes. The RNP matrix of the sperm LBC loops is both abundant and varied in morphology like that of newt LBCs (Figure 8, D and F). Similar variations in loop morphology occur on endogenous Xenopus LBCs but are less striking. A conspicuous feature is the staining of the loop axis with mAb H14 against RNA Pol II. In normal newt LBCs and in Xenopus sperm LBCs in the newt GV this axis is unusually prominent (Figure 8, A and C), whereas in normal Xenopus LBCs it is less conspicuous (Figure 6A).
We used two newt-specific antibodies to examine proteins associated with Xenopus sperm LBCs in the newt GV. mAb A33 detects a zinc-finger protein (A33) originally described from Pleurodeles waltl (Lacroix et al., 1985; Bellini et al., 1995), and mAb SE5 detects an unrelated protein (SE5) from N. viridescens (Roth and Gall, 1987). Both antibodies stain the RNP matrix of most but not all loops on LBCs of N. viridescens, whereas neither reacts with normal LBCs of Xenopus. In injection experiments, however, Xenopus sperm LBCs stained indistinguishably from the endogenous N. viridescens chromosomes in the same GV (Figure 8G). This observation demonstrates that newt proteins are used for the assembly of Xenopus sperm LBCs in newt GVs.
We have carried out other heterologous injection experiments with limited success. Lysolecithin-treated sperm heads from the mouse M. musculus and the cricket A. domesticus were injected into GVs of Xenopus. The sperm heads of both species underwent initial swelling and began to stain with mAb H14. In neither case, however, did individual chromosomes become visible, although a few short loops extended from some of the mouse nuclei. Samples of the same sperm heads formed typical in vitro nuclei after 30–60 min of incubation in Xenopus egg extract, suggesting that competence in the latter assay is not a sufficient criterion for competence in the GV system.
DISCUSSION
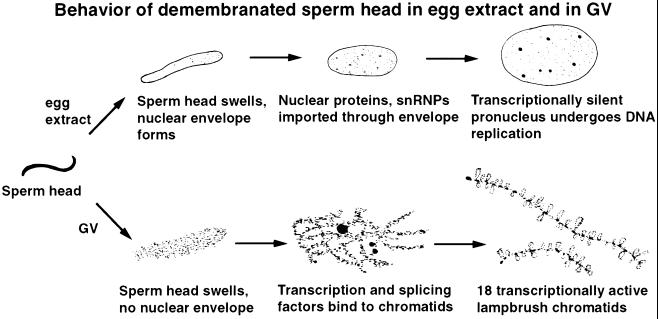
We have shown that typical transcriptionally active lampbrush chromosomes can be assembled from sperm chromatin when demembranated sperm heads of Xenopus are injected into the GV of intact Xenopus oocytes. About 15 years ago, Lohka and Masui (1983, 1984) found that such sperm heads transform into essentially normal pronuclei when incubated in an extract of Xenopus eggs, and the egg extract system has since been used to investigate many aspects of DNA replication and the cell cycle. The GV and egg extract systems are fundamentally different. In the egg extract, demembranated sperm chromatin becomes surrounded by a nuclear envelope and gives rise to a transcriptionally silent nucleus that may subsequently undergo DNA replication and enter mitosis. In the GV, however, the same demembranated sperm chromatin remains freely exposed to the surrounding milieu, does not undergo replication, and transforms into separate transcriptionally active giant chromosomes (Figure 11).
Figure 11.
Comparison of the behavior of demembranated sperm heads after incubation in egg extract (top row) or injection into the GV (bottom row).
It has been documented many times that amphibian GVs contain large quantities of stored materials destined for the early embryo, including transcription and splicing factors. These factors allow the GV to transcribe injected DNA templates and to process the resulting RNA (reviewed in Gurdon and Melton, 1981; Davidson, 1986; Almouzni and Wolffe, 1993). GV extracts have been used to support Pol III transcription from DNA templates in vitro, as in the extensive studies of Brown and his colleagues (Birkenmeier et al., 1978) on 5S RNA. On the other hand, aqueous GV extracts have been of limited use for the study of Pol II transcription, although GVs isolated under oil, and therefore retaining soluble cofactors, support efficient transcription from U1 snRNA templates (Lund and Dahlberg, 1989). In early experiments by Gurdon (1976), somatic nuclei were injected into both cytoplasm and GVs of Xenopus oocytes, and the subsequent morphological and transcriptional events examined in considerable detail. Nuclei injected into the GV swelled and became transcriptionally active but retained some type of investing material, presumably the nuclear lamina. LBCs may well have formed inside these nuclei without being detected as such.
The assembly of 18 giant LBCs from the chromatin of a minute sperm head within a matter of hours is a dramatic phenomenon, yet aspects of this transformation could have been anticipated from the studies just cited and from observations on LBCs themselves. As early as 1969, Snow and Callan (1969) studied the loss and reformation of lampbrush loops when newt oocytes were treated with actinomycin D. Within a few hours after oocytes were exposed to actinomycin D, the loops lost their RNP matrix and retracted into the chromomeres, which themselves coalesced to form shorter chromosomes. After removal of the drug, RNA synthesis resumed, and typical LBC morphology was regained over a period of 2–4 d. These experiments were carried out on ovaries of living animals. In a later study, Scheer (1987) carefully documented a similar course of events when excised newt oocytes were treated with actinomycin D in vitro. Even when oocytes are removed from a female Xenopus and placed immediately in a balanced salt solution, the LBCs exhibit similar signs of transcriptional shutdown during the first few hours (Gall et al., 1991). After 18–24 h, however, the chromosomes increase in length and the loops return. Thus the behavior of endogenous LBCs should have suggested that exogenous chromatin might undergo a similar transformation.
The final form of LBCs derived from sperm chromatin must be influenced by features of the chromatin itself as well as by factors in the GV where the transformation takes place. It is not surprising that Xenopus sperm chromatin in a Xenopus GV gives rise to LBCs that are in many respects indistinguishable from the endogenous LBCs. On the other hand, the results of heterologous injections are not easy to predict. At the outset we were strongly influenced by an interpretation of LBC structure derived from comparative cytological studies. Some organisms, particularly salamanders, have enormous LBCs with very long loops, whereas others like Xenopus have intermediate sized chromosomes with modest loops, and still others have very short chromosomes with barely detectable loops. These differences correlate roughly with the genomic DNA content or C value of the various organisms (Macgregor, 1980). One expects overall chromosome length to correlate with C value, but why should loop length do so as well, if loops simply correspond to one or a small number of transcription units? One interpretation is based on the existence of read-through transcription at the lampbrush stage (Gall et al., 1983). The argument in its simplest form is that the transcription unit includes much or all of the spacer region between genes, which on average will increase in length with C value; therefore, transcription units, at least in LBCs, will be longer in organisms with high C values. This model places almost all emphasis on DNA organization and predicts that the sperm of a given organism should form similar LBCs regardless of the GV into which they are placed. However, our preliminary results with heterologous injections, especially Xenopus sperm in the Notophthalmus GV, suggest that specific features of the loops, including the prominence of the Pol II axis, the morphology of the RNP matrix, and even overall loop length, depend in part on trans-acting factors from the GV.
In future experiments we will extend the range of organisms used as sperm donors. Our first attempts with a mammal (mouse) and an insect (cricket) have been disappointing. At this time we do not know whether we are dealing with a relatively trivial experimental problem, such as incomplete removal of material investing the sperm chromatin, or a more fundamental incompatibility between widely divergent species. Because sperm heads from both mouse and cricket form typical pronuclei when placed in egg extract, a phenomenon that requires considerable restructuring of the chromatin (Wolffe and Schild, 1991), we remain optimistic that almost any type of chromatin can be converted to transcriptionally active LBCs. It will also be of interest to inject chromatin from somatic nuclei into the GV. In earlier experiments Gurdon (1976) injected HeLa cell nuclei into Xenopus GVs, which then underwent a dramatic expansion and carried out transcription. Because the nuclei remained intact, it was not possible to see what morphological changes took place in the chromosomes. It should be possible to disrupt the envelope before injection, in which case LBCs might become evident.
To examine factors important in the assembly of LBCs, it will be necessary to add and subtract components from the GV contents. Antisense oligonucleotides can be used effectively to degrade RNA in the GV (Prives and Foukal, 1991). In earlier experiments we used knockout of U2 snRNA to show that LBC transcription does not require concomitant splicing (Tsvetkov et al., 1992), and the same holds true for the transformation of sperm chromatin into LBCs. Depletion or addition of proteins is more problematic, although injection of antibodies into the GV has been used with some success (Bona et al., 1981; Scheer et al., 1984). It would be highly desirable to have an in vitro system for assembly of LBCs comparable to the egg extract for assembly of interphase nuclei. Efforts will be made to repeat our experiments with GVs isolated under oil (Lund and Dahlberg, 1989; Paine et al., 1992), and if these are successful, to prepare cell-free extracts from GVs or oocytes that will support the transformation of sperm into LBCs.
ACKNOWLEDGMENTS
We thank the following individuals for antibodies: M. Bellini (mAb A33), L. Etkin (rabbit serum L24), A. Krainer (mAb K121), J. Steitz (mAb Y12), and S. Warren (mAb H14). R. Grainger kindly provided specimens of S. tropicalis and M. Halpern sperm of D. rerio. This work was supported by research grant GM-33397 from the National Institute of General Medical Sciences. J.G.G. is American Cancer Society Professor of Developmental Genetics.
Footnotes
Abbreviations used: DAPI, 4′,6-diamidino-2-phenylindole; GV, germinal vesicle; LBC, lampbrush chromosome; mAb, monoclonal antibody.
REFERENCES
- Almouzni G, Wolffe AP. Nuclear assembly, structure, and function: the use of Xenopus in vitro systems. Exp Cell Res. 1993;205:1–15. doi: 10.1006/excr.1993.1051. [DOI] [PubMed] [Google Scholar]
- Bellini M, Lacroix J-C, Gall JG. A zinc-binding domain is required for targeting the maternal nuclear protein PwA33 to lampbrush chromosome loops. J Cell Biol. 1995;131:563–570. doi: 10.1083/jcb.131.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier EH, Brown DD, Jordan E. A nuclear extract of Xenopus laevis oocytes that accurately transcribes 5S RNA genes. Cell. 1978;15:1077–1086. doi: 10.1016/0092-8674(78)90291-x. [DOI] [PubMed] [Google Scholar]
- Bona M, Scheer U, Bautz EKF. Antibodies to RNA polymerase II (B) inhibit transcription in lampbrush chromosomes after microinjection into living amphibian oocytes. J Mol Biol. 1981;151:81–89. doi: 10.1016/0022-2836(81)90222-9. [DOI] [PubMed] [Google Scholar]
- Bregman DB, Du L, van der Zee S, Warren SL. Transcription-dependent redistribution of the large subunit of RNA polymerase II to discrete nuclear domains. J Cell Biol. 1995;129:287–298. doi: 10.1083/jcb.129.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DD, Dawid IB. Specific gene amplification in oocytes. Science. 1968;160:272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Callan HG. Fine Structure of Cells: Symposium of the VIIIth Congress in Cell Biology, Leiden 1954. Groningen: Noordhof; 1955. Recent work on the structure of cell nuclei; pp. 89–109. [Google Scholar]
- Callan HG. In: Lampbrush Chromosomes. Solioz M, editor. Berlin: Springer-Verlag; 1986. [Google Scholar]
- Callan HG, Gall JG, Berg CA. The lampbrush chromosomes of Xenopus laevis: preparation, identification, and distribution of 5S DNA sequences. Chromosoma. 1987;95:236–250. doi: 10.1007/BF00294780. [DOI] [PubMed] [Google Scholar]
- Callan HG, Gall JG, Murphy C. The distribution of oocyte 5S, somatic 5S and 18S + 28S rDNA sequences in the lampbrush chromosomes of Xenopus laevis. Chromosoma. 1988;97:43–54. [Google Scholar]
- Callan HG, Lloyd L. Lampbrush chromosomes of crested newts Triturus cristatus (Laurenti) Philos Trans R Soc Lond B Biol Sci. 1960;243:135–219. [Google Scholar]
- Carnoy JB, Lebrun H. La vésicule germinative et les globules polaires chez les batraciens. I. Salamandre et Pleurodèle. Cellule. 1897;12:191–295. [Google Scholar]
- Davidson EH. Gene Activity in Early Development. 3rd ed. Orlando, FL: Academic Press; 1986. [Google Scholar]
- Dawid IB. Deoxyribonucleic acid in amphibian eggs. J Mol Biol. 1965;12:581–599. doi: 10.1016/s0022-2836(65)80313-8. [DOI] [PubMed] [Google Scholar]
- Flemming W. Zellsubstanz, Kern und Zelltheilung. Leipzig: F.C.W. Vogel; 1882. [Google Scholar]
- Gall JG. Differential synthesis of the genes for ribosomal RNA during amphibian oogenesis. Proc Natl Acad Sci USA. 1968;60:553–560. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall JG. Spread preparations of Xenopus germinal vesicle contents. In: Spector D, Goldman R, Leinwand L, editors. Cell Biology: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1998. pp. 3.1–3.3. [Google Scholar]
- Gall JG, Callan HG, Wu Z, Murphy C. Lampbrush chromosomes. In: Kay BK, Peng HB, editors. Xenopus laevis: Practical Uses in Cell and Molecular Biology. San Diego, CA: Academic Press; 1991. pp. 149–166. [Google Scholar]
- Gall JG, Diaz MO, Stephenson EC, Mahon KA. Gene Structure and Regulation in Development. S. Subtelny and F. Kafatos, New York: Alan R. Liss; 1983. The transcription unit of lampbrush chromosomes; pp. 137–146. [Google Scholar]
- Gurdon JB. Injected nuclei in frog oocytes: fate, enlargement, and chromatin dispersal. J Embryol Exp Morphol. 1976;36:523–540. [PubMed] [Google Scholar]
- Gurdon JB, Melton DA. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. doi: 10.1146/annurev.ge.15.120181.001201. [DOI] [PubMed] [Google Scholar]
- Izawa M, Allfrey VG, Mirsky AL. The relationship between RNA synthesis and loop structure in lampbrush chromosomes. Proc Natl Acad Sci USA. 1963;49:544–551. doi: 10.1073/pnas.49.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainer A. Pre-mRNA splicing by complementation with purified human U1, U2, U4/U6 and U5 snRNPs. Nucleic Acids Res. 1988;16:9415–9429. doi: 10.1093/nar/16.20.9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix J-C, Azzouz R, Boucher D, Abbadie C, Pyne CK, Charlemagne J. Monoclonal antibodies to lampbrush chromosome antigens of Pleurodeles waltlii. Chromosoma. 1985;92:69–80. doi: 10.1007/BF00327246. [DOI] [PubMed] [Google Scholar]
- Lerner EA, Lerner MR, Janeway CA, Steitz JA. Monoclonal antibodies to nucleic acid-containing cellular constituents: Probes for molecular biology and autoimmune disease. Proc Natl Acad Sci USA. 1981;78:2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka MJ, Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983;220:719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- Lohka MJ, Masui Y. Role of cytosol and cytoplasmic particles in nuclear envelope assembly and sperm pronuclear formation in cell-free preparations from amphibian eggs. J Cell Biol. 1984;98:1222–1230. doi: 10.1083/jcb.98.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E, Dahlberg JE. In vitro synthesis of vertebrate U1 snRNA. EMBO J. 1989;8:287–292. doi: 10.1002/j.1460-2075.1989.tb03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macgregor HC. Recent developments in the study of lampbrush chromosomes. Heredity. 1980;44:3–35. [Google Scholar]
- Maréchal J. Sur l’ovogénèse des sélaciens et de quelques autres chordates. Cellule. 1907;24:5–239. [Google Scholar]
- Müller W. The lampbrush chromosomes of Xenopus laevis (Daudin) Chromosoma. 1974;47:283–296. doi: 10.1007/BF00328862. [DOI] [PubMed] [Google Scholar]
- Newmeyer DD, Wilson KL. Egg extracts for nuclear import and nuclear assembly reactions. In: Kay BK, Peng HB, editors. Xenopus laevis: Practical Uses in Cell and Molecular Biology. San Diego, CA: Academic Press; 1991. pp. 607–634. [DOI] [PubMed] [Google Scholar]
- Paine PL, Johnson ME, Lau Y-T, Tluczek LJM, Miller DS. The oocyte nucleus isolated in oil retains in vivo structure and functions. Biotechniques. 1992;13:238–245. [PubMed] [Google Scholar]
- Pan Z-Q, Prives C. Assembly of functional U1 and U2 human–amphibian hybrid snRNPs in Xenopus laevis oocytes. Science. 1988;241:1328–1331. doi: 10.1126/science.2970672. [DOI] [PubMed] [Google Scholar]
- Paulson JR, Laemmli UK. The structure of histone-depleted metaphase chromosomes. Cell. 1977;12:817–828. doi: 10.1016/0092-8674(77)90280-x. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH, Talbot WS. Zebrafish genomics: from mutants to genes. Trends Genet. 1997;13:183–190. doi: 10.1016/s0168-9525(97)01129-3. [DOI] [PubMed] [Google Scholar]
- Prives C, Foukal D. The use of oligonucleotides for antisense experiments in Xenopus laevis oocytes. In: Kay BK, Peng HB, editors. Xenopus laevis: Practical Uses in Cell and Molecular Biology. San Diego, CA: Academic Press; 1991. pp. 185–210. [DOI] [PubMed] [Google Scholar]
- Reddy BA, Kloc M, Etkin L. The cloning and characterization of a maternally expressed novel zinc finger nuclear phosphoprotein (xnf7) in Xenopus laevis. Dev Biol. 1991;148:107–116. doi: 10.1016/0012-1606(91)90321-s. [DOI] [PubMed] [Google Scholar]
- Roth MB, Gall JG. Monoclonal antibodies that recognize transcription unit proteins on newt lampbrush chromosomes. J Cell Biol. 1987;105:1047–1054. doi: 10.1083/jcb.105.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückert J. Zur Entwickelungsgeschichte des Ovarialeies bei Selachiern. Anatomische Anzeiger. 1892;7:107–158. [Google Scholar]
- Saitoh Y, Laemmli U. From the chromosomal loops and the scaffold to the classic bands of metaphase chromosomes. Cold Spring Harbor Symp Quant Biol. 1993;58:755–765. doi: 10.1101/sqb.1993.058.01.083. [DOI] [PubMed] [Google Scholar]
- Scheer U. Structure of lampbrush chromosome loops during different states of transcriptional activity as visualized in the presence of physiological salt concentrations. Biol Cell. 1987;59:33–42. [Google Scholar]
- Scheer U, Hinssen H, Franke WW, Jockusch BM. Microinjection of actin-binding proteins and actin antibodies demonstrates involvement of nuclear actin in transcription of lampbrush chromosomes. Cell. 1984;39:111–122. doi: 10.1016/0092-8674(84)90196-x. [DOI] [PubMed] [Google Scholar]
- Schultz LD, Kay BK, Gall JG. In vitro RNA synthesis in oocyte nuclei of the newt Notophthalmus. Chromosoma. 1981;82:171–187. doi: 10.1007/BF00286102. [DOI] [PubMed] [Google Scholar]
- Snow MHL, Callan HG. Evidence for a polarized movement of the lateral loops of newt lampbrush chromosomes during oogenesis. J Cell Sci. 1969;5:1–25. doi: 10.1242/jcs.5.1.1. [DOI] [PubMed] [Google Scholar]
- Tsvetkov A, Jantsch M, Wu Z, Murphy C, Gall JG. Transcription on lampbrush chromosome loops in the absence of U2 snRNA. Mol Biol Cell. 1992;3:249–261. doi: 10.1091/mbc.3.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RA, Jared DW, Dumont JN, Sega MW. Protein incorporation by isolated amphibian oocytes: III. Optimum incubation conditions. J Exp Zool. 1973;184:321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Wolffe AP, Schild C. Chromatin assembly. In: Kay BK, Peng HB, editors. Xenopus laevis: Practical Uses in Cell and Molecular Biology. San Diego, CA: Academic Press; 1991. pp. 541–559. [Google Scholar]
- Wu Z, Murphy C, Callan HG, Gall JG. Small nuclear ribonucleoproteins and heterogeneous nuclear ribonucleoproteins in the amphibian germinal vesicle: loops, spheres, and snurposomes. J Cell Biol. 1991;113:465–483. doi: 10.1083/jcb.113.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota H, van den Engh G, Hearst J, Sachs R, Trask B. Evidence for the organization of chromatin in megabase pair-sized loops arranged along a random walk path in the human G0/G1 interphase nucleus. J Cell Biol. 1995;130:1239–1249. doi: 10.1083/jcb.130.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]