Abstract
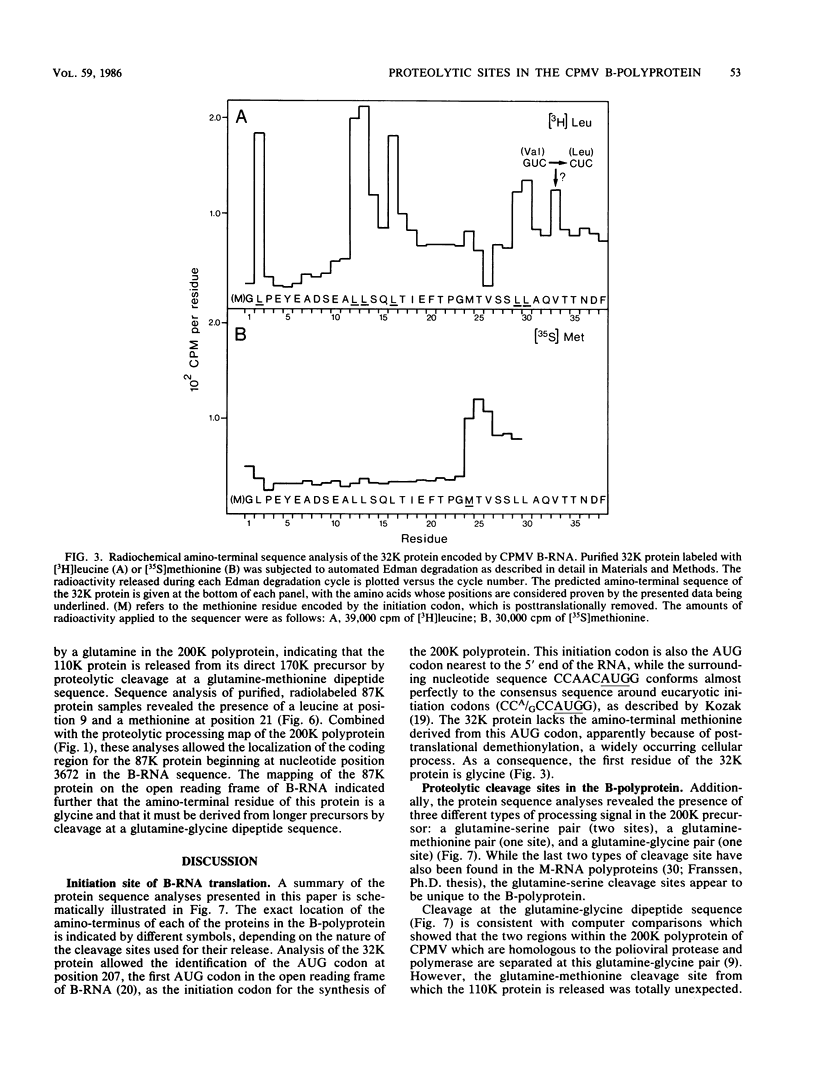
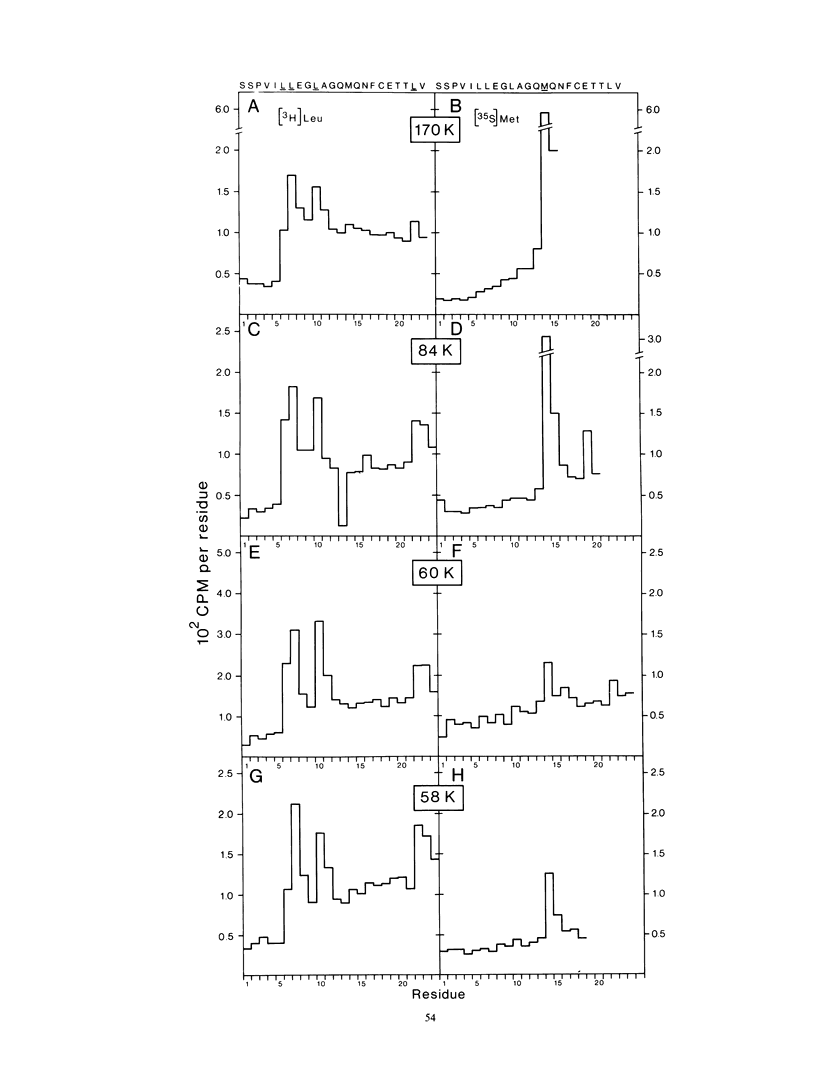
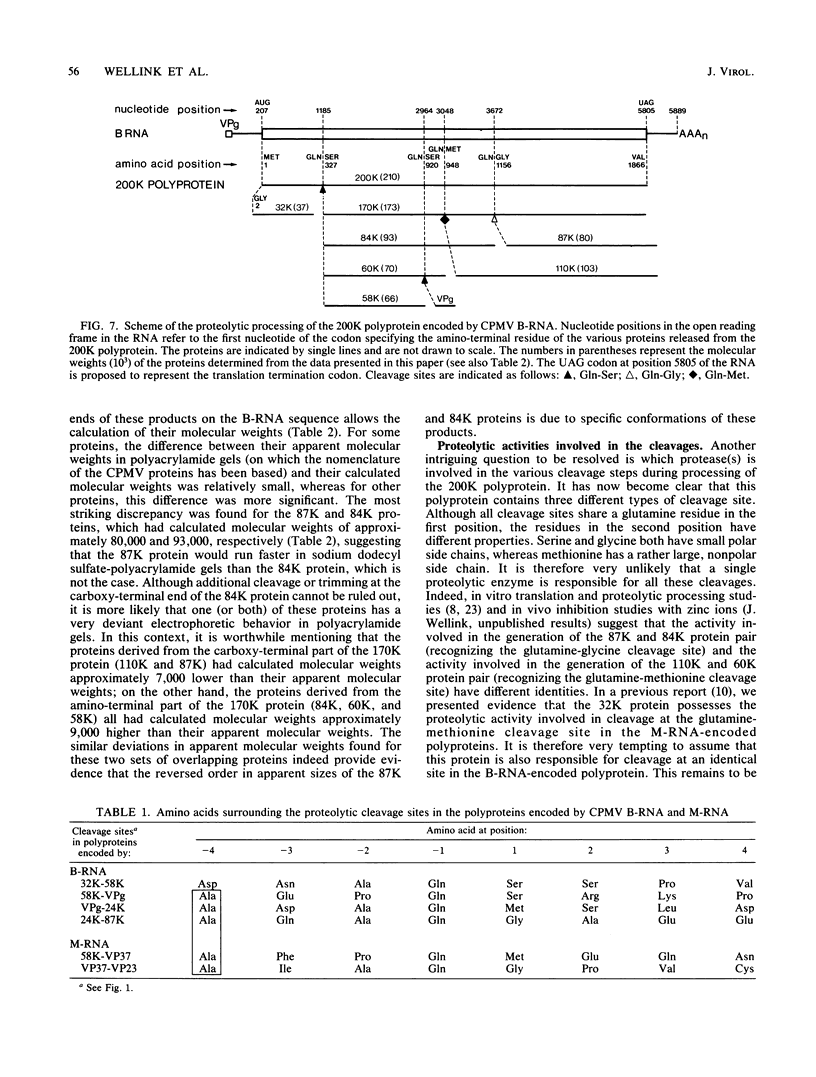
The bottom-component RNA (B-RNA) of cowpea mosaic virus is expressed by the production of a ∼200,000-dalton polyprotein (200K polyprotein), from which the functional proteins are formed by specific proteolytic cleavages. Partial amino-terminal sequences of the various B-RNA-encoded proteins have now been determined. Comparison of the information obtained with the B-RNA sequence allowed the localization of the coding regions for these proteins on B-RNA, the calculation of their precise molecular weights, and the determination of the cleavage sites at which they are released from the polyprotein precursor. Sequence analysis of the 32K protein, which is derived from the amino-terminal end of the 200K polyprotein, indicated that the AUG codon at nucleotide position 207 of the RNA sequence is the translation initiation codon. Sequence analysis of the 170K, 110K, 87K, 84K, 60K, and 58K proteins revealed the existence of three types of cleavage site in the 200K polyprotein: glutamine-serine (two sites), glutamine-methionine (one site), and glutamine-glycine (one site) amino acid pairs. The nature of these cleavage sites suggested that two different viral proteases are involved in the processing of the B-RNA-encoded polyprotein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhown A. S., Mole J. E., Hunter F., Bennett J. C. High-sensitivity sequence determination of proteins quantitatively recovered from sodium dodecyl sulfate gels using an improved electrodialysis procedure. Anal Biochem. 1980 Mar 15;103(1):184–190. doi: 10.1016/0003-2697(80)90254-7. [DOI] [PubMed] [Google Scholar]
- Forss S., Schaller H. A tandem repeat gene in a picornavirus. Nucleic Acids Res. 1982 Oct 25;10(20):6441–6450. doi: 10.1093/nar/10.20.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forss S., Strebel K., Beck E., Schaller H. Nucleotide sequence and genome organization of foot-and-mouth disease virus. Nucleic Acids Res. 1984 Aug 24;12(16):6587–6601. doi: 10.1093/nar/12.16.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H., Goldbach R., Broekhuijsen M., Moerman M., van Kammen A. Expression of Middle-Component RNA of Cowpea Mosaic Virus: In Vitro Generation of a Precursor to Both Capsid Proteins by a Bottom-Component RNA-Encoded Protease from Infected Cells. J Virol. 1982 Jan;41(1):8–17. doi: 10.1128/jvi.41.1.8-17.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H., Leunissen J., Goldbach R., Lomonossoff G., Zimmern D. Homologous sequences in non-structural proteins from cowpea mosaic virus and picornaviruses. EMBO J. 1984 Apr;3(4):855–861. doi: 10.1002/j.1460-2075.1984.tb01896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H., Moerman M., Rezelman G., Goldbach R. Evidence That the 32,000-Dalton Protein Encoded by Bottom-Component RNA of Cowpea Mosaic Virus is a Proteolytic Processing Enzyme. J Virol. 1984 Apr;50(1):183–190. doi: 10.1128/jvi.50.1.183-190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach R. W., Schilthuis J. G., Rezelman G. Comparison of in vivo and in vitro translation of cowpea mosaic virus RNAs. Biochem Biophys Res Commun. 1981 Mar 16;99(1):89–94. doi: 10.1016/0006-291x(81)91716-2. [DOI] [PubMed] [Google Scholar]
- Goldbach R., Rezelman G. Orientation of the cleavage map of the 200-kilodalton polypeptide encoded by the bottom-component RNA of cowpea mosaic virus. J Virol. 1983 May;46(2):614–619. doi: 10.1128/jvi.46.2.614-619.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach R., Rezelman G., Zabel P., van Kammen A. Expression of the Bottom-Component RNA of Cowpea Mosaic Virus: Evidence that the 60-Kilodalton VPg Precursor Is Cleaved into Single VPg and a 58-Kilodalton Polypeptide. J Virol. 1982 May;42(2):630–635. doi: 10.1128/jvi.42.2.630-635.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi T., Rezelman G., Van Kammen A. Infection of cowpea mesophyll protoplasts with cowpea mosaic virus. Virology. 1975 Apr;64(2):308–318. doi: 10.1016/0042-6822(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Adler C. J., Rothberg P. G., Martinko J., Nathenson S. G., Wimmer E. The genome-linked protein of picornaviruses. VII. Genetic mapping of poliovirus VPg by protein and RNA sequence studies. Cell. 1980 Aug;21(1):295–302. doi: 10.1016/0092-8674(80)90137-3. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Klootwijk J., Klein I., Zabel P., van Kammen A. Cowpea mosaic virus RNAs have neither m7GpppN ... nor mono-, di- or triphosphates at their 5' ends. Cell. 1977 May;11(1):73–82. doi: 10.1016/0092-8674(77)90318-x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonossoff G. P., Shanks M. The nucleotide sequence of cowpea mosaic virus B RNA. EMBO J. 1983;2(12):2253–2258. doi: 10.1002/j.1460-2075.1983.tb01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Kirby E. M., Janda M. R., Drake N. L., Duke G. M., Potratz K. F., Collett M. S. The nucleotide and deduced amino acid sequences of the encephalomyocarditis viral polyprotein coding region. Nucleic Acids Res. 1984 Mar 26;12(6):2969–2985. doi: 10.1093/nar/12.6.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Synthesis and proteolytic processing of cowpea mosaic virus proteins in reticulocyte lysates. Virology. 1979 Jul 30;96(2):463–477. doi: 10.1016/0042-6822(79)90104-1. [DOI] [PubMed] [Google Scholar]
- Peng X. X., Shih D. S. Proteolytic processing of the proteins translated from the bottom component RNA of cowpea mosaic virus. The primary and secondary cleavage reactions. J Biol Chem. 1984 Mar 10;259(5):3197–3201. [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezelman G., Goldbach R., Van Kammen A. Expression of bottom component RNA of cowpea mosaic virus in cowpea protoplasts. J Virol. 1980 Nov;36(2):366–373. doi: 10.1128/jvi.36.2.366-373.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Anderson C. W., Kitamura N., Rothberg P. G., Wishart W. L., Wimmer E. Poliovirus replication proteins: RNA sequence encoding P3-1b and the sites of proteolytic processing. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3464–3468. doi: 10.1073/pnas.78.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skern T., Sommergruber W., Blaas D., Gruendler P., Fraundorfer F., Pieler C., Fogy I., Kuechler E. Human rhinovirus 2: complete nucleotide sequence and proteolytic processing signals in the capsid protein region. Nucleic Acids Res. 1985 Mar 25;13(6):2111–2126. doi: 10.1093/nar/13.6.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanway G., Hughes P. J., Mountford R. C., Minor P. D., Almond J. W. The complete nucleotide sequence of a common cold virus: human rhinovirus 14. Nucleic Acids Res. 1984 Oct 25;12(20):7859–7875. doi: 10.1093/nar/12.20.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P., Moerman M., Lomonossoff G., Shanks M., Beyreuther K. Cowpea mosaic virus VPg: sequencing of radiochemically modified protein allows mapping of the gene on B RNA. EMBO J. 1984 Jul;3(7):1629–1634. doi: 10.1002/j.1460-2075.1984.tb02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kammen A. Purification and properties of the components of cowpea mosaic virus. Virology. 1967 Apr;31(4):633–642. doi: 10.1016/0042-6822(67)90192-4. [DOI] [PubMed] [Google Scholar]
- van Wezenbeek P., Verver J., Harmsen J., Vos P., van Kammen A. Primary structure and gene organization of the middle-component RNA of cowpea mosaic virus. EMBO J. 1983;2(6):941–946. doi: 10.1002/j.1460-2075.1983.tb01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]