Abstract
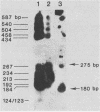
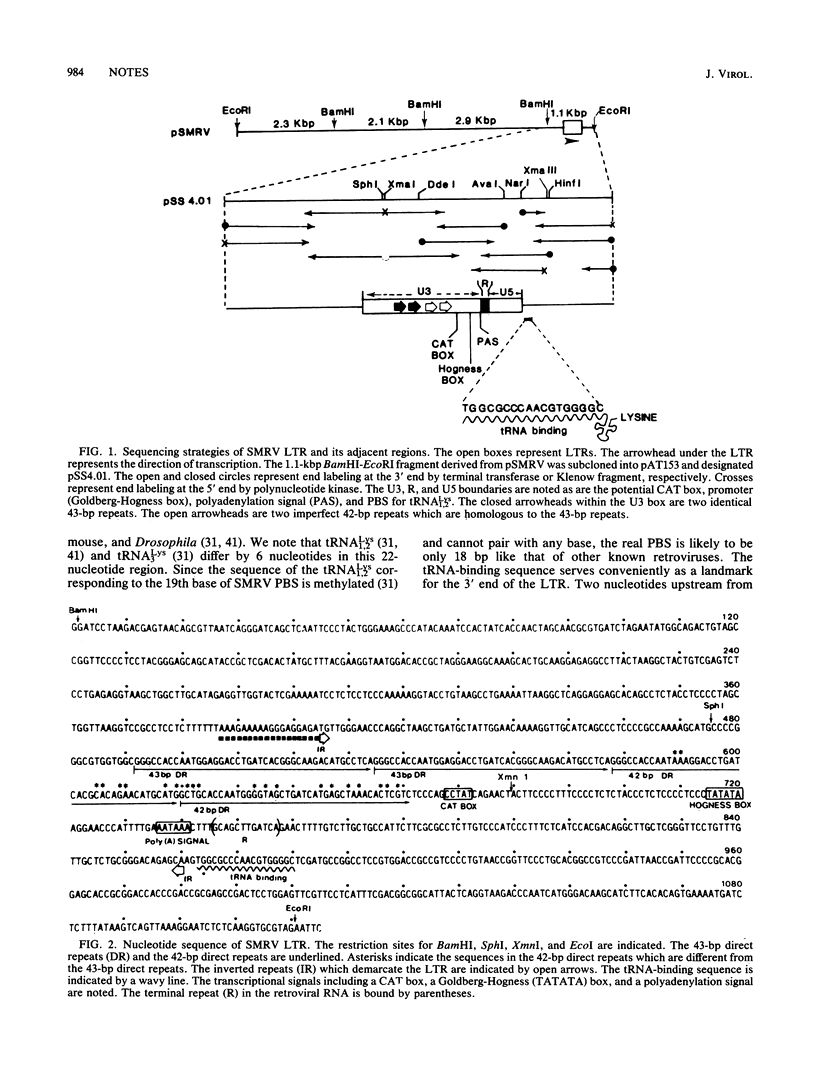
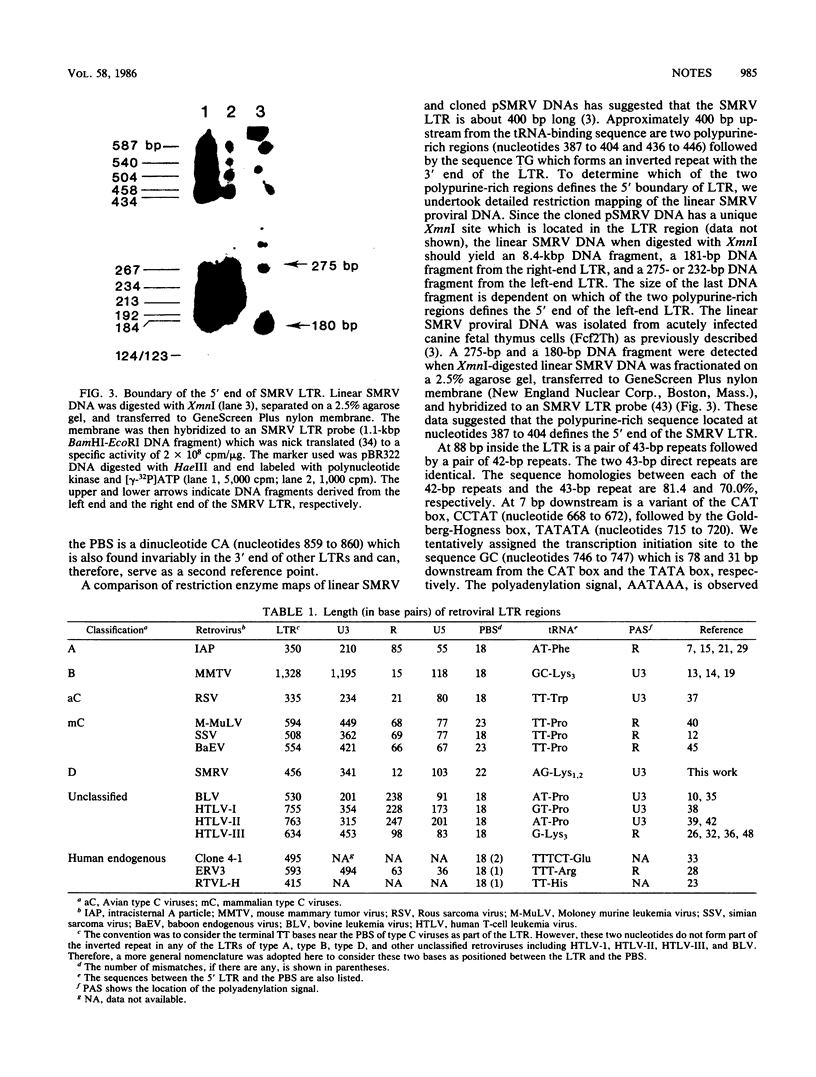
Nucleotide sequences of a DNA fragment containing the long terminal repeat (LTR) of squirrel monkey retrovirus (SMRV) were determined. Sequence analysis showed that the SMRV LTR is 456 base pairs (bp) long and is bounded by 2-bp inverted repeats. Within the U3 region, there are two 43-bp repeats and two 42-bp repeats which are homologous to each other. These repeats are likely to provide enhancer activities commonly observed in other enhancer sequences. Following the repeats are transcriptional regulatory sequences including a CAT box, a Goldberg-Hogness box, and a polyadenylation signal, all positioned within the U3 region of SMRV LTR. A 22-nucleotide sequence immediately downstream from the LTR was found to be complementary to tRNALys1,2, suggesting that tRNALys1,2 serves as the primer for the reverse transcription of SMRV viral RNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Long L. K., Aaronson S. A. Major structural proteins of type B, type C, and type D oncoviruses share interspecies antigenic determinants. Proc Natl Acad Sci U S A. 1980 Jan;77(1):72–76. doi: 10.1073/pnas.77.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. R., Barker W. C. Nucleotide sequences of the retroviral long terminal repeats and their adjacent regions. Nucleic Acids Res. 1984 Feb 24;12(4):1767–1778. doi: 10.1093/nar/12.4.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu I. M., Andersen P. R., Aaronson S. A., Tronick S. R. Molecular cloning of the unintegrated squirrel monkey retrovirus genome: organization and distribution of related sequences in primate DNAs. J Virol. 1983 Sep;47(3):434–441. doi: 10.1128/jvi.47.3.434-441.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu I. M., Callahan R., Tronick S. R., Schlom J., Aaronson S. A. Major pol gene progenitors in the evolution of oncoviruses. Science. 1984 Jan 27;223(4634):364–370. doi: 10.1126/science.6197754. [DOI] [PubMed] [Google Scholar]
- Chiu I. M., Huang R. C., Aaronson S. A. Genetic relatedness between intracisternal A particles and other major oncovirus genera. Virus Res. 1985 Jul;3(1):1–11. doi: 10.1016/0168-1702(85)90036-x. [DOI] [PubMed] [Google Scholar]
- Chiu I. M., Yaniv A., Dahlberg J. E., Gazit A., Skuntz S. F., Tronick S. R., Aaronson S. A. Nucleotide sequence evidence for relationship of AIDS retrovirus to lentiviruses. 1985 Sep 26-Oct 2Nature. 317(6035):366–368. doi: 10.1038/317366a0. [DOI] [PubMed] [Google Scholar]
- Christy R. J., Brown A. R., Gourlie B. B., Huang R. C. Nucleotide sequences of murine intracisternal A-particle gene LTRs have extensive variability within the R region. Nucleic Acids Res. 1985 Jan 11;13(1):289–302. doi: 10.1093/nar/13.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M., Rice N., Stephens R., O'Connell C. DNA sequence relationship of the baboon endogenous virus genome to the genomes of other type C and type D retroviruses. J Virol. 1982 Mar;41(3):801–812. doi: 10.1128/jvi.41.3.801-812.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcher D., Heberling R. L., Kalter S. S., Schlom J. Squirrel monkey retrovirus: an endogenous virus of a new world primate. J Virol. 1977 Aug;23(2):294–301. doi: 10.1128/jvi.23.2.294-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couez D., Deschamps J., Kettmann R., Stephens R. M., Gilden R. V., Burny A. Nucleotide sequence analysis of the long terminal repeat of integrated bovine leukemia provirus DNA and of adjacent viral and host sequences. J Virol. 1984 Feb;49(2):615–620. doi: 10.1128/jvi.49.2.615-620.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M. D., King N. W., Letvin N. L., Hunt R. D., Sehgal P. K., Desrosiers R. C. A new type D retrovirus isolated from macaques with an immunodeficiency syndrome. Science. 1984 Feb 10;223(4636):602–605. doi: 10.1126/science.6695172. [DOI] [PubMed] [Google Scholar]
- Devare S. G., Reddy E. P., Law J. D., Aaronson S. A. Nucleotide sequence analysis of the long terminal repeat of integrated simian sarcoma virus: evolutionary relationship with other mammalian retroviral long terminal repeats. J Virol. 1982 Jun;42(3):1108–1113. doi: 10.1128/jvi.42.3.1108-1113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasel N., Pearson K., Buetti E., Diggelmann H. The region of mouse mammary tumor virus DNA containing the long terminal repeat includes a long coding sequence and signals for hormonally regulated transcription. EMBO J. 1982;1(1):3–7. doi: 10.1002/j.1460-2075.1982.tb01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Hozumi N. Transposition of two different intracisternal A particle elements into an immunoglobulin kappa-chain gene. Mol Cell Biol. 1984 Dec;4(12):2565–2572. doi: 10.1128/mcb.4.12.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberling R. L., Barker S. T., Kalter S. S., Smith G. C., Helmke R. J. Oncornavirus: isolation from a squirrel monkey (Saimiri sciureus) lung culture. Science. 1977 Jan 21;195(4275):289–292. doi: 10.1126/science.63993. [DOI] [PubMed] [Google Scholar]
- Hess J. L., Clements J. E., Narayan O. cis- and trans-acting transcriptional regulation of visna virus. Science. 1985 Aug 2;229(4712):482–485. doi: 10.1126/science.2990051. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Knedlitschek G., Groner B., Hynes N. E., Herrlich P., Michalides R., van Ooyen A. J. Long terminal repeats of endogenous mouse mammary tumour virus contain a long open reading frame which extends into adjacent sequences. Nature. 1982 Feb 18;295(5850):622–624. doi: 10.1038/295622a0. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Rechavi G., Givol D., Canaani E. Homology between an endogenous viral LTR and sequences inserted in an activated cellular oncogene. Nature. 1983 Apr 7;302(5908):547–548. doi: 10.1038/302547a0. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Smith L., Hawley R., Hozumi N., Shulman M. Intracisternal A-particle genes as movable elements in the mouse genome. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1992–1996. doi: 10.1073/pnas.80.7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager D. L., Henthorn P. S. Identification of a retrovirus-like repetitive element in human DNA. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7510–7514. doi: 10.1073/pnas.81.23.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx P. A., Maul D. H., Osborn K. G., Lerche N. W., Moody P., Lowenstine L. J., Henrickson R. V., Arthur L. O., Gilden R. V., Gravell M. Simian AIDS: isolation of a type D retrovirus and transmission of the disease. Science. 1984 Mar 9;223(4640):1083–1086. doi: 10.1126/science.6695196. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Goodwin R. G., Rottman F. M., Crittenden L. B., Raines M. A., Kung H. J. c-erbB activation in ALV-induced erythroblastosis: novel RNA processing and promoter insertion result in expression of an amino-truncated EGF receptor. Cell. 1985 Jul;41(3):719–726. doi: 10.1016/s0092-8674(85)80052-0. [DOI] [PubMed] [Google Scholar]
- O'Connell C. D., Cohen M. The long terminal repeat sequences of a novel human endogenous retrovirus. Science. 1984 Dec 7;226(4679):1204–1206. doi: 10.1126/science.6505687. [DOI] [PubMed] [Google Scholar]
- Ono M., Ohishi H. Long terminal repeat sequences of intracisternal A particle genes in the Syrian hamster genome: identification of tRNAPhe as a putative primer tRNA. Nucleic Acids Res. 1983 Oct 25;11(20):7169–7179. doi: 10.1093/nar/11.20.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Raba M., Limburg K., Burghagen M., Katze J. R., Simsek M., Heckman J. E., Rajbhandary U. L., Gross H. J. Nucleotide sequence of three isoaccepting lysine tRNAs from rabbit liver and SV40-transformed mouse fibroblasts. Eur J Biochem. 1979 Jun;97(1):305–318. doi: 10.1111/j.1432-1033.1979.tb13115.x. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Repaske R., Steele P. E., O'Neill R. R., Rabson A. B., Martin M. A. Nucleotide sequence of a full-length human endogenous retroviral segment. J Virol. 1985 Jun;54(3):764–772. doi: 10.1128/jvi.54.3.764-772.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Ogawa Y., Tsuzuku-Kawamura J., Ikawa Y. Bovine leukemia virus: unique structural features of its long terminal repeats and its evolutionary relationship to human T-cell leukemia virus. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4741–4745. doi: 10.1073/pnas.81.15.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Golde D. W., Miwa M., Sugimura T., Chen I. S. Nucleotide sequence analysis of the long terminal repeat of human T-cell leukemia virus type II. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1079–1083. doi: 10.1073/pnas.81.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Silverman S., Gillam I. C., Tener G. M., Söll D. The nucleotide sequence of lysine tRNA2 from Drosophila. Nucleic Acids Res. 1979 Feb;6(2):435–442. doi: 10.1093/nar/6.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Trus M., Perkins D., Patarca R., Wong-Staal F., Gelmann E., Gallo R., Haseltine W. A. Repetitive structure in the long-terminal-repeat element of a type II human T-cell leukemia virus. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4617–4621. doi: 10.1073/pnas.81.15.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stromberg K., Benveniste R. E., Arthur L. O., Rabin H., Giddens W. E., Jr, Ochs H. D., Morton W. R., Tsai C. C. Characterization of exogenous type D retrovirus from a fibroma of a macaque with simian AIDS and fibromatosis. Science. 1984 Apr 20;224(4646):289–282. doi: 10.1126/science.6200929. [DOI] [PubMed] [Google Scholar]
- Tamura T., Noda M., Takano T. Structure of the baboon endogenous virus genome: nucleotide sequences of the long terminal repeat. Nucleic Acids Res. 1981 Dec 11;9(23):6615–6626. doi: 10.1093/nar/9.23.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]