Abstract
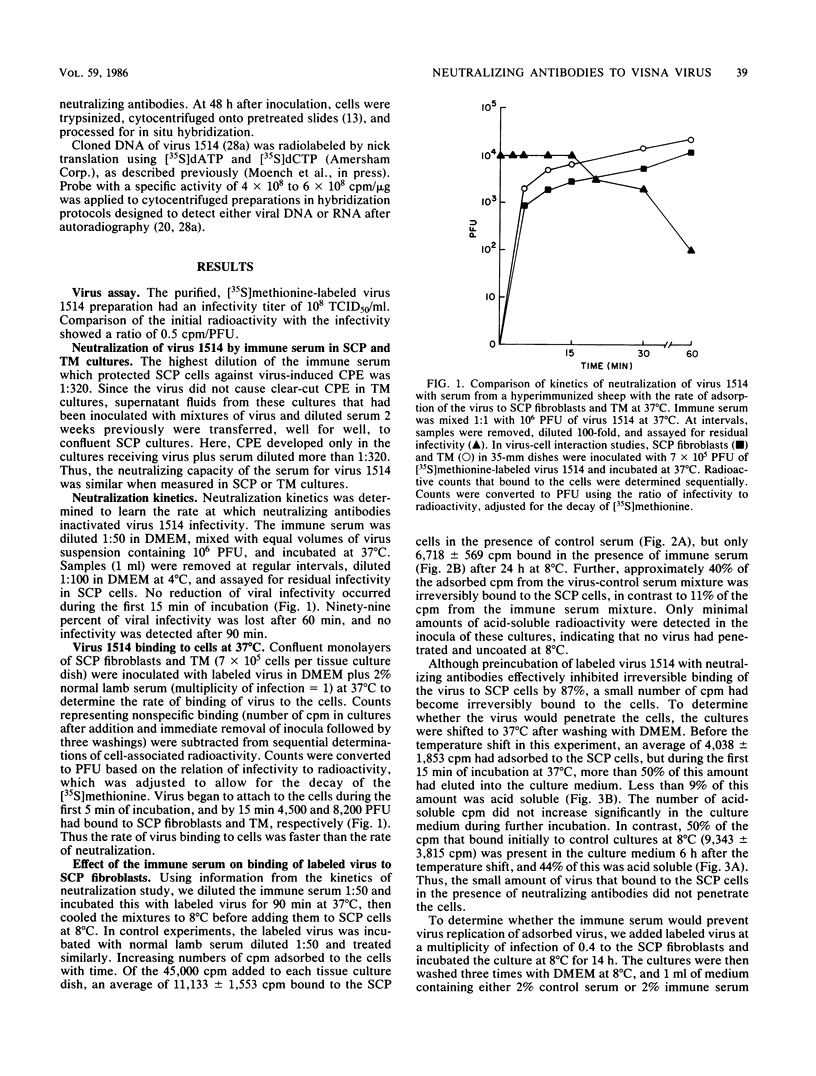
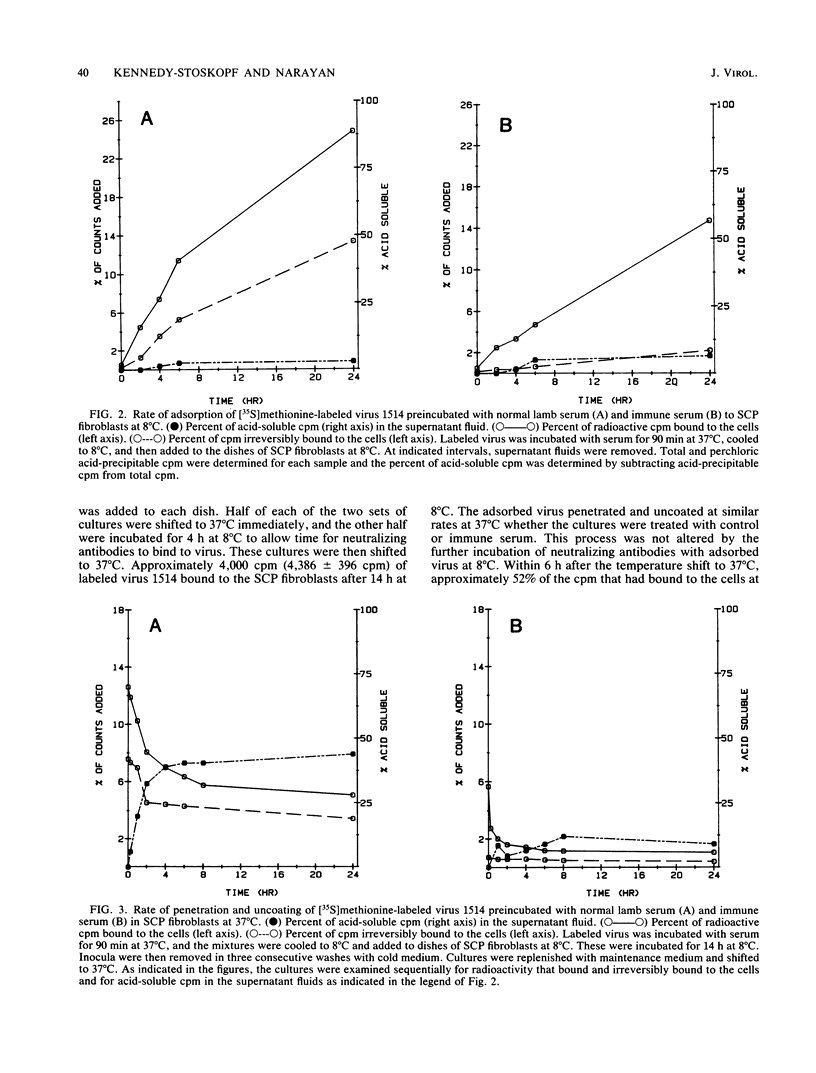
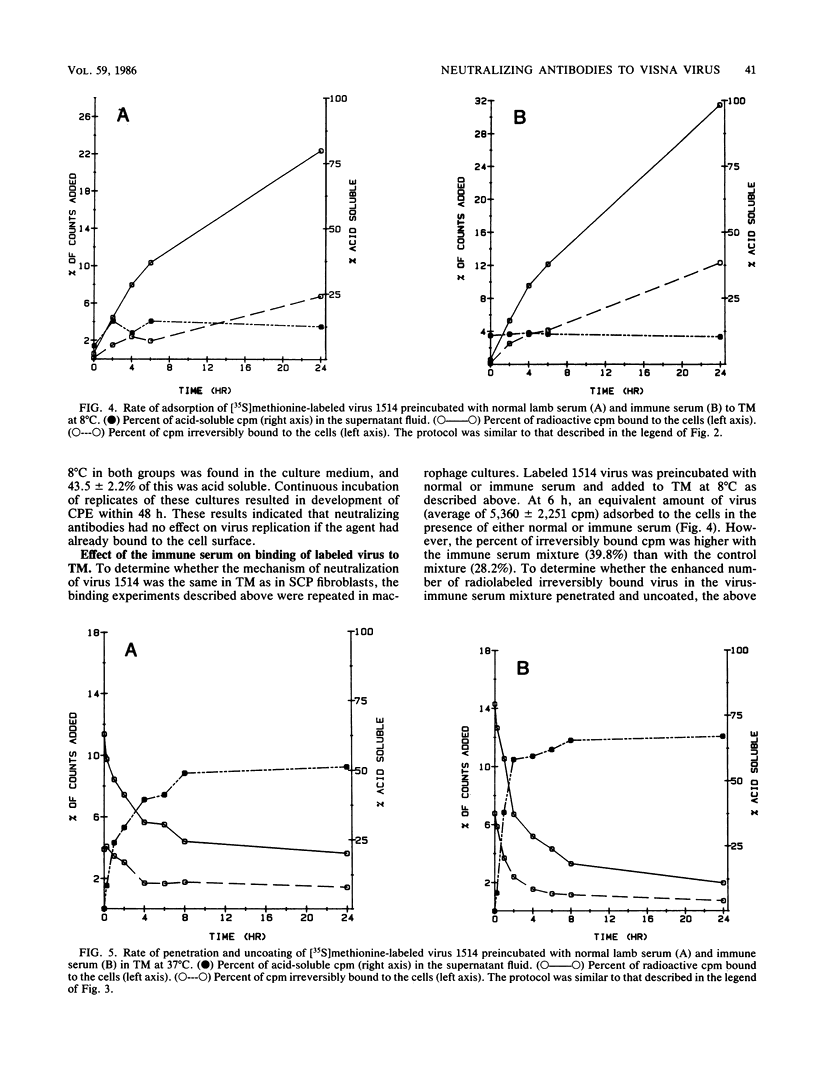
Lentiviruses are nononcogenic retroviruses that cause persistent infections and slowly progressive diseases. Visna virus, a lentivirus of sheep, persists in cells of the macrophage lineage despite the presence of neutralizing antibodies in the animal. These antibodies are measured by prevention of virus replication in sheep fibroblast cell cultures. In this study we have compared the antiviral properties of the antibodies in sheep fibroblast and macrophage cell cultures, the latter being more relevant to infection in the animal. Using infectivity assays, binding of radiolabeled virus to cell membranes, cellular processing of labeled virus into acid-precipitable and acid-soluble components, and in situ hybridization of viral nucleic acid, we show that the antibodies prevented virus replication in both fibroblasts and macrophages. However, the site of neutralization differed between the two cell types. In fibroblasts, the site of virus neutralization was at the cell membrane, when the antibodies prevented virus attachment. In macrophages, virus incubated with the antibodies was phagocytized rapidly, followed by uncoating of the virions. However, virus RNA was not transcribed. Despite this ability of the antibodies to abort virus replication in macrophages, the kinetics of binding of the antibodies to the virus was much slower than the binding of virus to the macrophages. Therefore, persistent virus replication in immune sheep may be the result of virus spreading from macrophage to macrophage before the agent can be neutralized by antibodies in the plasma.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. B., Nexø B. A. Entry of murine retrovirus into mouse fibroblasts. Virology. 1983 Feb;125(1):85–98. doi: 10.1016/0042-6822(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Bowen D. L., Lane H. C., Fauci A. S. Immunopathogenesis of the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Nov;103(5):704–709. doi: 10.7326/0003-4819-103-5-704. [DOI] [PubMed] [Google Scholar]
- Cheevers W. P., Archer B. G., Crawford T. B. Characterization of RNA from equine infectious anemia virus. J Virol. 1977 Nov;24(2):489–497. doi: 10.1128/jvi.24.2.489-497.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheevers W. P., McGuire T. C. Equine infectious anemia virus: immunopathogenesis and persistence. Rev Infect Dis. 1985 Jan-Feb;7(1):83–88. doi: 10.1093/clinids/7.1.83. [DOI] [PubMed] [Google Scholar]
- Chippaux-Hyppolite C., Taranger C., Tamalet J., Pautrat G., Brahic M. Aspects ultrastructuraux du virus visna en cultures cellulaires. Ann Inst Pasteur (Paris) 1972 Sep;123(3):409–420. [PubMed] [Google Scholar]
- Clements J. E., Narayan O., Cork L. C. Biochemical characterization of the virus causing leukoencephalitis and arthritis in goats. J Gen Virol. 1980 Oct;50(2):423–427. doi: 10.1099/0022-1317-50-2-423. [DOI] [PubMed] [Google Scholar]
- Cork L. C., Narayan O. The pathogenesis of viral leukoencephalomyelitis-arthritis of goats. I. Persistent viral infection with progressive pathologic changes. Lab Invest. 1980 Jun;42(6):596–602. [PubMed] [Google Scholar]
- Crawford T. B., Adams D. S. Caprine arthritis-encephalitis: clinical features and presence of antibody in selected goat populations. J Am Vet Med Assoc. 1981 Apr 1;178(7):713–719. [PubMed] [Google Scholar]
- Curran J. W. The epidemiology and prevention of the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Nov;103(5):657–662. doi: 10.7326/0003-4819-103-5-657. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Wong-Staal F. A human T-lymphotropic retrovirus (HTLV-III) as the cause of the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Nov;103(5):679–689. doi: 10.7326/0003-4819-103-5-679. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Moench T. R., Narayan O., Griffin D. E. Selection of a fixative for identifying T cell subsets, B cells, and macrophages in paraffin-embedded mouse spleen. J Immunol Methods. 1983 Dec 16;65(1-2):137–145. doi: 10.1016/0022-1759(83)90310-1. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Clements J. E., Pezeshkpour G. H. Slow virus-macrophage interactions. Characterization of a transformed cell line of sheep alveolar macrophages that express a marker for susceptibility to ovine-caprine lentivirus infections. Lab Invest. 1984 Nov;51(5):547–555. [PubMed] [Google Scholar]
- Gollins S. W., Porterfield J. S. Flavivirus infection enhancement in macrophages: radioactive and biological studies on the effect of antibody on viral fate. J Gen Virol. 1984 Aug;65(Pt 8):1261–1272. doi: 10.1099/0022-1317-65-8-1261. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Wong-Staal F., Gallo R. C., Clements J. E., Narayan O., Gilden R. V. Sequence homology and morphologic similarity of HTLV-III and visna virus, a pathogenic lentivirus. Science. 1985 Jan 11;227(4683):173–177. doi: 10.1126/science.2981428. [DOI] [PubMed] [Google Scholar]
- Griffin D. E., Narayan O., Adams R. J. Early immune responses in visna, a slow viral disease of sheep. J Infect Dis. 1978 Sep;138(3):340–350. doi: 10.1093/infdis/138.3.340. [DOI] [PubMed] [Google Scholar]
- Gudnadóttir M., Pálsson P. A. Host-virus interaction in visna infected sheep. J Immunol. 1965 Dec;95(6):1116–1120. [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Harris J. D., Traynor B., Ventura P., Peluso R., Brahic M. Visna DNA synthesis and the tempo of infection in vitro. Virology. 1982 Jun;119(2):399–410. doi: 10.1016/0042-6822(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Haase A. T. The slow infection caused by visna virus. Curr Top Microbiol Immunol. 1975;72:101–156. doi: 10.1007/978-3-642-66289-8_4. [DOI] [PubMed] [Google Scholar]
- Harter D. H., Choppin P. W. Cell-fusing activity of visna virus particles. Virology. 1967 Feb;31(2):279–288. doi: 10.1016/0042-6822(67)90172-9. [DOI] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevjer-Anderson P., McGuire T. C. Neutralizing antibody response of rabbits and goats to caprine arthritis-encephalitis virus. Infect Immun. 1982 Nov;38(2):455–461. doi: 10.1128/iai.38.2.455-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAFFERTY K. J. THE INTERACTION BETWEEN VIRUS AND ANTIBODY. I. KINETIC STUDIES. Virology. 1963 Sep;21:61–75. doi: 10.1016/0042-6822(63)90305-2. [DOI] [PubMed] [Google Scholar]
- Lutley R., Pétursson G., Pálsson P. A., Georgsson G., Klein J., Nathanson N. Antigenic drift in visna: virus variation during long-term infection of Icelandic sheep. J Gen Virol. 1983 Jul;64(Pt 7):1433–1440. doi: 10.1099/0022-1317-64-7-1433. [DOI] [PubMed] [Google Scholar]
- Mandel B. The interaction of neutralized poliovirus with HeLa cells. I. Adsorption. Virology. 1967 Feb;31(2):238–247. doi: 10.1016/0042-6822(67)90167-5. [DOI] [PubMed] [Google Scholar]
- Mehta P. D., Thormar H. Neutralizing activity in isolated serum antibody fractions from visna-infected sheep. Infect Immun. 1974 Sep;10(3):678–680. doi: 10.1128/iai.10.3.678-680.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench T. R., Gendelman H. E., Clements J. E., Narayan O., Griffin D. E. Efficiency of in situ hybridization as a function of probe size and fixation technique. J Virol Methods. 1985 Jun;11(2):119–130. doi: 10.1016/0166-0934(85)90035-7. [DOI] [PubMed] [Google Scholar]
- Molineaux S., Clements J. E. Molecular cloning of unintegrated visna viral DNA and characterization of frequent deletions in the 3' terminus. Gene. 1983 Aug;23(2):137–148. doi: 10.1016/0378-1119(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Narayan O., Clements J. E., Griffin D. E., Wolinsky J. S. Neutralizing antibody spectrum determines the antigenic profiles of emerging mutants of visna virus. Infect Immun. 1981 Jun;32(3):1045–1050. doi: 10.1128/iai.32.3.1045-1050.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Chase J. Antigenic shift of visna virus in persistently infected sheep. Science. 1977 Jul 22;197(4301):376–378. doi: 10.1126/science.195339. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Clements J. E. Virus mutation during 'slow infection': temporal development and characterization of mutants of visna virus recovered from sheep. J Gen Virol. 1978 Nov;41(2):343–352. doi: 10.1099/0022-1317-41-2-343. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Silverstein A. M. Slow virus infection: replication and mechanisms of persistence of visna virus in sheep. J Infect Dis. 1977 May;135(5):800–806. doi: 10.1093/infdis/135.5.800. [DOI] [PubMed] [Google Scholar]
- Narayan O., Kennedy-Stoskopf S., Sheffer D., Griffin D. E., Clements J. E. Activation of caprine arthritis-encephalitis virus expression during maturation of monocytes to macrophages. Infect Immun. 1983 Jul;41(1):67–73. doi: 10.1128/iai.41.1.67-73.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Sheffer D., Griffin D. E., Clements J., Hess J. Lack of neutralizing antibodies to caprine arthritis-encephalitis lentivirus in persistently infected goats can be overcome by immunization with inactivated Mycobacterium tuberculosis. J Virol. 1984 Feb;49(2):349–355. doi: 10.1128/jvi.49.2.349-355.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Wolinsky J. S., Clements J. E., Strandberg J. D., Griffin D. E., Cork L. C. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol. 1982 Apr;59(Pt 2):345–356. doi: 10.1099/0022-1317-59-2-345. [DOI] [PubMed] [Google Scholar]
- Nathanson N., Panitch H., Palsson P. A., Petursson G., Georgsson G. Pathogenesis of visna. II. Effect of immunosuppression upon early central nervous system lesions. Lab Invest. 1976 Nov;35(5):444–451. [PubMed] [Google Scholar]
- Pétursson G., Nathanson N., Georgsson G., Panitch H., Pálsson P. A. Pathogenesis of visna. I. Sequential virologic, serologic, and pathologic studies. Lab Invest. 1976 Oct;35(4):402–412. [PubMed] [Google Scholar]
- RUBIN H., FRANKLIN R. M. On the mechanism of Newcastle disease virus neutralization by immune serum. Virology. 1957 Feb;3(1):84–95. doi: 10.1016/0042-6822(57)90025-9. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Brown M., Gallo R. C. HTLV-III-neutralizing antibodies in patients with AIDS and AIDS-related complex. Nature. 1985 Jul 4;316(6023):72–74. doi: 10.1038/316072a0. [DOI] [PubMed] [Google Scholar]
- SIGURDSSON B., PALSSON P., GRIMSSON H. Visna, a demyelinating transmissible disease of sheep. J Neuropathol Exp Neurol. 1957 Jul;16(3):389–403. doi: 10.1097/00005072-195707000-00010. [DOI] [PubMed] [Google Scholar]
- THORMAR H. The growth cycle of visna virus in monolayer cultures of sheep cells. Virology. 1963 Mar;19:273–278. doi: 10.1016/0042-6822(63)90064-3. [DOI] [PubMed] [Google Scholar]
- Thormar H., Barshatzky M. R., Arnesen K., Kozlowski P. B. The emergence of antigenic variants is a rare event in long-term visna virus infection in vivo. J Gen Virol. 1983 Jul;64(Pt 7):1427–1432. doi: 10.1099/0022-1317-64-7-1427. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Clapham P. R., Cheingsong-Popov R., Dalgleish A. G., Carne C. A., Weller I. V., Tedder R. S. Neutralization of human T-lymphotropic virus type III by sera of AIDS and AIDS-risk patients. Nature. 1985 Jul 4;316(6023):69–72. doi: 10.1038/316069a0. [DOI] [PubMed] [Google Scholar]



