Abstract
Gene targeting techniques and early mouse embryos have been used to produce immortalized fibroblasts genetically deficient in phospholipase C (PLC)-γ1, a ubiquitous tyrosine kinase substrate. Plcg1−/− embryos die at embryonic day 9; however, cells derived from these embryos proliferate as well as cells from Plcg1+/+ embryos. The null cells do grow to a higher saturation density in serum-containing media, as their capacity to spread out is decreased compared with that of wild-type cells. In terms of epidermal growth factor receptor activation and internalization, or growth factor induction of mitogen-activated protein kinase, c-fos, or DNA synthesis in quiescent cells, PLcg1−/− cells respond equivalently to PLcg1+/+ cells. Also, null cells are able to migrate effectively in a wounded monolayer. Therefore, immortalized fibroblasts do not require PLC-γ1 for many responses to growth factors.
INTRODUCTION
Activation of growth factor receptor tyrosine kinases by ligand binding provokes intracellular signaling through a variety of molecules to various points of signal reception and response generation. Proximal to activated receptors are a number of signaling molecules that serve as tyrosine phosphorylation substrates and/or adaptor molecules. Most of these utilize SH2 domains to facilitate interaction with receptor autophosphorylation sites (Pawson, 1995). Molecules proximal to the epidermal growth factor (EGF) receptor include the enzymes phospholipase C-γ1, the phosphotyrosine phosphatase SHP-1, the coreceptor tyrosine kinases ErbB-2 and ErbB-3, the intracellular tyrosine kinase src, the transcription factor STAT3, and the adaptor molecules Shc, Grb-2, and Nck. These receptor-proximal molecules lead to the activation of several signal transduction pathways. The details of these pathways and manner in which they interact to produce biological responses, such as cell proliferation, is not understood.
Mammalian cells contain at least ten genes that encode various phosphatidylinositol 4,5-bisphosphate (PIP2)-specific phospholipase C (PLC) isozymes (Rhee and Bae, 1997). All mammalian PLC isozymes are characterized by the presence of sequence motifs termed X and Y, which in the native enzyme are folded together to constitute the active site for PIP2 hydrolysis (Essen et al., 1996). Tyrosine kinases are known to exclusively activate two PLC-γ isozymes, while agonists for heptahelical receptors utilize heterotrimeric G protein to activate four PLC-β isozymes. Cellular control of the four PLC-δ isozymes is unclear. The γ subfamily is distinguished by the presence of src homology domains (two SH2 and one SH3) that are hallmarks of receptor tyrosine kinase-signaling pathways. The PLC-γ1 isoform is ubiquitously expressed, while the γ2 isoform is more limited in expression to hematopoietic cells (Emori et al., 1989).
PLC-γ1 associates with activated growth factor receptors by means of SH2 domains and is subsequently phosphorylated at tyrosine residues that modulate the molecule’s enzymatic activity (Kamat and Carpenter, 1997; Rhee and Bae, 1997). When activated, PLC-γ1 provokes the hydrolysis of PIP2 to produce two second messenger molecules: inositol 1,4,5-trisphosphate (IP3), which increases intracellular levels of free Ca2+, and diacylglycerol, an endogenous activator of protein kinase. PLC-γ1 activation occurs proximal to most all activated growth factor tyrosine kinase receptors, including fibroblast growth factor receptors 1 and 2, platelet-derived growth factor receptors α and β, glial-derived neurotropic factor receptor, Ret, the hepatocyte growth factor receptor, Met, and the neurotropin receptors, TrkA, B, and C.
A critical issue in signal transduction is understanding the relationship of individual molecules to the generation of downstream signaling elements and the generation of biologic responses. Attempts to define PLC-γ1 as a necessary component for mitogenic signaling have, in cell culture systems, not produced a consistent view of the significance of this molecule. Recently, gene targeting and disruption have shown that, in mice, PLC-γ1 is essential during early to midgestation, as Plcg1−/− embryos die at approximately embryonic day 9 (E9.0) (Ji et al., 1997). This targeted disruption of the Plcg1 locus removes exons that encode the entire X domain, which is necessary for catalysis, plus both SH2 domains, which facilitate PLC-γ1 association with activated receptor tyrosine kinases. It was shown that mouse embryo fibroblasts (MEF) produced from Plcg1−/− embryos are unable to mobilize intracellular Ca2+ when challenged with an agonist that is known to activate the PLC-γ isozyme. In this report we have explored various EGF-dependent mitogenic responses in cells genetically deficient in this signaling element.
MATERIALS AND METHODS
Cell Culture
MEF were prepared from E9.5 embryos with and without targeted disruption of the Plcg1 gene (Ji et al., 1997). Plcg1−/− MEF have been characterized by Southern blotting, Western blotting, and Ca2+ mobilization in response to EGF (Ji et al., 1997). The cells were established in culture following the procedures of Todaro and Green (1963) and maintained as immortalized nontransformed cell lines. The cells are routinely grown in DMEM supplemented with 10% fetal bovine serum. To prepare cells for growth factor stimulation, subconfluent cultures were incubated in DMEM containing 0.5% fetal bovine serum for 24–72 h before the addition of growth factor. Mouse EGF, isolated as previously described by Savage and Cohen (1972), was added to a final concentration of 20 ng/ml unless otherwise noted. Cell numbers were determined by Coulter counting, and cell morphology was examined with an Olympus inverted phase contrast microscope.
Western Blotting
After treatment without or with EGF (50 ng/ml), cells were lysed in TGH buffer (1% Triton X-100, 10% glycerol, 50 mM HEPES, pH 7.2) supplemented with 100 mM NaCl and protease and phosphatase inhibitors (10 μg/ml aprotinin, 10 μg/ml leupeptin, 100 μM phenylmethylsulfonyl fluoride, 1 mM Na3VO4). After brief centrifugation, aliquots (100 μg protein) of each lysate were subjected to gel electrophoresis (10% SDS-PAGE). Aftertransfer to nitrocellulose, the samples were incubated in blocking solution (Baulida et al., 1996) and then blotted with antibodies to tyrosine-phosphorylated mitogen-activated protein (MAP) kinase to detect the activated enzyme. After washing, bound antibody was detected by enhanced chemiluminescence (ECL). Data were quantitated using densitometric scanning with molecular analyst software (Bio-Rad, Hercules, CA).
To detect tyrosine phosphorylated EGF receptor, cells were treated without or with EGF (20 ng/ml) and cell extracts prepared as previously described (Baulida et al., 1996). Antisera 986 to the EGF receptor (Stoscheck and Carpenter, 1983) was employed to precipitate the receptor. After 7.5% SDS-PAGE and transfer to nitrocellulose, activated receptors were detected by blotting with antiphosphotyrosine (Zymed, South San Francisco, CA) and ECL.
DNA Synthesis
Cells were grown to confluence and then incubated in DMEM plus 0.5% fetal bovine serum for 36 h before the addition of EGF (20 ng/ml). At each indicated time point thereafter, 3H-thymidine (0.5 μCi/ml) was added for 1 h. Cell cultures were then processed as previously described (Carpenter and Cohen, 1976a) to measure the amount of cold 5% trichoroacetic acid-insoluble radioactivity. Protein was measured by the modified Bradford procedure (Bio-Rad) and radioactivity expressed as counts per min per μg cell protein. Bromodeoxyuridine incorporation was employed to determine the labeling index of EGF-stimulated cells. Cells were arrested in 0.5% fetal calf serum for 36 h before the addition of EGF. At each indicated time point (0 h, 19 h, 36 h) bromodeoxyuridine was added for 1 h and cell labeling, fixation, reaction with antibody, and counter staining were performed using a Cell Proliferation kit (Amersham, Arlington Heights, IL) according to the manufacturer’s instructions. At each time point, approximately 1000 cells in each of three separate fields were counted to determine the labeling index.
Northern Blotting
Total cellular RNA was isolated using a Qiagen kit according to the manufacturer’s instructions (QIAGEN, Chatsworth, CA). For each data point, an aliquot (15 μg) of total RNA was electrophoresed, transferred to nylon membrane (Bio-Rad), and probed according to standard procedures. To detect c-fos mRNA, a 1.0-kilobase (kb) EcoRI-BamHI fragment of c-fos (Dr. Ronald Wisdom, Vanderbilt University) was used. A probe for glyceraldehyde-3-phosphate dehydrogenase was also obtained from Dr. Wisdom (Vanderbilt University). The probes were labeled with α32P-dATP using the Prime-It II random primer labeling kit (Stratagene, La Jolla, CA).
Cell Migration
Cells were grown to confluence and then incubated in DMEM plus 0.5% fetal bovine serum for 24 h. A plastic pipette tip was then used to scrape a wound in the center of the monolayer. Thereafter, the monolayers were washed and DMEM plus 0.1% BSA without (control) or with EGF (20 ng/ml) was added. Mitomycin C (Chen et al., 1994) was added to a final concentration of 0.5 μg/ml to prevent cell proliferation. Small wounded areas were then identified and marked, and photographs of the same area were made after 0, 11, and 22 h.
125I-EGF Binding
Mouse-derived EGF was radiolabeled with 125I− as previously described (Carpenter and Cohen, 1976b), and specific ligand binding or ligand internalization was measured using published procedures (Baulida et al., 1996). Total specific cell surface binding was assessed after a 2-h incubation at 5°C (to prevent receptor internalization) with 125I-EGF (100 ng/ml). To assess ligand internalization rate, cells were incubated with a low concentration of 125I-EGF (2 ng/ml) for a brief period of time, 1–5 min. After washing, surface and internalized 125I-EGF was determined by washing with cold acidic (pH 2.8) buffer. Nonspecific binding was always measured in parallel cultures exposed to 125I-EGF and a 200-fold excess of unlabeled EGF. Specific internalized 125I-EGF is reported for each time point.
Materials
DMEM was purchased from Grand Island Biological (Grand Island, NY) and fetal bovine serum was purchased from Hyclone (Logan, Utah). Cell culture dishes were obtained from Costar (Cambridge, MA). Inhibitors (aprotinin, leupeptin, phenylmethylsulfonyl fluoride, Mitomycin C) were Sigma (St. Louis, MO) products. Antibody to tyrosine phosphorylated MAP kinase was purchased from New England Biolabs (Beverly, MA). Antibody to phosphotyrosine was from Zymed. 3H-thymidine, 125I− and α32P-dATP were purchased from New England Nuclear-Dupont (Boston, MA). RNA isolation kit was a Qiagen product.
RESULTS
Growth and Morphology
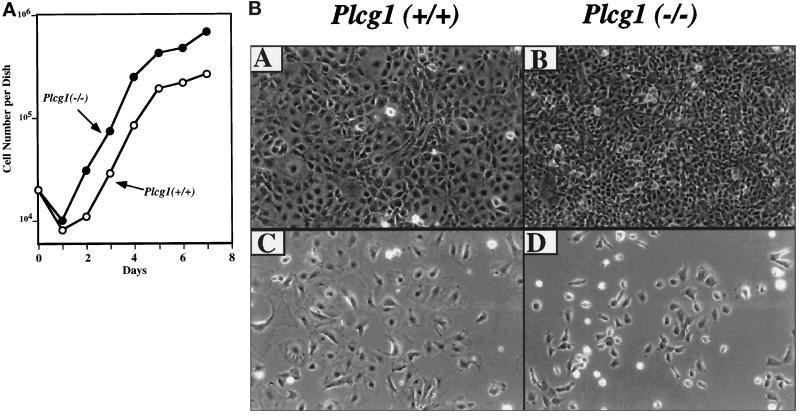
MEF from Plcg1+/+ and Plcg1−/− were established in culture according to the methods of Todaro and Green (1963) and used after crisis when the cells were immortalized. None of the cell lines were transformed as judged by morphology, presence of density-restricted growth (monolayer contact inhibition), and the absence of colony- forming activity in soft agar. To assess the consequence of the absence of PLC-γ1 on growth, cells were plated at low density (2 × 103 per cm2) in media containing 10% serum, and cell numbers were determined. As shown in Figure 1A, Plcg1−/− subconfluent cells grew at a rate comparable to that of Plcg1+/+ cells under these standard growth media conditions. The most noticeable difference was the higher (2.5-fold) saturation density achieved by Plcg1−/−cells, approximately 6.8 × 104 cells per cm2 compared with 2.7 × 104 cells per cm2 for Plcg1+/+ cells. This difference was observed in three independent lines of Plcg1−/− and two independent Plcg1+/+ lines of cells. To characterize the increased saturation density of Plcg1−/− cells, their morphology compared with Plcg1+/+ cells was examined. The results shown in Figure 1B indicate that this growth behavior is, in part, correlated with an altered morphology, as Plcg1−/− cells are smaller or less spread out in morphology compared with Plcg1+/+ cells. This was apparent in sparse cultures as well as confluent cultures where Plcg1 null cells are more dense than wild-type cells. Plcg1−/− cells, however, were density inhibited and did not exhibit characteristics of transformation, such as colony formation in soft agar.
Figure 1.
Growth and morphology of Plcg1+/+ and Plcg1−/− embryonic fibroblasts. (A) Cells were seeded at an initial density of 2 × 103 cells per cm2 in DMEM plus 10% fetal bovine serum. Thereafter cells were counted at the indicated times. Each point represents the average of duplicate cultures. (B) Cells were plated at a low density in DMEM plus 10% fetal bovine serum. When the cells were sparse (C and D) or postconfluent (A and B) photographs were taken (180 × total magnification).
Receptor Phosphorylation and Trafficking
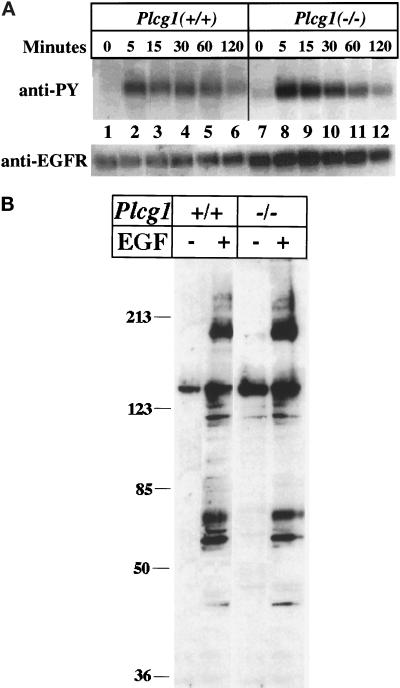
To compare the response of Plcg1+/+ and Plcg1−/− cells, the level of surface EGF receptors were assayed by ligand binding (125I-labeled EGF at 5°C) to determine approximate cell surface numbers. The results indicate that Plcg1+/+ cells have approximately 2.8 × 104 EGF receptors per cell, while the Plcg1−/− cells have approximately 5.2 × 104 receptors per cell. In each cell type, EGF addition provokes a rapid but transient increase in receptor autophosphorylation (Figure 2A). The apparent higher level of receptor autophosphorylation observed in Plcg1−/− cells reflects the higher level of receptors present in these cells, as shown by Western blotting with anti-EGF receptor. After activation of the EGF receptor, a number of other intracellular proteins become tyrosine phosphorylated. This has been assessed in Plcg1+/+ and Plcg1−/− cells by Western blotting cell lysates from control and EGF-treated cells with antiphosphotyrosine. The results, shown in Figure 2B, do not indicate distinctions in the profile of tyrosine-phosphorylated proteins detectable in the two cell types.
Figure 2.
Influence of PLC-γ1 on EGF receptor autophosphorylation and protein tyrosine phosphorylation. (A) Confluent Plcg1+/+ and Plcg1−/− cells were incubated overnight in DMEM plus 0.5% fetal bovine serum and then treated at 37°C with EGF (20 ng/ml) for the indicated times. Cells were then lysed, and the EGF receptor was immunoprecipitated and probed by Western blotting with antiphosphotyrosine as described in MATERIALS AND METHODS. (B) Confluent Plcg1+/+ and Plcg1−/− cells were incubated in DMEM plus 0.5% fetal calf serum overnight, and the cells were then treated for 5 min at 37° without or with EGF (50 ng/ml). After cell lysis, equal aliquots (40 μg) of each lysate were subjected to SDS-PAGE and Western blotting with antiphosphotyrosine. Bound antibody was detected by ECL.
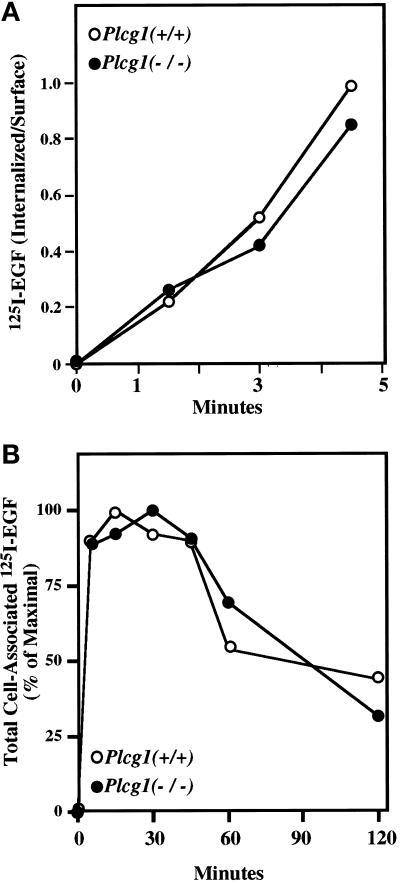
After ligand binding and activation of EGF receptor tyrosine kinase activity, an initial step in receptor desensitization is its rapid internalization as part of the endocytic pathway leading to receptor degradation in lysosomes (Sorkin and Waters, 1993). Therefore, we determined whether PLC-γ1 activity is a required part of the internalization mechanism as suggested for the fibroblast growth factor (FGF) receptor (Sorokin et al., 1994). As shown by the data in Figure 3A, PLC-γ1 is not required for rapid EGF receptor internalization in Plcg1 null cells. The rates of receptor internalization were equivalent in wild-type and null cells. Also, when ligand binding was followed over a longer time period, the typical down-regulation of receptor-binding capacity was detected in both cell types (Figure 3B). Therefore, the activation of PLC-γ1 and the mobilization of intracellular Ca2+ after growth factor addition is not essential for these receptor trafficking functions.
Figure 3.
EGF receptor internalization and down-regulation. (A) Confluent Plcg1+/+ and Plcg1−/− cells were placed in binding medium (DMEM plus 0.1% BSA) and incubated with I125-EGF (2 ng/ml) for the indicated times (1–4.5 min) at 37°C. Low concentrations of I125-EGF and brief incubation times were used to maximize internalization while minimizing recycling. Internalized ligand was measured as described in MATERIALS AND METHODS. (B) Confluent cells were treated as in panel A, but incubated for the indicated periods of time with a saturating concentration of 125I-EGF (20 ng/ml). At each time point, total cell-associated radioactivity was determined by washing with pH 7.0 buffer.
EGF-Induced Mitogenesis and Signaling
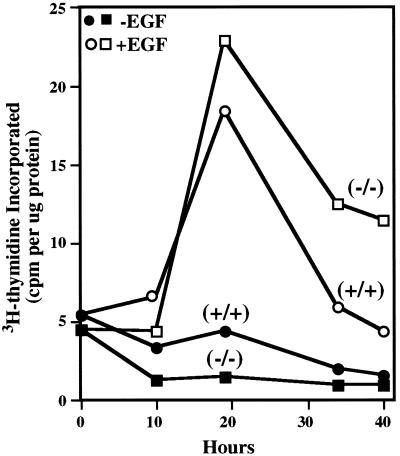
Reports in the literature indicate that PLC-γ1 function is either dispensable or essential for growth factor induction of DNA synthesis in quiescent cells (see DISCUSSION). Therefore, we tested the capacity of serum-arrested Plcg1+/+ or Plcg1−/− cells to enter S phase when stimulated by EGF. The results shown in Figure 4 demonstrate that PLC-γ1 is not required in these cells for EGF to stimulate these quiescent cells to enter DNA synthesis, as judged by the incorporation of 3H-thymidine during 1-h pulse labeling. In this and other experiments, however, DNA synthesis is more prolonged in Plcg1−/− cells than Plcg1+/+cells. 3H-thymidine incorporation at 34–40 h after growth factor addition remains well above basal levels in Plcg1−/− cells, while the incorporation of 3H-thymidine in wild-type cells has returned to basal levels. Clearly, the data show that PLC-γ1 is not essential for growth factor-induced S phase entry of these quiescent cells in culture. DNA synthesis also was measured by bromodeoxyuridine incorporation for 1-h periods, and the results were expressed as the labeling index at 19–20 h, when growth factor-induced DNA synthesis is near maximal, and at 36–37 h, when the stimulation of DNA synthesis has declined. Before the addition of EGF the labeling index was 3–4% for Plcg1+/+ and Plcg1−/− cells. In wild-type cells, the labeling index then increased to 20% at 19 h and subsequently declined to 4% at 36 h. The labeling index for Plcg1 null cells was slightly higher (34%) at 19 h, but declined only slightly to 24% at 36 h. At this later time, therefore, the labeling index for the null cells is about 8-fold higher than that of wild-type cells. Hence, Plcg1 null cells demonstrate a prolonged S phase under these conditions. However, when cycling cells grown in standard media (10% serum) are analyzed, there is no increase in the percentage of Plcg1 null cells in S phase.
Figure 4.
EGF-Induction of DNA Synthesis in Plcg1+/+ and Plcg1−/− cells. Confluent cells were incubated in DMEM plus 0.5% fetal bovine serum for 36 h before the addition of EGF (20 ng/ml) or no addition. At each indicated time, cells were incubated for 1 h with 3H-thymidine, and the radioactivity incorporated into trichloroacetic acid-insoluble material was determined as described in MATERIALS AND METHODS.
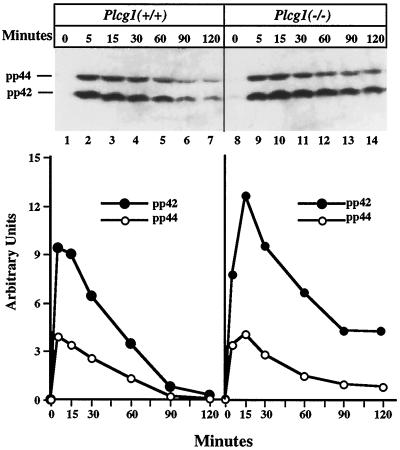
The main pathway for the transduction of intracellular signals generated by growth factors is the activation of MAP kinase, which Huang et al. (1995) have reported is attenuated after activation of FGF receptors unable to associate with and activate PLC-γ1. Therefore, we have assessed the activation of this signaling intermediate in EGF-treated cells. Cells were treated with EGF for varying periods of time, and lysates were probed with antibody to tyrosine-phosphorylated MAP kinase to detect its activation. The results shown in Figure 5 demonstrate that MAP kinase is rapidly activated after growth factor addition to both Plcg1+/+ and Plcg1−/− cells. The time course data suggest that MAP kinase activation is transient in both cell types, but more prolonged in Plcg1−/− cells compared with the wild-type cells. This result is not unlike that observed for the stimulation of DNA synthesis.
Figure 5.
EGF activation of MAP kinase in Plcg1+/+ and Plcg1−/− cells. Cells were incubated overnight in media containing 0.5% fetal bovine serum and then stimulated with EGF (50 ng/ml) for the indicated times. The tyrosine phosphorylated forms of MAP kinase (pp42, pp44) were detected by immunoblotting cell lysates with antibody specific for activated MAP kinase as described in MATERIALS AND METHODS. The upper panel shows the direct blots, while the lower panel presents scanning densitometric quantitation of the primary data.
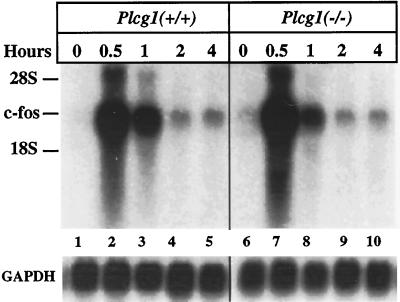
Recently, it has been reported that PLC-γ1 is required for the activation of the c-fos promoter after the addition of platelet-derived growth factor (PDGF) or EGF to different cell lines (Roche et al., 1996; Wang et al., 1998). We have, therefore, measured the growth factor induction c-fos mRNA in Plcg1+/+ and Plcg1−/− cells (Figure 6). Northern blots clearly demonstrate that c-fos is rapidly induced after EGF addition to both cell types. Similar results have been obtained with cells stimulated by PDGF (our unpublished results).
Figure 6.
Induction of c-fos mRNA in Plcg1+/+ and Plcg1−/− cells. Confluent cells were incubated in media containing 0.5% fetal bovine serum for 72 h before the addition of EGF (50 ng/ml) for the indicated times. At each time point, total RNA was isolated and c-fos mRNA was detected by Northern blotting as described in MATERIALS AND METHODS. The blot was also probed for glyceraldehyde-3-phosphate dehydrogenase as a control for loading variation (lower panel).
Cell Migration
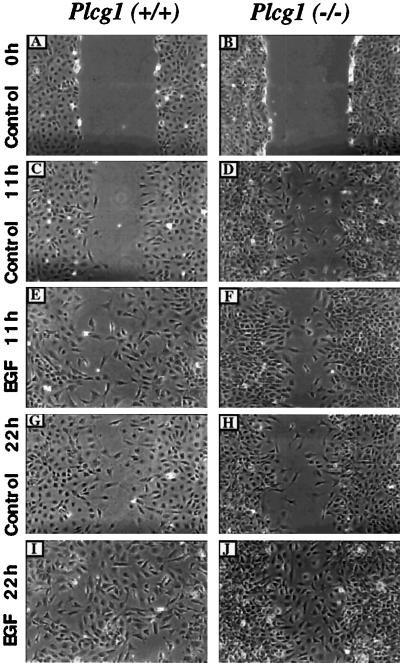
Based on EGF receptor mutants and the application of a PLC inhibitor, several reports (Chen et al., 1994, 1996a) indicate that PLC-γ1 is required for EGF-dependent cell migration. Given these data and the fact that migration is part of the proliferation response, we have assessed the migration capacity of Plcg1+/+ and Plcg1−/− cells in a wound-healing assay. Monolayers of both cell types were “wounded” with a pipette tip (Figure 7, top panel). The cells were then treated with or without EGF and their capacity to migrate into the wound was determined after 11 h and 22 h. In this experiment, Mitomycin C was added at 0 h to prevent cell division. Hence, the movement of cells into the wounded areas depends only on cell migration. As shown at both time points, migration was enhanced by the presence of EGF in both cell lines and by 22 h the wounded areas of Plcg1+/+ and Plcg1−/− cell monolayers were nearly completely occupied for each cell type incubated in the presence of EGF. Therefore, the capacity of cells to migrate under these conditions is not attenuated by the absence of PLC-γ1.
Figure 7.
Influence of PLC-γ1 on cell migration. Plcg1+/+ and Plcg1−/− cells were grown to confluence, incubated overnight in media containing 0.5% fetal bovine serum, and then the monolayer was wounded, as described in MATERIALS AND METHODS. The cultures were washed to remove scraped cells, and DMEM plus 0.1% BSA was added. Mitomycin C (0.5 μg/ml) was also added to prevent subsequent cell division. The indicated cultures were then stimulated with EGF (20 ng/ml) or with no addition (control). At the indicated times, the same wounded area was examined and photographed as described in MATERIALS AND METHODS. Total magnification, 100×.
DISCUSSION
Nearly all growth factor receptor tyrosine kinases produce, when activated, tyrosine phosphorylation and activation of PLC-γ1, which gives rise to a transient increase in IP3, followed by the IP3-dependent mobilization of intracellular free Ca2+. Immortalized MEF prepared from Plcg1−/− mice fail to mobilize intracellular Ca2+ when provoked with EGF (Ji et al., 1997). No growth factor-dependent increase in intracellular free Ca2+ is produced in Plcg1−/− cells in the presence or absence of extracellular Ca2+. When exposed to 1% fresh serum, however, Ca2+ is mobilized in these cells—presumably due to serum components such as lysophosphatidic acid, which activate PLC-β isoforms. Therefore, Plcg1−/− cells do have a functional system for the mobilization of intracellular Ca2+.
Although a sphingosine-dependent, IP3-independent mechanism has been reported to mobilize Ca2+ in response to PDGF (Spiegel et al., 1996), we have not observed Ca2+ mobilization in Plcg1−/− MEF treated with PDGF (our unpublished results). Also, PLC-γ2, which is tyrosine phosphorylated and activated when expressed in fibroblasts (Sultzman et al., 1991; Totzke et al., 1992a, 1992b) or smooth muscle cells (Homma et al., 1993) treated with PDGF, is not detectable by immunoblotting in Plcg1−/− cells. Therefore, we have not observed a PLC-γ1–independent mechanism for Ca2+ mobilization in Plcg1−/− cells when either EGF or PDGF is used as the activating agent.
Intracellular Ca2+ mobilization occurs at both the G1→S and G2→M transitions in the cell cycle (Berridge, 1995). Little is known of the latter, but various indirect experiments suggest a putatively important role for Ca2+ in the G1→S transition (Berridge, 1995). More specifically, various approaches have been employed to assess the role of PLC-γ1, a ubiquitous tyrosine kinase substrate, in mitogenic responses. However, conflicting results have been reported. Initially, mutagenesis of PLC-γ1–specific phosphotyrosine association sites in the FGF receptor, expressed in L6 myoblasts (Mohammadi et al., 1992; Peters et al., 1992), and PDGF receptors, expressed in aortic endothelial cells (Rönnstrand et al., 1992), showed no decrease in growth factor-dependent entry of quiescent cells into DNA synthesis. Mutagenesis of these specific autophosphorylation sites did prevent growth factor-induced PLC-γ1 tyrosine phosphorylation and stimulation of phosphoinositide turnover. These results lead to the conclusion that PLC-γ1 was a dispensable signaling element for the mitogenic response. Others, however, have reported dissimilar conclusions in terms of whether PLC-γ1 is dispensable for this mitogenic response. Two groups (Valius et al., 1993; Alimandi et al., 1997) have reported that mutagenesis of PLC-γ1 association sites on the PDGF receptor decreases DNA synthesis when receptors are expressed in a kidney epithelial cell line or the FDC-P2 myeloid progenitor line. Also, microinjections of antibody to PLC-γ1 (Smith et al., 1990; Roche et al., 1996) or GST-SH2 fusion proteins, which would be expected to act as dominant negative competitive inhibitors of PLC-γ1 (Roche et al., 1996; Wang et al., 1998), are reported to block PDGF or serum induction of DNA synthesis in a manner specific for PLC-γ1. These reports have argued that PLC-γ1 is essential for growth factor induction of DNA synthesis. Reconciling these divergent approaches, results, and conclusions is complicated by the fact that none is entirely specific for PLC-γ1, and the extent of interference in the PLC-γ1 activation is difficult to judge. Our analysis of Plcg1−/− cells, which have no capacity for PLC-γ1 functions, known or unknown, support the view that this signaling molecule is dispensable for mitogenesis, at least in immortalized cell lines.
Interestingly, in some reports DNA synthesis is increased in cells expressing receptors mutated at the PLC-γ1 association site compared with cells expressing wild-type receptors (Peters et al., 1992; Rönnstrand et al., 1992). This observation is not unlike our results with Plcg1−/− cells. Growth factor induction of DNA synthesis is not attenuated in the absence of PLC-γ1, but is consistently increased relative to control Plcg1+/+ cells. Similarly, Chen et al. (1996b) concluded that inhibition of PLC-γ1 activity by a pharmacologic agent, or overexpression of a PLC-γ1 SH2-SH2-SH3 fragment, augmented EGF-induced DNA synthesis, as measured by 3H-thymidine incorporation. Additional experiments are underway to explore the basis of the enhanced level of growth factor-stimulated DNA synthesis during S phase in Plcg1−/− cells.
The experiments of Roche et al. (1996) and Wang et al. (1998) found that PLC-γ1 is required for the PDGF or EGF activation of the c-fos promoter, as measured by a reporter construct. Previously, Schalasta and Doppler (1990) have shown that a putative inhibitor (D609) of phospholipase C activity attenuates EGF-induced c-fos transcription. Our results, in contrast, indicate that EGF or PDGF effectively stimulates the induction of c-fos in quiescent cells in the absence of PLC-γ1 and Ca2+ mobilization.
Cell movement is an essential biological process that growth factors are able to regulate and for which phosphoinositides, free Ca2+, and phospholipase C activity have been implicated as signaling elements to the cytoskeleton (Stossel, 1993; Lauffenburger and Horwitz, 1996). Chen et al. (1994) investigated the role of PLC-γ in EGF-induced cell movement or migration into an acellular area created by wounding a monolayer of the NR-6 variant of 3T3 cells. Based on analysis of transfected EGF receptor deletion mutants, pharmacological inhibition of PLC activity with the drug U73122, antisense reduction of PLC-γ1 levels, and expression of a dominant negative fragment of PLC-γ1, this group concluded that PLC-γ1 function is required for cell migration. Subsequently, gelsolin was identified as a downstream target of EGF-induced PLC-γ1 activity (Chen et al., 1996a). Our assay of cell migration is similar to that employed by Chen et al., and the cell lines used in both studies are mouse fibroblasts; however, the results are, at least qualitatively, discordant. We do not find that PLC-γ1 is necessary for EGF-induced migration.
In summary, our results do not indicate that PLC-γ1 has an essential role in several physiological responses to EGF, including receptor activation and internalization kinetics, the induction of MAP kinase activity and c-fos, the stimulation of DNA synthesis, or cell migration. However, a more general interpretation or extrapolation of the results may have to be limited to these in vitro conditions for several possible reasons. Obviously, immortalized cell lines may have a relaxed requirement for PLC-γ1 signaling compared with the intact organism. Fibroblasts have been recovered from the targeted disruption of several signal-transducing elements and only in the case of c-jun do the nullizygous cells fail to grow in cell culture (Karin et al., 1997). Perhaps the putative redundancy in signaling elements is less frequent in vivo than in established cell lines, due to requirements such as spatial and temporal relationships. Also, cells in culture may, during crisis and the immortalization process, adapt to the absence of a signaling element such as PLC-γ1. At this point, primary cells from Plcg1−/− embryos have not been analyzed due to the very small size of the embryos. Obviously, cell culture limits the cell type that can be examined, and the cell type most affected by the absence of PLC-γ1 in the organism may not be represented in cell lines or even in primary cells. Therefore, the apparent discordance of the PLC-γ1 requirement in vivo and in vitro could reflect the level of its signaling essential for the proliferation of different cell types.
ACKNOWLEDGMENTS
The authors appreciate the efforts of Sue Carpenter in preparation of the manuscript. Drs. Laura Rudolph-Owen and Debra Horstman are thanked for reading the manuscript and contributing helpful comments. The technical assistance of Dr. Heping Yan is appreciated. The grant support of the National Cancer Institute (CA-75195 and CA-68485) is acknowledged.
REFERENCES
- Alimandi M, Heideran MA, Gutkind JS, Zhang J, Ellmore N, Valius M, Kazlauskas A, Pierce JH, Li W. PLC-γ activation is required for PDGF-βR-mediated mitogenesis and monocytic differentiation of myeloid progenitor cells. Oncogene. 1997;15:585–593. doi: 10.1038/sj.onc.1201221. [DOI] [PubMed] [Google Scholar]
- Baulida J, Kraus MH, Alimandi M, Di Fiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium signalling and cell proliferation. BioEssays. 1995;17:491–500. doi: 10.1002/bies.950170605. [DOI] [PubMed] [Google Scholar]
- Carpenter G, Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol. 1976a;88:227–238. doi: 10.1002/jcp.1040880212. [DOI] [PubMed] [Google Scholar]
- Carpenter G, Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976b;71:159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Xie H, Sekar MC, Gupta K, Wells A. Epidermal growth factor receptor-mediated cell motility: phospholipase C activity is required, but mitogen-activated protein kinase activity is not sufficient for induced cell movement. J Cell Biol. 1994;127:847–857. doi: 10.1083/jcb.127.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Murphy-Ullrich JE, Wells A. A role for gelsolin in actuating epidermal growth factor receptor-mediated cell motility. J Cell Biol. 1996a;134:689–698. doi: 10.1083/jcb.134.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Xie H, Wells A. Mitogenic signaling from the EGF receptor is attenuated by a phospholipase C-γ/protein kinase C feedback mechanism. Mol Biol Cell. 1996b;7:871–881. doi: 10.1091/mbc.7.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emori Y, Sorimachi H, Suzuki K. Tissue- and cell type-specific expression of mRNAs for four types of inositol phospholipid-specific phospholipase C. Biochem Biophys Res Commun. 1989;164:406–412. doi: 10.1016/0006-291x(89)91734-8. [DOI] [PubMed] [Google Scholar]
- Essen L-O, Perisic O, Cheung R, Katan M, Williams RL. Crystal structure of a mammalian phosphoinositide-specific phospholipase Cδ. Nature. 1996;380:595–602. doi: 10.1038/380595a0. [DOI] [PubMed] [Google Scholar]
- Homma U, Sakamoto H, Tsunoda M, Aoki M, Takenawa, Ooyama T. Evidence of involvement of phospholipase C-γ2 in signal transduction of platelet-derived growth factor in vascular smooth-muscle cells. Biochem J. 1993;290:649–653. doi: 10.1042/bj2900649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Mohammadi M, Rodrigues GA, Schlessinger J. Reduced activation of RAF-1 and MAP kinase by a fibroblast growth factor receptor mutant deficient in stimulation of phosphatidylinositol hydrolysis. J Biol Chem. 1995;270:5065–5072. doi: 10.1074/jbc.270.10.5065. [DOI] [PubMed] [Google Scholar]
- Ji Q-s, Winnier GE, Niswender KD, Horstman D, Wisdom R, Magnuson MA, Carpenter G. Essential role of the tyrosine kinase substrate phospholipase C-γ1 in mammalian growth and development. Proc Natl Acad Sci USA. 1997;94:2999–3003. doi: 10.1073/pnas.94.7.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat A, Carpenter G. Phospholipase C-γ1: regulation of enzyme function and role in growth factor-dependent signal transduction. Cytokine Growth Factor Rev. 1997;8:109–117. doi: 10.1016/s1359-6101(97)00003-8. [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Z-g, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Dionne CA, Li W, Li N, Spivak T, Honegger AM, Jaye M, Schlessinger J. Point mutation in FGF receptor eliminates phosphatidylinositol hydrolysis without affecting mitogenesis. Nature. 1992;358:681–684. doi: 10.1038/358681a0. [DOI] [PubMed] [Google Scholar]
- Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Peters KG, Marie J, Wilson E, Ives HE, Escobedo J, Del Rosario M, Mirda D, Williams LT. Point mutation of an FGF receptor abolishes phosphatidylinositol turnover and Ca2+ flux but not mitogenesis. Nature. 1992;358:678–681. doi: 10.1038/358678a0. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- Roche S, McGlade J, Jones M, Gish GD, Pawson T, Courtneidge SA. Requirement of phospholipase Cγ, the tyrosine phosphatase Syp and the adaptor proteins Shc and Nck for PDGF-induced DNA synthesis: evidence for Ras-independent pathways. EMBO J. 1996;15:4940–4948. [PMC free article] [PubMed] [Google Scholar]
- Rönnstrand L, Mori S, Arridsson A-K, Eriksson A, Wernstedt C, Hellman U, Claesson-Welsh L, Heldin C-H. Identification of two C-terminal autophosphorylation sites in the PDGF β-receptor: involvement in the interaction with phospholipase C-γ. EMBO J. 1992;11:3911–3919. doi: 10.1002/j.1460-2075.1992.tb05484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage CR, Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972;247:7609–7611. [PubMed] [Google Scholar]
- Schalasta G, Doppler C. Inhibition of c-fos transcription and phosphorylation of the serum response factor by an inhibitor of phospholipase C-type reactions. Mol Cell Biol. 1990;10:5558–5561. doi: 10.1128/mcb.10.10.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Liu Y-L, Kim H, Rhee SG, Kung H-F. Inhibition of serum- and ras-stimulated DNA synthesis by antibodies to phospholipase C. Science. 1990;247:1074–1077. doi: 10.1126/science.2408147. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Waters CM. Endocytosis of growth factor receptors. Bioessays. 1993;15:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- Sorokin A, Mohammadi M, Huang J, Schlessinger J. Internalization of fibroblast growth factor receptor is inhibited by a point mutation at tyrosine 766. J Biol Chem. 1994;269:17056–17061. [PubMed] [Google Scholar]
- Spiegel S, Foster D, Kolesnick R. Signal transduction through lipid second messengers. Curr Opin Cell Biol. 1996;8:159–167. doi: 10.1016/s0955-0674(96)80061-5. [DOI] [PubMed] [Google Scholar]
- Stoscheck CM, Carpenter G. Characteristics of antibodies to the epidermal growth factor receptor-kinase. Arch Biochem Biophys. 1983;227:457–468. doi: 10.1016/0003-9861(83)90476-9. [DOI] [PubMed] [Google Scholar]
- Stossel TP. On the crawling of animal cells. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Sultzman L, Ellis C, Lin L-L, Pawson T, Knopf J. Platelet-derived growth factor increases the in vivo activity of phospholipase C-γ1 and phospholipase C-γ2. Mol Cell Biol. 1991;11:2018–2025. doi: 10.1128/mcb.11.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G, Green H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totzke F, Hug H, Fitzke E, Marmé D, Dieter P. Over-expression of human phospholipase-C-γ2 enhances platelet-derived growth factor-induced mobilization of intracellular Ca2+ and the release of arachidonic acid and prostaglandins in NIH 3T3 fibroblasts. FEBS Lett. 1992;308:125–129. doi: 10.1016/0014-5793(92)81258-n. [DOI] [PubMed] [Google Scholar]
- Totzke F, Marmé D, Hug H. Inducible expression of human phospholipase C-γ2 and its activation by platelet-derived growth factor B-chain homodimer and platelet-derived growth factor a-chain homodimer in transfected NIH 3T3 fibroblasts. Eur J Biochem. 1992;203:633–639. doi: 10.1111/j.1432-1033.1992.tb16593.x. [DOI] [PubMed] [Google Scholar]
- Valius M, Bazenet C, Kazlauskas A. Tyrosines 1021 and 1009 are phosphorylation sites in the carboxy terminus of the platelet-derived growth factor receptor β subunit and are required for binding of phospholipase Cγ and a 64-kilodalton protein, respectively. Mol Cell Biol. 1993;13:133–143. doi: 10.1128/mcb.13.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Glück S, Zhang L, Moran MF. Requirement for phospholipase C-γ1 enzymatic activity in growth factor induced mitogenesis. Mol Cell Biol. 1998;18:590–597. doi: 10.1128/mcb.18.1.590. [DOI] [PMC free article] [PubMed] [Google Scholar]