Abstract
Interleukin 1β (IL-1β), a secretory protein lacking a signal peptide, does not follow the classical endoplasmic reticulum-to-Golgi pathway of secretion. Here we provide the evidence for a “leaderless” secretory route that uses regulated exocytosis of preterminal endocytic vesicles to transport cytosolic IL-1β out of the cell. Indeed, although most of the IL-1β precursor (proIL-1β) localizes in the cytosol of activated human monocytes, a fraction is contained within vesicles that cofractionate with late endosomes and early lysosomes on Percoll density gradients and display ultrastructural features and markers typical of these organelles. The observation of organelles positive for both IL-1β and the endolysosomal hydrolase cathepsin D or for both IL-1β and the lysosomal marker Lamp-1 further suggests that they belong to the preterminal endocytic compartment. In addition, similarly to lysosomal hydrolases, secretion of IL-1β is induced by acidotropic drugs. Treatment of monocytes with the sulfonylurea glibenclamide inhibits both IL-1β secretion and vesicular accumulation, suggesting that this drug prevents the translocation of proIL-1β from the cytosol into the vesicles. A high concentration of extracellular ATP and hypotonic medium increase secretion of IL-1β but deplete the vesicular proIL-1β content, indicating that exocytosis of proIL-1β–containing vesicles is regulated by ATP and osmotic conditions.
INTRODUCTION
Interleukin 1 (IL-1)1 is a multifunctional cytokine and a major soluble mediator of inflammation (Dinarello, 1991). Although its biological activity is extracellular, this protein lacks a secretory signal sequence (Rubartelli and Sitia, 1997), raising the question of how it can be transported out of the cell. Two forms of IL-1 exist, α and β; however, studies on IL-1 secretion mostly focused on IL-1β, which is the major extracellular form in humans (Dinarello, 1991). IL-1β is synthesized by monocytes upon activation as a 35-kDa precursor, which accumulates in the cytosol (Singer et al., 1988) and is proteolytically processed to the mature form of 17 kDa by the caspase IL-1β–converting enzyme (ICE) (Cerretti et al., 1992; Thornberry et al., 1992). ICE is present in the cell cytosol (Ayala et al., 1994; Singer et al., 1995) as a p45 inactive polypeptide. Where and how maturation of ICE to the active (p10/p20) form and the processing of IL-1β take place are unclear; however, the mature form of IL-1β seems to be either immediately secreted after cleavage or immediately cleaved after secretion, because 17-kDa IL-1β is undetectable inside the cell (Rubartelli et al., 1990). Although the levels of basal release of IL-1β are quite low (Rubartelli et al., 1990), secretion is dramatically induced by extracellular ATP (Hogquist et al., 1991; Rubartelli et al., 1993; Perregaux and Gabel, 1994), which can be autocrinally produced by activated monocytes (Ferrari et al., 1997) and interact with P2Z purinoreceptors expressed on their membrane (Hickman et al., 1994; Di Virgilio, 1995). The cellular pathway underlying this ATP-driven secretion is, however, unknown. We have previously shown that IL-1β is secreted by an active mechanism, which does not involve protein transit through endoplasmic reticulum and Golgi (Rubartelli et al., 1990). Similarly to IL-1β, a number of leaderless secretory (LLS) proteins, both in prokaryotes and eukaryotes (Rubartelli and Sitia, 1997), are known to be externalized via nonclassical pathways. Although in the case of many LLS proteins cell lysis and damage as a nonspecific mechanism of release can be ruled out (Cooper and Barondes, 1990; Rubartelli et al., 1990, 1992; Mignatti et al., 1991; Florkiewicz et al., 1995), their mechanism of export is still undefined. Either translocation (of the plasma membrane or of intracellular membranes) or pinching off from the plasma membrane of vesicles enriched in a given LLS protein has been proposed (Rubartelli and Sitia, 1997). In unicellular organisms, ATP-binding cassette (ABC) membrane transporters have been shown to mediate secretion of most LLS proteins (Kuchler et al., 1997), with a notable exception in yeast (Cleves et al., 1996). In mammals, a role for ABC proteins in leaderless secretion has not been demonstrated so far; however, it is of note that the sulfonylurea glibenclamide, a potent blocker of the anion exchange activity of the murine ABC transporter ABC1 (Becq et al., 1997), inhibits secretion of IL-1β by activated monocytes in mouse and human (Hamon et al., 1997). In Dictyostelium discoideum, the transport from the cytosol to the cell surface of DdCAD-1, a leaderless adhesion protein, involves its translocation into contractile vacuoles, acidic vesicles whose exocytosis is modulated by extracellular osmotic conditions (Sesaki et al., 1997). We have previously observed that, although most IL-1β precursor (proIL-1β) localizes in the cytosol of activated human monocytes, a fraction is contained within vesicles of unknown nature, which protect it from protease digestion (Rubartelli et al., 1990). This raises the possibility that these vesicles are part of the IL-1β secretory route; cytosolic proIL-1β might translocate across their membrane, undergo maturation, and be released after fusion of the organelle membrane with the plasma membrane. Interestingly, a pathway of translocation for cytosolic proteins to lysosomes under stress conditions has been recently described, and the molecular transporter mediating translocation has been characterized (Cuervo and Dice, 1996). In that case, however, the fate of the translocated proteins is degradation; moreover, the membrane protein mediating the import into lysosomes does not belong to the family of ABC transporters. Here we provide biochemical and morphological evidence that, in activated monocytes, particulated IL-1β is contained in endolysosomalrelated organelles, whose exocytosis leads to the extracellular release of the cytokine. The modulation of protected and secreted IL-1β under different culture conditions suggests that these organelles represent a specialized subset of lysosomes whose membrane is equipped with a glibenclamide-sensitive transporter.
MATERIALS AND METHODS
Cell Cultures
Human monocytes were isolated from buffy coats from healthy donors, enriched by adherence and activated with 1 μg/ml lipopolysaccharide (LPS; Sigma Chemical, St. Louis, MO) for 1 h in RPMI medium (Biochrom, Berlin, Germany) supplemented with 10% FCS (Biochrom) as described (Rubartelli et al., 1990, Hamon et al., 1997), in the presence or absence of 100 μM glibenclamide, 1 μM bafilomycin A1 (BafA1), 50 μM cloroquine, or 50 mM NH4Cl (all from Sigma). Supernatants were then replaced with RPMI medium supplemented with 1% Nutridoma-HU (Boehringer Mannheim, Mannheim, Germany). When indicated, medium was supplemented with the same substances as above or with 5 mM EDTA, 5 mM MgCl2, 5 mM EDTA plus 10 mM MgCl2 or 0.1 M sucrose (Sigma, for hypertonic medium) or diluted with H2O at 50:50 ratio (for hypotonic medium). Incubation was carried out for 3 h or for 2 h 30 min followed by 30 min in the presence of 1 mM ATP (Boehringer Mannheim), in the absence or presence of the same compounds as above. At the end of incubation, supernatants were concentrated by 10% trichloroacetic acid (TCA) (Hamon et al., 1997); cells were detached by scraping.
Subcellular Fractionation by Differential Ultracentrifugation
Subcellular fractionation was carried out as described by Pitt et al. (1992). Briefly, cells were washed, resuspended in homogenizing buffer (250 mM sucrose, 5 mM EGTA, 20 mM HEPES-KOH, pH 7.2) at 5 × 107/ml and broken in a Dounce homogenizer. Unbroken cells, debris, and nuclei were discharged by three cycles of centrifugation at 800, 1000, and 1200 × g, and the postnuclear supernatant (PNS) obtained was treated with 0.1 mg/ml proteinase K (Sigma) for 30 min on ice, followed by addition of protease inhibitors (Rubartelli et al., 1990), diluted 10-fold in homogenizing buffer, and centrifuged at 35,000 × g for 1 min. The pellet was kept as pellet 1 (P1); P1 supernatant was centrifuged at 50,000 × g for 5min, leading to a second pellet (P2). In some experiments, PNS was not protease treated, and the P1 and P2 fractions were washed once by ultracentrifugation in homogenizing buffer. Under these conditions, however, the amount of proIL-1β present in the two fractions was increased twofold (our unpublished results). Because we could not discriminate between the proIL-1β molecules in the way to translocate inside the vesicles and those nonspecifically bound, we performed all the analyses on protease-treated P1 and P2 fractions. The P2 supernatant, containing cytosolic IL-1β, was concentrated by 10% TCA precipitation and used as control of efficient protease digestion (our unpublished results). When indicated, P1 from undigested PNS was treated with proteinase K, 0.1 mg/ml, for 30 min on ice in the presence or absence of 0.1% Triton X-100 (Bio-Rad, Milan, Italy).
Pulse–Chase Analyses
Human monocytes enriched by adherence as above were activated with 1 μg/ml LPS for 1 h in RPMI medium supplemented with 10% FCS, washed three times in methionine-free medium (ICN Biomedicals, Costa Mesa, CA), and starved for 20 min at 37°C in 5% CO2 in the same medium supplemented with dialyzed FCS. After starvation, medium was replaced with fresh methionine-free medium containing 5% dialyzed FCS and 1 mCi/ml Redivue Promix L-[35S] (Amersham Pharmacia Biotech, Milan, Italy), and cells were pulsed for 15 min. At the end of the pulse, cells were washed three times with medium containing an excess (2×) of cold methionine and cysteine and chased in complete medium for 1, 3, or 15 h. In parallel experiments, 100 μg of pepstatin and leupeptin (Sigma) were added in the last 30 min of LPS activation and then in all the steps of the experiment. At the end of each period of chase, supernatants was removed, cells were washed, and subcellular fractionation was carried out as above, with the difference that protease treatment was performed on the pooled P1 and P2 fractions, rather than on the PNS, and that inhibitors of proteases were added before solubilization of P1 and P2 fractions with lysis buffer containing 0.1% Triton X-100. Cytosolic and particulated fractions and supernatants were precleared by Sepharose-bound protein A (Amersham Pharmacia Biotech) and immunoprecipitated with anti-IL-1β antiserum (a kind gift from F. Cozzolino, University of Torvergata, Rome, Italy) followed by Sepharose-bound protein A. Immunoprecipitates were washed, eluted, and loaded on 12% SDS-PAGE followed by autoradiography as described (Rubartelli et al., 1990). Densitometric analyses were performed on at least two different exposures of the same autoradiograph.
Subcellular Fractionation on Percoll Density Gradients
P1 and P2 pellets obtained as above were resuspended in 1 ml of a buffer containing 3 mM imidazole and 250 mM sucrose, pH 7, treated with trypsin (2 μg/mg protein) for 30 min on ice, and mixed with 9 ml of the same buffer containing Percoll (Sigma) up to 25% (Diment et al., 1988; Pierre et al., 1996; Morkowski et al., 1997). After centrifugation for 2 h at 90,000 × g (TiSW41 rotor, 27,000 rpm; Beckman Instruments, Fullerton, CA), fractions were collected using a needle connected to a peristaltic pump (Amersham Pharmacia Biotech), membranes were lysed with 0.5% Triton X-100, and an aliquot (one-fourth) was assayed for the presence of β-hexosoaminidase activity as described (Rodriguez et al., 1997). The remaining was diluted 5× in the same buffer, ultracentrifuged 30 min at 100,000 × g to remove Percoll (Morkowski et al., 1997), and concentrated by TCA precipitation.
Western Blot Analysis
An aliquot of PNS (5–10%, corresponding to 100 μg of proteins; protein dosage performed with the Bio-Rad kit based on the colorimetric Lowry method) and the corresponding whole P1, P2, TCA-concentrated P2 supernatants, culture media from 106 cells, or aliquots from the different Percoll gradient fractions were solubilized in reducing sample buffer and resolved on 12% SDS-PAGE under reducing conditions (Rubartelli et al., 1990; Hamon et al., 1997). Gels were electrotransferred onto nitrocellulose filters (Hybond ECL, Amersham Pharmacia Biotech), which were stained with Ponceau Red (Sigma), to confirm equal protein loading and to rule out degradation (our unpublished results) and destained before blocking overnight with 10% nonfat dry milk in PBS. Filters were hybridized with the following antibodies: the anti-IL-1β mAb 3ZD (provided by the National Cancer Institute Biological Resources Branch, Frederick, MD), the rabbit anti-cathepsin D (CD) antiserum (a gift from C. Isidoro, University of Alessandria, Alessandria, Italy), and the rabbit anti-Rab7 antiserum (a gift from S. Méresse, CIML, Marseille, France), followed by an HRP-conjugated goat anti-mouse immunoglobulin G (IgG) or goat anti-rabbit IgG (DAKO, Milan, Italy), and developed by ECL-Plus (Amersham Pharmacia Biotech) according to the manufacturer’s instructions. When stated, densitometric analyses of the blots were performed as above.
Conventional and Immunoelectron Microscopy
P1 and P2 fractions were processed for postembedding immunocytochemistry as described (Lotti et al., 1992). Briefly, fractions were fixed in 1.0% glutaraldehyde (Life Technologies, Grand Island, NY) in PBS for 1 h at room temperature, partially dehydrated in ethanol, and embedded in LR White resin (Electron Microscopy Sciences, Fort Washington, PA). Thin sections were collected on nickel grids and immunolabeled with anti-IL-1β mAb, rabbit anti-CD antiserum, or rabbit anti-Lamp-1 antiserum (a gift from S. Méresse) and then with protein-A gold (18 nm) prepared by the citrate method (Slot and Geuze, 1981). In double-labeling experiments, the sections were first incubated with anti-IL-1β followed by 10 nm of goat anti-mouse IgG gold conjugates (British BioCell International, Carditt, UK) and then incubated with anti-CD or anti-Lamp-1 followed by 18 nm of protein-A gold. All sections were finally stained with uranyl acetate and lead citrate.
Alternatively, P1 and P2 fractions fixed in glutaraldehyde as above were processed for conventional thin section electron microscopy as described (Lotti et al., 1992). Thin sections were examined unstained and poststained with uranyl acetate and lead hydroxide.
Quantitative evaluation of immunolabeling was performed by comparing the number of small (10-nm) and large (18-nm) gold particles present inside two different structures, identified according to their size and morphology: small (<200-nm) dense vesicles, and large (>200-nm) organelles displaying the ultrastructural appearance of late endosomes and lysosomes. Fifty images of each type of structures, randomly photographed from three different immunolabeling experiments, were analyzed.
RESULTS
ProIL-1β Is Present in Vesicles Cofractionating with Endolysosomes: Inhibition by Glibenclamide
To investigate the subcellular localization of protected IL-1β, LPS-activated human monocytes were homogenized, and two fractions (P1 and P2) were obtained by the differential ultracentrifugation protocol described by Pitt et al. (1992) and subjected to Western blot analysis. The endolysosomal hydrolase CD was used as a marker of these compartments (Figure 1A). Three molecular forms of CD (Rijnboutt et al., 1992) are detected by Western blot analysis in the PNSs (Figure 1A, lane 1): prepro-CD, corresponding to the 51-kDa endoplasmic reticulum precursor polypeptide; proCD, the partially processed 45-kDa form typical of endosomes, and the mature, most abundant, lysosomal form of 31-kDa CD. Prepro-CD is absent from the two pellets; proCD and CD are present in both pellets (Figure 1A, lanes 2 and 3) although proCD predominates in P2 (Figure 1A, lane 3). When the same blot was hybridized with an anti-IL-1β antibody, a protease-protected proIL-1β band was found in P1 and barely detectable in P2 (Figure 1B, lanes 1–3). The amount of protected IL-1β varied in the different cell preparations from 5 to 10% of the total cellular IL-1β and disappeared, like CD, if protease digestion was performed in the presence of Triton X-100 (Figure 1, A and B, lanes 9 and 10). Although secreted IL-1β is mostly in the mature, 17-kDa molecular form, only the precursor form of 35 kDa is present intracellularly; the 17-kDa IL-1β band detected in P1 is erratic and probably due to nonspecific endoproteases, activated during the preparation of the samples, which may give rise to fragments of apparent molecular weight similar to that of ICE-processed IL-1β (Hazuda et al., 1991).
Figure 1.
ProIL-1β is contained in part in vesicles cofractionating with lysosomes. Western blot analysis of PNS (5% of total, lanes 1 and 4), P1 (lanes 2 and 5), and P2 (lanes 3 and 6) or supernatants from 106 cells (lanes 7 and 8) obtained from activated monocytes cultured in the absence (−) or presence (+) of 100 μM glibenclamide (glib). After removal of 5% PNS, PNS were treated with proteinase K (PK) before the ultracentrifugation. Lanes 9 and 10, P1 from undigested PNS was untreated (lane 9) or solubilized (+TX100, lane 10) with Triton X-100 before proteinase K (PK) digestion. Filters were hybridized with rabbit anti-CD (A), melted, and rehybridized with mouse anti-IL-1β (B) antibodies. The migration of the three molecular forms of CD and proIL-1β and IL-1β is indicated.
The sulfonylurea glibenclamide blocks both the basal and the ATP-induced secretion of IL-1β by activated monocytes (Hamon et al., 1997) but has no effect on the release of CD (Figure 1, A and B, lanes 7 and 8). After treatment of monocytes with this drug, proIL-1β disappears from P1 and P2 (Figure 1B, lanes 5 and 6), whereas particulated CD remains unaffected (Figure 1A, lanes 5 and 6).
These findings suggest that cytosolic proIL-1β molecules translocate from the cytosol into a dense vesicular compartment; glibenclamide may block the entry of proIL-1β into the vesicles, possibly interfering with a membrane transporter.
Inhibition of Endolysosomal Proteases Results in Increased Secretion of IL-1β
To further elucidate the involvement of proIL-1β-containing vesicles in the secretory pathway of this cytokine, we performed pulse–chase experiments aimed at following the fate of newly synthesized proIL-1β molecules. Human monocytes, activated with LPS, were biosynthetically labeled with a [35S]methionine-cysteine mix for 15 min and chased in cold medium for different periods. As shown in Figure 2, open symbols, after 15 min of pulse (time 0) 10% of the newly synthesized proIL-1β is already protected (in the different experiments performed, this percentage varied from 5 to 12%). The amount of IL-1β present in this particulated fraction increases slightly after 1 h of chase and decreases thereafter (Figure 2A). Cytosolic proIL-1β is maximal at time 0 and reaches 50% of the initial labeled protein after 6 h (Figure 2B), in agreement with previous experiments (Rubartelli et al., 1990). Secretion starts to be detected after 1 h and increases slowly (Figure 2C). To discriminate whether the decrease in the particulated proIL-1β is due to degradation or to secretion, the same kinetic experiments were performed in the presence of excess protease inhibitors. Figure 2, closed symbols, shows that the kinetics of disappearance of IL-1β from the particulated fraction (Figure 2A) or the cell cytosol (Figure 2B) of cells incubated in the presence of 100 μM pepstatin and leupeptin are similar to those of control monocytes. In contrast, the amount of secreted, fully processed IL-1β by cells treated with the protease inhibitors was consistently much higher than that secreted by control cells at all the different times of chase (Figure 2C).
Figure 2.
Kinetic analyses of cytosolic and particulated IL-1β. LPS-activated monocytes were pulsed with 1 mCi/ml methionine-cysteine Promix [35S] for 15 min (time of chase, 0) and chased in complete medium for 1, 3, 6, or 15 h in the absence (open symbols) or presence (closed symbols) of 100 μg of pepstatin and leupeptin. At the end of each period of chase subcellular fractionation was carried out as above, P1 and P2 were treated with proteinase K, followed by protease inhibitors and solubilization, and the IL-1β present in the pooled P1 and P2 fractions (A), in the cytosol (B), and in the supernatants (C) was analyzed by immunoprecipitation with anti-IL-1β antiserum followed by SDS-PAGE and autoradiography as described (Rubartelli et al., 1990). Data are expressed as densitometric areas (AU, arbitrary units). One representative experiment of three performed is shown.
Particulated IL-1β Colocalizes Partially with CD or Lamp-1 in Endolysosomal-related Structures
Two different experimental approaches were used to characterize the vesicular compartment containing proIL-1β: 1) immunoelectron microscopy analysis of proIL-1β–containing fractions and 2) fractionation of trypsin-treated P1 and P2 pellets on Percoll density gradients.
Electron microscopy analysis revealed that P1 is enriched in lysosomes displaying different degrees of density, smaller dense vesicles (diameter < 200 nm), late endosomes, and multivesicular bodies (our unpublished results). In contrast, P2 is selectively enriched for vesicular structures of different size, having a uniform, electron-lucent appearance, thus fitting the classical morphological features of endosomes (our unpublished results). As shown in Figure 3, IL-1β (small gold particles) is detected in structures displaying the morphology of late endosomes or lysosomes (Figure 3, A, B, and D, arrows) and in small dense vesicles (Figure 3, E–G, arrowheads). Colocalization with CD (large gold particles; Figure 3, A, B, and F) or with the lysosomal membrane protein Lamp-1 (Kornfeld and Mellman, 1989; Méresse et al., 1995) (large gold particles; Figure 3, D and G) is observed in both structures; however, a large number of organelles stained with either anti-IL-1β alone (Figure 3E) or anti-CD alone (Figure 3C) are detected. Quantitative analysis performed by counting IL-1β or CD gold particles revealed that structures displaying IL-1β labeling only predominate among the dense vesicles: of 50 vesicles analyzed, 30 were labeled with anti-IL-1β only, 16 with both antibodies, and 4 with anti-CD only. In contrast, a consistent portion of mature lysosomes (12 of 50) displayed only CD staining. Parallel analyses performed by counting IL-1β or Lamp-1 gold particles confirmed the prevalence of dense vesicles positively labeled for IL-1β only (31 of 50) with respect to vesicles double stained (14 of 50) or single stained for Lamp-1 (1 of 50). In keeping with the CD data, 15 of 50 lysosomes displayed Lamp-1 staining only. These data indicate that proIL-1β is contained in organelles displaying the morphology of endolysosome-related organelles; however, the partial but not complete colocalization with CD and with Lamp-1 suggests that proIL-1β–containing structures belong to a specialized subset of endolysosomes.
Figure 3.
Immunoelectron microscopy analysis of the colocalization of IL-1β and CD (A–C, E, and F) and of IL1β and Lamp-1 (D and G) in P1 fraction. Double immunolabeling with anti-IL-1β (10-nm gold particles) and anti-CD (18-nm gold particles) antibodies reveals the presence of both molecules in organelles (>200 nm in diameter), which display the typical morphology of late endosomes and early lysosomes (A, arrow) or more dense, mature lysosomes (B, arrow). These organelles appear double immunolabeled also for IL-1β (10-nm gold particles) and Lamp-1 (18-nm gold particles) (D, arrow). Mature lysosomes showing positive staining for IL-1β alone are also found (C, arrow). Dense vesicles, <200 nm in diameter, appear either positively immunolabeled for IL-1β only (E, arrowheads), for both IL-1β and CD (F, arrowhead), or for both IL-1β and Lamp-1 (G, arrowhead). Bars, 200 nm.
The migration of proIL-1β–containing organelles with respect to lysosomes and endosomes was then investigated in Percoll density gradients. Trypsin-treated P1 and P2 fractions were pooled and centrifuged on a 25% Percoll density gradient, and fractions obtained were analyzed for their content in CD or IL-1β by Western blotting. Figure 4A shows that the 31-kDa mature form of CD accumulates at the bottom of the gradient (fractions 16–18), where heavy density lysosomes migrate, with a shoulder in fractions 14–16. A second peak is next to, and partially overlapping, the peak containing the endosomal marker proCD (fractions 5–9). After hybridization of the same blots with anti-IL-1β, proIL-1β is absent from the highest density, CD-enriched fractions, whereas it is detected as a double peak in fractions 5–9, thus colocalizing with both proCD and the minor peak of CD (Figure 4B). Furthermore, a second peak of proIL-1β is found in high-density fractions 15–16, where the shoulder of lysosomal CD is detected (Figure 4B). The late endosome marker Rab7 (Méresse et al., 1995) is present in the same fractions of proIL-1β, whereas the β-hexosoaminidase activity, taken as an indicator of lysosomal enzymes, predominates in the high-density fractions (Figure 4C). This distribution of lysosomal and endosomal markers is in keeping with those described by others in the same or in different cell types (Diment et al., 1988; Pierre et al., 1996; Morkowski et al., 1997).
Figure 4.
Migration of proIL-1β on Percoll density gradient. P1 and P2 fractions were pooled and further fractionated by centrifugation on a 25% Percoll density gradient under conditions that separate heavy-density lysosomes from lower-density endosomes. Membranes were collected from the individual fractions and tested for β-hexosoaminidase activity or analyzed by Western blot using anti-CD, anti-IL-1β, or anti-Rab7 antibodies. Fraction 1 represents the lowest density; fraction 18 is the highest density. The reactivity of proCD and CD (A), IL-1β (B), and Rab7 (C) was quantified by image digitalization and plotted as arbitrary units (a.u.); β-hexosoaminidase activity (C) is expressed as percent of total. The Western blots hybridized with anti-CD and anti-IL-1β are shown as insets in A and B, respectively. One representative experiment four is shown.
Thus, in agreement with the immunoelectron microscopy data, proIL-1β colocalizes only partially with lysosomal markers; IL-1β–containing structures display a density lower than that of most mature lysosomes, which are highly enriched in CD and in β-hexosoaminidase activity but are devoid of IL-1β.
Increased Endoluminal pH Inhibits Vesicular Accumulation and Secretion of IL-1β
Increases in lysosomal pH result in lysosome exocytosis, with enhanced secretion of preformed hydrolases (Brown et al., 1985; Tapper and Sundler, 1990, 1995). Therefore, we studied whether IL-1β–containing vesicles behave as secretory lysosomes and compared the effects of lysosomotropic drugs on CD and IL-1β secretion. Figure 5A, lower panel, shows that, in keeping with previous reports (Brown et al., 1985; Tapper and Sundler, 1990, 1995), CD secretion is enhanced by NH4Cl treatment (Figure 5A, lower panel, lanes 8 and 9). Similarly, chasing LPS-activated monocytes in the presence of the same drug results in increased IL-1β secretion (Figure 5A, upper panel, lane 9) with decreased vesicular IL-1β (Figure 5A, upper panel, lane 6). Interestingly however, when monocytes are treated with NH4Cl before activation with LPS, secretion of IL-1β is inhibited (Figure 5A, upper panel, lane 8), and a concomitant decrease of protected proIL-1β is observed (Figure 5A, upper panel, lane 5), whereas the accumulation of the cytosolic precursor protein is almost unaffected (Figure 5A, upper panel, lane 2). Similarly to NH4Cl, BafA1 and chloroquine, two drugs that raise endoluminal pH with different mechanisms (Bowman et al., 1988), increase IL-1β secretion when added to LPS-activated monocytes, whereas they prevent secretion if the treatment is carried out before induction of IL-1β synthesis by LPS (Figure 5B).
Figure 5.
Effects of lysosomotropic drug treatment on secretion and vesicular accumulation of IL-1β. A, Monocytes were stimulated 1 h with LPS without (nil; lanes 1, 4, and 7) or with 50 mM NH4Cl (NH4Cl pre; lanes 2, 5, and 8) and cultured 2.5 h in the absence or presence of the same drug as indicated. Alternatively, monocytes were stimulated 1 h with LPS without NH4Cl, followed by 2.5 h of culture with the drug (NH4Cl chase; lanes 3, 6, and 9). Filters were hybridized with anti-IL-1β (upper panels) or anti-CD (lower panels) antibodies. Cyt, cytosolic fractions (lanes 1–3); P1, P1 pellet (lanes 4–6); sec, supernatants (lanes 7–9). (B) Supernatants from monocytes untreated (nil, lane 1), chased (lanes 2–4), or pretreated (lanes 5–7) as in A with 50 mM NH4Cl (lanes 2 and 5), 50 μM chloroquine (chlor; lanes 3 and 6), or 1 μM BafA1 (lanes 4 and 7). Filters were hybridized with anti-IL-1β antibody.
Altogether these results indicate that lysosomotropic drugs stimulate secretion of CD and of preformed, particulated IL-1β. However, when proIL-1β synthesis is induced in cells in which the endoluminal pH is already increased by the same drugs, both IL-1β secretion and vesicular accumulation are prevented, suggesting that the abolition of ΔpH between cytosol and vesicle lumen affects the entry of proIL-1β into the vesicles and consequently IL-1β secretion.
Osmotic Conditions and Extracellular ATP Regulate the Exocytosis of ProIL-1β–containing Vesicles
Recently, it has been proposed that ATP, autocrinally secreted by monocytes upon LPS stimulation, activates its purinergic receptors, leading to accelerated IL-1β secretion (Ferrari et al., 1997). Because Mg2+ chelates ATP4−, the active component of extracellular ATP (Lammas et al., 1997), we studied the effect of extracellular Mg2+ modulation on secreted and particulated IL-1β. Figure 6A, upper panel, shows that both the basal and the ATP-induced IL-1β secretion are blocked by addition of 10 mM MgCl2 (lanes 2 and 6). Conversely, exposure to the Mg2+ chelator EDTA (5 mM) results in a dramatic enhancement of IL-1β secretion (Figure 6A, upper panel, lanes 3 and 7). This effect is indeed due to Mg2+ chelation, because it can be reversed by the simultaneous addition of 20 mM MgCl2 (Figure 6A, upper panel, lanes 4 and 8). The inhibition of secretion induced by Mg2+ is paralleled by an increase of particulated IL-1β (Figure 6B, upper panel, lane 4 vs. lane 2); in turn, cells treated with EDTA have only traces of protected proIL-1β (Figure 6B, upper panel, lane 6). On the contrary, CD secretion, which is induced by ATP at a much lesser extent than IL-1β secretion (Figure 6A, lower panel, lane 5) is only slightly influenced by variations in extracellular [Mg2+] (Figure 6A, lower panel), which, similarly, have no or little effects on CD content in P1 (Figure 6B, lower panel).
Figure 6.
Exocytosis of IL-1β–containing vesicles is regulated by extracellular [Mg2+]. (A) Supernatants from monocytes incubated 2.5 h with LPS in medium alone (lanes 1 and 5) or plus 5 mM EDTA (lanes 3 and 7), 5 mM MgCl2 (lanes 2 and 6), or 5 mM EDTA plus 5 mM MgCl2 (lanes 4 and 8). When indicated (+ATP, lanes 5–8), 1 mM ATP was added during the last 30 min. (B) PNS (5%) and P1 from activated monocytes cultured 1 h in the absence (−, lanes 1 and 2) or presence of 5 mM MgCl2 (Mg2+, lanes 3 and 4) or 5 mM EDTA (lanes 5 and 6). Filters were hybridized with anti-IL-1β (upper panels) or anti-CD (lower panels).
Osmotic conditions, known to regulate accumulation and externalization of DdCAD-1 into vacuoles in D. discoideum (Sesaki et al., 1997), modulate vesicular content and secretion of IL-1β as well. Indeed, when activated monocytes are incubated in hypertonic medium, both the basal and the ATP-induced secretion of IL-1β are inhibited, whereas hypotonic conditions dramatically stimulate secretion (Figure 7A, upper panel). Conversely, the same conditions affect only barely the release of CD (Figure 7A, lower panel). Moreover, protected IL-1β increases upon incubation in hyperosmotic conditions, whereas it almost disappears after exposure to hypotonic medium (Figure 7B), suggesting that osmotic conditions, similarly to extracellular ATP levels, modulate the exocytosis of IL-1β-containing organelles.
Figure 7.
Exocytosis of IL-1β–containing vesicles is regulated by extracellular osmotic conditions. (A) Supernatants from monocytes incubated 2.5 h with LPS in isotonic conditions (medium alone, iso, lanes 1 and 4) or plus 0.1 M sucrose (hyper, lanes 2 and 5) or diluted 1:2 with water (hypo, lanes 3 and 6) in the absence (−, lanes 1–3) or presence of 1 mM ATP (+, lanes 4–6) for the last 30 min. Filters were hybridized with anti-IL-1β (upper panels) or anti-CD (lower panels). (B) PNS and P1 from monocytes incubated 2.5 h with LPS in isotonic conditions (medium alone, iso, lanes 1 and 2) or plus 0.1 M sucrose (hyper, lanes 3 and 4) or diluted 1:2 with water (hypo, lanes 5 and 6).
DISCUSSION
Here we describe a nonclassical pathway of secretion involving regulated exocytosis of endolysosomal-related vesicles for the LLS protein IL-1β, as indicated by the following lines of evidence: 1) IL-1β is contained in part within organelles cofractionating with Rab-7–positive structures and displaying ultrastructural features of late endosomes and dense vesicles; 2) a fraction of IL-1β-containing organelles costains with the endolysosomal protein CD or the lysosomal marker Lamp-1; 3) IL-1β secretion is pH dependent; and 4) the amount of protected proIL-1β and the secretion of IL-1β are modulated by osmotic conditions and extracellular ATP concentration.
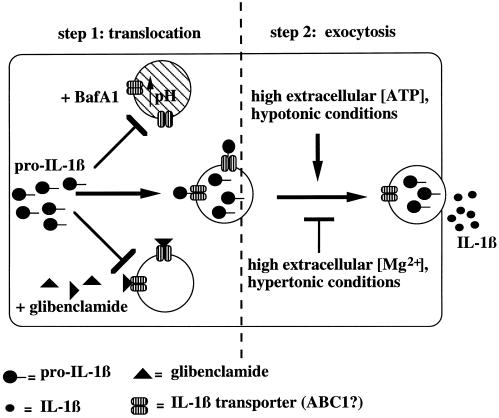
On these bases, a two-step model for the IL-1β secretory pathway is proposed in Figure 8. The first step is translocation of a fraction of cytosolic proIL-1β across the membrane of specialized vesicles. The vesicles deputated to vehicle IL-1β extracellularly may be a subset of late endosomes and lysosomes, expressing on their cytosolic surface a dedicated translocation machinery. The translocation requires a ΔpH between the cytosol and the lysosomal lumen, which may furnish at least part of the energy necessary for the translocation process. In step 2, IL-1β is released upon fusion of the vesicle membrane with the plasma membrane. This exocytosis is driven by exogenous ATP and by hypotonic conditions.
Figure 8.
Two step model for IL-1β secretion, showing vesicle-mediated transport from the cytosol to the extracellular space. The translocation step is blocked by glibenclamide and when the ΔpH between cytosol and vesicles is abolished; the exocytosis is induced by high extracellular ATP and by hypotonic medium, whereas it is inhibited by low extracellular ATP and by hypertonic conditions.
IL-1β Translocation within Endolysosomal-related Vesicles
Particulated IL-1β is contained in structures belonging to the endolysosomal compartment, as indicated by their ultrastructural features and by the partial colocalization with CD and Lamp-1. However, although mature lysosomes stain preferentially for CD or Lamp-1 alone, IL-1β is found both in structures displaying the morphology of late endosomes and early lysosomes and in smaller dense vesicles, which possibly correspond to prelysosomes (Pieters et al., 1991). The immunoelectron microscopy findings are paralleled by the results obtained with Percoll density gradients showing that proIL-1β is unequally distributed along the gradient; indeed, proIL-1β is scarcely represented in the highest density fractions enriched with mature lysosomes, in which the 31-kDa form of CD is most abundant, is present with a minor peak cosedimenting with less dense, immature lysosomes, and displays a clear accumulation at lower density, at which it overlaps with both the minor peak of 31-kDa CD and the peak of the endosomal marker proCD. The belonging of IL-1β–containing vesicles to the preterminal endocytic compartment is further confirmed by the cofractionation with Rab7, which is known to define late endosome vesicles connected to lysosomes (Méresse et al., 1995).
The hypothesis that proIL-1β–containing vesicles are intermediates of IL-1β secretion is supported by the results obtained by treating monocytes with lysosomotropic drugs before or after induction of IL-1β synthesis and of accumulation of vesicular IL-1β. Indeed, pretreating resting monocytes, which do not express IL-1β, with lysosomotropic drugs does not affect LPS-induced proIL-1β synthesis but inhibits IL-1β secretion and vesicular accumulation, suggesting that, as in other systems (Chaddock et al., 1995; Santini et al., 1998), translocation of proIL-1β from cytosol to vesicles require a ΔpH and therefore is impaired by increases in the endoluminal pH. In contrast, if LPS-activated monocytes are treated with lysosomotropic drugs, and hence the endoluminal pH is raised when proIL-1β is already stored in the vesicles, increased secretion of IL-1β with emptying of proIL-1β–containing vesicles is observed. In this case IL-1β behaves like lysosome-stored hydrolases, whose secretion is induced by lysosomotropic drugs (Figure 5; Brown et al., 1985; Tapper and Sundler, 1990, 1995). NH4Cl, weak bases such as cloroquine, and inhibitors of the vacuolar proton pump such as BafA1, which raise pH with different mechanisms, gave similar results, allowing us to reasonably exclude that they may influence IL-1β export by affecting parameters other than pH (Mellman et al., 1986; Hunke et al., 1995; van Weert et al., 1995). Moreover, when pulse–chase experiments were performed in the presence of endolysosomal protease inhibitors, the kinetics of disappearance of IL-1β from the vesicles was almost unchanged compared with control cells, but the amount of secreted IL-1β was enhanced: these data strongly suggest that the protease inhibitors rescue a portion of vesicular IL-1β from degradation, allowing its secretion.
Pretreatment of monocytes with glibenclamide prevents both IL-1β secretion and accumulation of protected proIL-1β. Glibenclamide is a functional blocker of a few ABC transporters, namely ABC1, the cystic fibrosis transmembrane regulator, and the sulfonylurea receptor. Although the involvement of the cystic fibrosis transmembrane regulator and sulfonylurea receptor in IL-1β secretion can be excluded, a role for ABC1 is suggested by the observations that the secretion of IL-1β and the function of ABC1 as an anion exchanger are sensitive to the same drugs, including glibenclamide (Hamon et al., 1997). In addition, ABC1 localizes on vesicles belonging to the phagolysosome compartment in mouse macrophages (Luciani and Chimini, 1996) and in transfected human cells (Hamon and Chimini, unpublished data). Glibenclamide could interfere with the translocation machinery on the vesicle membrane, thus impairing proIL-1β translocation from the cytosol and consequently blocking IL-1β secretion. As for pretreatment with lysosomotropic drugs, the effects of glibenclamide on IL-1β and CD secretion and vesicular accumulation are different because of the different way of access to the organelle lumen of the two proteins: by translocation in the case of proIL-1β and by endoluminal transport in the case of CD.
Unlike extracellular IL-1β, which is mostly in the 17-kDa molecular form, particulated IL-1β is in the precursor form, ruling out that it derives from secreted IL-1β, taken up by monocytes after its release. However, the site and the modality of the proteolytic processing remain to be clarified.
Regulation of the Exocytosis of IL-1β–containing Vesicles
The secretion of lysosomal enzymes by macrophages and their role in inflammation and tissue repair are long-standing evidence (Unanue, 1976). In many cells, including activated monocytes, lysosomes behave as secretory organelles (Page et al., 1998) and release hydrolytic enzymes in a regulated manner (Unanue, 1976; Schnyder and Baggiolini, 1978; Keeling and Henson, 1982; Tapper and Sundler, 1990, 1995; Rodriguez et al., 1997). Furthermore, it is interesting to recall that a number of specialized vesicles present in different cell types (including the azurophyl granules of neutrophils, the specific granules of mast cells, and the lytic granules of T lymphocytes) display a common biogenesis with endolysosomes (Jamur et al., 1986; Peters et al., 1991; Borregaard et al., 1993) and undergo regulated exocytosis.
Here we show that extracellular ATP regulates IL-1β secretion: a dramatic release of IL-1β occurs soon after the exposure to ATP; similarly, high extracellular ATP levels lead to depletion of protected proIL-1β, whereas the opposite is observed in the absence of ATP, strongly suggesting that exogenous ATP triggers the exocytosis of IL-1β–containing organelles.
Monocytic cells can release ATP, which in turn may interact with P2Z receptors on their plasma membrane, eventually leading to IL-1β secretion (Ferrari et al., 1997). Indeed, chelation of ATP4−, the active component of extracellular ATP, by Mg2+ ions (Lammas et al., 1997) results in complete inhibition of IL-1β secretion, whereas the contrary is observed by Mg2+ chelation. These data indicate that the basal IL-1β secretion is due to autocrinally produced extracellular ATP; the partial inactivation of secreted ATP by the Mg2+ ions present in the extracellular medium keeps IL-1β secretion at low levels. The release of CD is only slightly induced by extracellular ATP, and the intracellular level of CD is almost unaffected, suggesting that the IL-1β–containing organelles are highly sensitive to the exocytotic stimulus of extracellular ATP, whereas the mature, CD-enriched dense lysosomes are insensitive.
Exocytosis of proIL-1β–containing vesicles also undergoes osmotic regulation; secretion of IL-1β and emptying of the vesicles are induced in hypotonic medium, whereas hypertonic conditions inhibit secretion and induce intravesicular accumulation. Again, CD release is much less modulated by the same conditions, indicating that mature lysosomes are insensitive to osmotic regulation. In D. discoideum, the exocytosis of the endolysosomal-related conctractile vacuoles enriched with the leaderless membrane protein DdCAD-1 is pH dependent and modulated by extracellular osmotic conditions (Sesaki et al., 1997). Thus, the organelles involved in IL-1β secretion and the contractile vacuoles of Dictyostelium possess common features and display a similar behavior. These similarities suggest that a mechanism of export of LLS proteins that uses intracellular acidic vesicles as a vehicle to the extracellular space may have been conserved during evolution.
Because of the low levels of IL-1β secretion in the absence of stimuli, our data cannot formally exclude that, in basal conditions, secretion of the cytokine occurs through a mechanism different from endolysosome exocytosis and that protected IL-1β is destined to degradation. Even in this case, however, perturbations of the microenvironment could possibly rescue protected IL-1β from degradation, inducing its secretion, as supported by the observation that an increased release of IL-1β is triggered by treatment with lysosomotropic drugs, ATP, and hypotonic conditions. All these may mimic in vitro conditions arising in vivo in the extracellular milieu of monocytes in the course of inflammation, when secretion of IL-1β has a physiopathological relevance. Support for this hypothesis comes from the finding that, in basal conditions, inhibition of endolysosomal proteases results in secretion of a fraction of particulated IL-1β, otherwise destined to degradation. Thus, this novel pathway of secretion, involving regulated exocytosis of endolysosomerelated vesicles, gives the macrophage the potential to exert a regulatory influence in the course of inflammation, infection, and induction of immune response by modulating the release of IL-1β in its surrounding environment. The full characterization of IL-1β–containing organelles deserves further investigation. Interestingly, recent studies point to the existence in antigen-presenting cells of specialized endolysosomal compartments, where antigen processing and peptide loading occur. These compartments are heterogeneous in morphology and density and not always distinct from conventional endosomes and lysosomes (Mellman et al., 1998). It is tempting to speculate that the secretory route of IL-1β and the endocytotic pathway of exogenous antigens intersect in activated monocytes in the same endolysosomal compartment from which recycling of antigenic peptides and secretion of mature IL-1β may occur.
ACKNOWLEDGMENTS
We thank S. Costigliolo for excellent technical assistance, R. Sitia and M.R. Zocchi for critical comments, F. Cozzolino, C. Isidoro, and S. Méresse for sharing their reagents, and the Blood Center of Gaslini Scientific Institute for providing buffy coats. The 3ZD monoclonal antibody was obtained through the National Cancer Institute Biological Resources Branch, Frederick Cancer Research and Development Center (Frederick, MD). This work was supported in part by grants from Consiglio Nazionale delle Ricerche (Target Project on Biotechnology), Associazione Italiana per la Ricerca sul Cancro, and Ministero Sanità (Progetto Finalizzato Oncology and Special Project AIDS). C.A. is the recipient of a fellowship from “Fondazione Levi-Montalcini.”
Abbreviations used:
- ABC
ATP-binding cassette
- BafA1
bafilomycin A1
- CD
cathepsin D
- ICE
interleukin-1β–converting enzyme
- IgG
immunoglobulin G
- IL-1
interleukin 1
- LLS
leaderless secretory
- LPS
lipopolysaccharide
- P
pellet
- PNS
postnuclear supernatant
- proIL-1β
IL-1β precursor
- TCA
trichloroacetic acid
REFERENCES
- Ayala JM, Yamin TT, Egger LA, Chin J, Kostura MJ, Miller DE. IL-1β-converting enzyme is present in monocytic cells as inactive precursor. J Immunol. 1994;153:2592–2599. [PubMed] [Google Scholar]
- Becq F, Hamon Y, Bajetto A, Gola M, Verrier B, Chimini G. ABC1, an ATP binding cassette transporter, required during apoptosis, generates a regulated ion flux after expression in Xenopus oocytes. J Biol Chem. 1997;272:2695–2701. doi: 10.1074/jbc.272.5.2695. [DOI] [PubMed] [Google Scholar]
- Borregaard N, Lollike K, Kjeldsen L, Sengelov H, Bastholm L, Nielsen MH, Baiton DF. Human neutrophil granules and secretory vesicles. Eur J Hematol. 1993;51:187–198. doi: 10.1111/j.1600-0609.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Bowman EJ, Siebers A, Alterdorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Novak EK, Swank RT. Effects of ammonia on processing and secretion of precursor and mature lysosomal enzyme from macrophages of normal and pale ear mice: evidence of two distinct pathways. J Cell Biol. 1985;100:1894–1904. doi: 10.1083/jcb.100.6.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerretti DP, et al. Molecular cloning of the Interleukin-1β converting enzyme. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- Chaddock AM, Mant A, Karnauchov I, Brink S, Herrmann RG, Klosgen RB, Robinson C. A new type of signal peptide: central role of a twin-arginine motif in transfer signals for the ΔpH-dependent thylakoidal protein translocase. EMBO J. 1995;14:2715–2722. doi: 10.1002/j.1460-2075.1995.tb07272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleves AE, Cooper DNW, Barondes SH, Kelly RB. A new pathway for protein export in Saccharomyces cerevisiae. J Cell Biol. 1996;133:1017–1026. doi: 10.1083/jcb.133.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DN, Barondes SH. Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J Cell Biol. 1990;110:1681–1691. doi: 10.1083/jcb.110.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- Diment S, Leech MS, Stahl PD. Cathepsin D is membrane-associated in macrophage endosomes. J Biol Chem. 1988;263:6901–6907. [PubMed] [Google Scholar]
- Dinarello CA. Interleukin 1 and Interleukin 1 antagonism. Blood. 1991;127:119–127. [PubMed] [Google Scholar]
- Di Virgilio F. The P2Z purinoreceptor: an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic stimulation of interleukin-1β release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florkiewicz RZ, Majack RA, Buechler RD, Florkiewicz E. Quantitative export of FGF-2 occurs through an alternative, energy-dependent, nonER/Golgi pathway. J Cell Physiol. 1995;162:388–399. doi: 10.1002/jcp.1041620311. [DOI] [PubMed] [Google Scholar]
- Hamon Y, Luciani MF, Becq F, Verrier B, Rubartelli A, Chimini G. Interleukin-1β secretion is impaired by inhibitors of the ATP binding cassette transporter, ABC1. Blood. 1997;90:2911–2915. [PubMed] [Google Scholar]
- Hazuda DJ, Strickler J, Simon P, Young PR. Structure-function mapping of Interleukin-1 precursor. Cleavage leads to a conformational change in the mature protein. J Biol Chem. 1991;266:7081–7086. [PubMed] [Google Scholar]
- Hickman SE, Khouri JE, Greenberg S, Silverstein SC. P2Z adenosine triphosphate receptor activity in cultured human monocyte-derived macrophages. Blood. 1994;84:2452–2457. [PubMed] [Google Scholar]
- Hogquist KA, Nett MA, Unanue ER, Chaplin DD. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci USA. 1991;88:8485–8489. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunke S, Drose S, Schneider E. Vanadate and bafilomycin A1 are potent inhibitors of the ATPase activity of the reconstituted bacterial ATP binding cassette transporter for maltose (MalFgK2) Biochem Biophys Res Commun. 1995;216:589–594. doi: 10.1006/bbrc.1995.2663. [DOI] [PubMed] [Google Scholar]
- Jamur MC, Vugman I, Hand AR. Ultrastructural and cytochemical studies of acid phosphatase and trimetaphosphatase in rat peritoneal mast cells developing in vitro. Cell Tissue Res. 1986;244:557–563. doi: 10.1007/BF00212533. [DOI] [PubMed] [Google Scholar]
- Keeling PJ, Henson PM. Lysosomal enzyme release from human monocytes in response to particulate stimuli. J Immunol. 1982;128:563–567. [PubMed] [Google Scholar]
- Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Kuchler K, Rubartelli A, Holland B, editors. Unusual secretory pathways: from bacteria to man. Austin: R.G. Landes; 1997. [Google Scholar]
- Lammas DA, Stober C, Hsarvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- Lotti LV, Torrisi MR, Pascale MC, Bonatti S. Immunocytochemical analysis of the transfer of vesicular stomatitis virus G glycoprotein from the intermediate compartment to the Golgi complex. J Cell Biol. 1992;118:43–50. doi: 10.1083/jcb.118.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani MF, Chimini G. The ATP binding cassette transporter ABC1 is required for the engulfment of corpses generated by apoptotic cell death. EMBO J. 1996;15:226–235. [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Mellman I, Turley SJ, Steinman RM. Antigen processing for amateurs and professionals. Trends Cell Biol. 1998;8:231–237. doi: 10.1016/s0962-8924(98)01276-8. [DOI] [PubMed] [Google Scholar]
- Méresse S, Gorvel JP, Chavrier P. The rab7 GTPase resides on a vesicular compartment connected to lysosomes. J Cell Sci. 1995;108:3349–3358. doi: 10.1242/jcs.108.11.3349. [DOI] [PubMed] [Google Scholar]
- Mignatti P, Morimoto T, Rifkin DB. Basic fibroblast growth factor released by single, isolated cells stimulates their migration in an autocrine manner. Proc Natl Acad Sci USA. 1991;88:11007–11011. doi: 10.1073/pnas.88.24.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkowski S, Raposo G, Kleijmeer M, Geuze HJ, Rudensky AY. Assembly of an abundant endogenous major hystocompatibility complex class II/peptide complex in class II compartments. Eur J Immunol. 1997;27:609–617. doi: 10.1002/eji.1830270306. [DOI] [PubMed] [Google Scholar]
- Page LJ, Darmon AJ, Uellner R, Griffiths GM. L is for lytic granules: lysosomes that kill. Biochim Biophys Acta. 1998;1401:146–156. doi: 10.1016/s0167-4889(97)00138-9. [DOI] [PubMed] [Google Scholar]
- Perregaux D, Gabel CA. Interleukin-1β maturation and release in response to ATP and nigericin. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- Peters PJ, Borst J, Oorschot V, Fukuda M, Krahenbuhl O, Tschopp J, Slot JW, Geuze HJ. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991;173:1099–1109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre P, Denzin LK, Hammond C, Drake JR, Amigorena S, Cresswell P, Mellman I. HLA-DM is localized to conventional and unconventional MHC class II-containing endocytic compartments. 1996. Immunity. 1996;4:229–239. doi: 10.1016/s1074-7613(00)80431-8. [DOI] [PubMed] [Google Scholar]
- Pieters J, Horstmann H, Bakke O, Griffiths G, Lipp J. Intracellular transport and localization of major histocompatibility complex class II molecules and associated invariant chain. J Cell Biol. 1991;115:1213–1223. doi: 10.1083/jcb.115.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt A, Mayorga LS, Schwartz AL, Stahl PD. Assay for phagosome-endosome fusion and phagosome protein recycling. Methods Enzymol. 1992;219:21–31. doi: 10.1016/0076-6879(92)19006-r. [DOI] [PubMed] [Google Scholar]
- Rijnboutt S, Stoorvogel W, Geuze HJ, Strous GJ. Identification of subcellular compartments involved in biosynthetic processing of cathepsin D. J Biol Chem. 1992;267:15665–15672. [PubMed] [Google Scholar]
- Rodriguez A, Webster P, Ortego J, Andrews NW. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J Cell Biol. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A, Bajetto A, Allavena G, Cozzolino F, Sitia R. Posttranslational regulation of Interleukin 1β secretion. Cytokine. 1993;5:117–124. doi: 10.1016/1043-4666(93)90050-f. [DOI] [PubMed] [Google Scholar]
- Rubartelli A, Bajetto A, Allavena G, Wollmann E, Sitia R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem. 1992;267:24161–24164. [PubMed] [Google Scholar]
- Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin 1β, a protein lacking a signal sequence. EMBO J. 1990;9:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A, Sitia R. Secretion of mammalian proteins that lack a signal sequence. In: Kuchler K, Rubartelli A, Holland BI, editors. Unusual Secretory Pathways: From Bacteria to Man. Austin: R.G. Landes; 1997. pp. 87–104. [Google Scholar]
- Santini CL, Bérèngere I, Chanal A, Muller M, Giordano G, Wu LF. A novel Sec-independent periplasmic protein translocation pathway in Escherichia coli. EMBO J. 1998;17:101–112. doi: 10.1093/emboj/17.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder J, Baggiolini M. Secretion of lysosomal hydrolases by stimulated and nonstimulated macrophages. J Exp Med. 1978;48:435–450. doi: 10.1084/jem.148.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesaki H, Wong EFS, Siu C-H. The cell adhesion molecule DdCAD-1 in Dictyostelium is targeted to the cell surface by a non classical transport pathway involving contractile vacuoles. J Cell Biol. 1997;137:939–951. doi: 10.1083/jcb.138.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer II, Scott S, Chin J, Bayn EK, Limjuco G, Weidner J, Miller DK, Chapman K, Kostura MJ. The interleukin 1 β converting enzyme (ICE) is localized on the external cell surface membrane and in the cytoplasmic ground substance of human monocytes by immuno-electron microscopy. J Exp Med. 1995;182:1447–1459. doi: 10.1084/jem.182.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer II, Scott S, Hall JL, Limjuco G, Chin J, Schmidt JA. Interleukin-1β is localized in the cytoplasmic ground substance but is largely absent from the Golgi apparatus and the plasma membrane of stimulated human monocytes. J Exp Med. 1988;167:389–407. doi: 10.1084/jem.167.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ. Sizing of protein A-colloidal gold probes for immunoelectron microscopy. J Cell Biol. 1981;90:553–536. doi: 10.1083/jcb.90.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper H, Sundler R. Role of lysosomal and cytosolic pH in the regulation of macrophage lysosomal secretion. Biochem J. 1990;272:407–414. doi: 10.1042/bj2720407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper H, Sundler R. Bafilomycin A1 inhibits lysosomal, phagosomal, and plasma membrane H+ ATPases and induces lysosomal enzyme secretion in macrophages. J Cell Physiol. 1995;163:137–144. doi: 10.1002/jcp.1041630116. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Unanue ER. Secretory function of mononuclear phagocytes. Am J Pathol. 1976;83:396–417. [PMC free article] [PubMed] [Google Scholar]
- van Weert AWM, Kenneth WD, Geuze HJ, Maxfield FR, Stoorvogel W. Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J Cell Biol. 1995;130:821–834. doi: 10.1083/jcb.130.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]