Abstract
The v-fgr oncogene codes for a unique transforming protein (P70gag-actin-fgr) that contains virus-specific determinants and cell-derived sequences for both a tyrosine-specific kinase domain and an actin domain. We examined the subcellular distribution of the v-fgr protein by immunofluorescence microscopy and various cell fractionation techniques. By immunofluorescence, the v-fgr protein was localized in a diffuse cytoplasmic pattern within transformed cells. The v-fgr protein was not detectable at substratum adhesion sites. Crude membrane preparations (P100) obtained from fgr-transformed cells contained elevated levels of P70gag-actin-fgr. Further analysis of membranes on discontinous sucrose gradients revealed that P70gag-actin-fgr cofractionated with plasma membranes. Using an alternate method of fractionation, we found that the majority of the v-fgr protein remained with the insoluble matrix obtained by treating cells with a buffer containing Triton X-100. When membranes were similarly treated with detergent, nearly all of v-fgr protein remained with the residual insoluble matrix. These results suggest that the transforming activity of P70gag-actin-fgr may be directed to subcellular cytoskeletal targets at or near the cytoplasmic face of the plasma membrane.
Full text
PDF


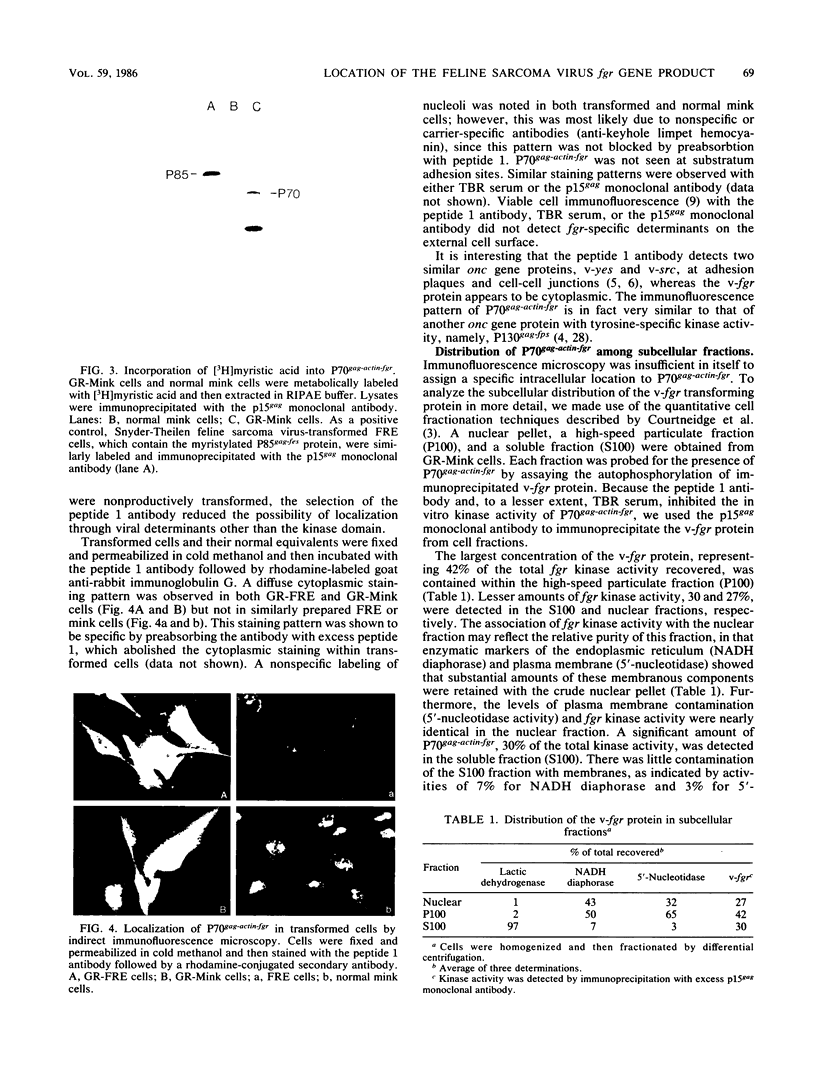



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ze'ev A., Duerr A., Solomon F., Penman S. The outer boundary of the cytoskeleton: a lamina derived from plasma membrane proteins. Cell. 1979 Aug;17(4):859–865. doi: 10.1016/0092-8674(79)90326-x. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Sefton B. M. Myristic acid, a rare fatty acid, is the lipid attached to the transforming protein of Rous sarcoma virus and its cellular homolog. J Virol. 1985 Jan;53(1):7–12. doi: 10.1128/jvi.53.1.7-12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. A., Wang E., Hanafusa H. Cytoplasmic localization of the transforming protein of Fujinami sarcoma virus: salt-sensitive association with subcellular components. J Virol. 1983 Feb;45(2):782–791. doi: 10.1128/jvi.45.2.782-791.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry L. E., Rohrschneider L. R., Casnellie J. E., Krebs E. G. Antibodies to a defined region of pp60src neutralize the tyrosine-specific kinase activity. J Biol Chem. 1983 Sep 25;258(18):11219–11228. [PubMed] [Google Scholar]
- Gentry L. E., Rohrschneider L. R. Common features of the yes and src gene products defined by peptide-specific antibodies. J Virol. 1984 Aug;51(2):539–546. doi: 10.1128/jvi.51.2.539-546.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman I. M., Pollard T. D. Comparison of purified anti-actin and fluorescent-heavy meromyosin staining patterns in dividing cells. J Cell Biol. 1979 Mar;80(3):509–520. doi: 10.1083/jcb.80.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Kitamura A., Toyoshima K., Hirayama Y., Yoshida M. Avian sarcoma virus Y73 genome sequence and structural similarity of its transforming gene product to that of Rous sarcoma virus. Nature. 1982 May 20;297(5863):205–208. doi: 10.1038/297205a0. [DOI] [PubMed] [Google Scholar]
- Manger R., Najita L., Nichols E. J., Hakomori S., Rohrschneider L. Cell surface expression of the McDonough strain of feline sarcoma virus fms gene product (gp 140fms). Cell. 1984 Dec;39(2 Pt 1):327–337. doi: 10.1016/0092-8674(84)90011-4. [DOI] [PubMed] [Google Scholar]
- Mescher M. F., Jose M. J., Balk S. P. Actin-containing matrix associated with the plasma membrane of murine tumour and lymphoid cells. Nature. 1981 Jan 15;289(5794):139–144. doi: 10.1038/289139a0. [DOI] [PubMed] [Google Scholar]
- Naharro G., Dunn C. Y., Robbins K. C. Analysis of the primary translational product and integrated DNA of a new feline sarcoma virus, GR-FeSV. Virology. 1983 Mar;125(2):502–507. doi: 10.1016/0042-6822(83)90223-4. [DOI] [PubMed] [Google Scholar]
- Naharro G., Robbins K. C., Reddy E. P. Gene product of v-fgr onc: hybrid protein containing a portion of actin and a tyrosine-specific protein kinase. Science. 1984 Jan 6;223(4631):63–66. doi: 10.1126/science.6318314. [DOI] [PubMed] [Google Scholar]
- Osborn M., Born T., Koitsch H. J., Weber K. Stereo immunofluorescence microscopy: I. Three-dimensional arrangement of microfilaments, microtubules and tonofilaments. Cell. 1978 Jul;14(3):477–488. doi: 10.1016/0092-8674(78)90234-9. [DOI] [PubMed] [Google Scholar]
- Radke K., Carter V. C., Moss P., Dehazya P., Schliwa M., Martin G. S. Membrane association of a 36,000-dalton substrate for tyrosine phosphorylation in chicken embryo fibroblasts transformed by avian sarcoma viruses. J Cell Biol. 1983 Nov;97(5 Pt 1):1601–1611. doi: 10.1083/jcb.97.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Barbacid M., Aaronson S., Gardner M. B. Origin and biological properties of a new feline sarcoma virus. Virology. 1982 Feb;117(1):238–244. doi: 10.1016/0042-6822(82)90522-0. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L. R. Adhesion plaques of Rous sarcoma virus-transformed cells contain the src gene product. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3514–3518. doi: 10.1073/pnas.77.6.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R., Najita L. M. Detection of the v-abl gene product at cell-substratum contact sites in Abelson murine leukemia virus-transformed fibroblasts. J Virol. 1984 Aug;51(2):547–552. doi: 10.1128/jvi.51.2.547-552.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M., van Blerkom J. Structural interaction of cytoskeletal components. J Cell Biol. 1981 Jul;90(1):222–235. doi: 10.1083/jcb.90.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A., Oroszlan S. Myristylation of gag-onc fusion proteins in mammalian transforming retroviruses. Virology. 1984 Mar;133(2):431–437. doi: 10.1016/0042-6822(84)90409-4. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Trowbridge I. S., Cooper J. A., Scolnick E. M. The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell. 1982 Dec;31(2 Pt 1):465–474. doi: 10.1016/0092-8674(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Unkeless J. C. Partial purification of a lipoprotein with 5'-nucleotidase activity from membranes of rat liver cells. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1050–1057. doi: 10.1073/pnas.61.3.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Yamada S. S., Davies P. J., Rutherford A. V., Gallo M. G., Pastan I. Intracellular localization of actin in cultured fibroblasts by electron microscopic immunocytochemistry. J Histochem Cytochem. 1981 Jan;29(1):17–37. doi: 10.1177/29.1.7009728. [DOI] [PubMed] [Google Scholar]
- Woolford J., Beemon K. Transforming proteins of fujinami and PRCII avian sarcoma viruses have different subcellular locations. Virology. 1984 May;135(1):168–180. doi: 10.1016/0042-6822(84)90127-2. [DOI] [PubMed] [Google Scholar]
- Yu J., Fischman D. A., Steck T. L. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct. 1973;1(3):233–248. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]