Abstract
In Saccharomyces cerevisiae, the Mps1p protein kinase is critical for both spindle pole body (SPB) duplication and the mitotic spindle assembly checkpoint. The mps1–1 mutation causes failure early in SPB duplication, and because the spindle assembly checkpoint is also compromised, mps1–1 cells proceed with a monopolar mitosis and rapidly lose viability. Here we report the genetic and molecular characterization of mps1–1 and five new temperature-sensitive alleles of MPS1. Each of the six alleles contains a single point mutation in the region of the gene encoding the protein kinase domain. The mutations affect several residues conserved among protein kinases, most notably the invariant glutamate in subdomain III. In vivo and in vitro kinase activity of the six epitope-tagged mutant proteins varies widely. Only two display appreciable in vitro activity, and interestingly, this activity is not thermolabile under the assay conditions used. While five of the six alleles cause SPB duplication to fail early, yielding cells with a single SPB, mps1–737 cells proceed into SPB duplication and assemble a second SPB that is structurally defective. This phenotype, together with the observation of intragenic complementation between this unique allele and two others, suggests that Mps1p is required for multiple events in SPB duplication.
INTRODUCTION
The MPS1 gene of Saccharomyces cerevisiae encodes an essential protein kinase with roles in spindle pole body (SPB)1 duplication and in the spindle assembly checkpoint (Winey et al., 1991; Weiss and Winey, 1996). The N terminus of Mps1p is unique, and its C-terminal kinase domain shares greatest homology with the catalytic domains of the mammalian kinases esk and PYT/TTK and the Arabidopsis thaliana kinase PPK1 (Poch et al., 1994; Lauzéet al., 1995; Kwart, personal communication). Across the kinase domain, these proteins exhibit approximately 40% identity and 60% similarity, but functional homology has not yet been demonstrated (Bachant and Winey, personal communication). Murine esk and human PYT/TTK are nearly identical kinases that are expressed primarily in proliferating cells, either transformed or stem cells (Douville et al., 1992; Mills et al., 1992; Lindberg et al., 1993; Hogg et al., 1994). Like these mammalian enzymes, Mps1p is a dual-specificity kinase. Mps1p will phosphorylate itself and the exogenous substrate myelin basic protein (MBP) on serine and threonine and, to a lesser extent, will autophosphorylate tyrosine residues (Lauzéet al., 1995).
Duplication of the SPB, the organelle that acts as the centrosome or microtubule-organizing center in budding yeast, requires the Mps1p kinase. The SPB is a trilaminar structure that remains embedded in the nuclear envelope throughout the life cycle of the yeast, and it is duplicated once during G1 of each cell cycle to provide the two poles of the mitotic spindle. Electron microscopic examination reveals several visible events in duplication (Byers and Goetsch, 1974; Winey and Byers, 1993). The first step observed is satellite formation, the appearance of a clump of material thought to be the precursor of the new SPB. The satellite forms on the cytoplasmic surface of the half-bridge, a thickened region of nuclear envelope adjacent to the SPB. This satellite-bearing SPB morphology is also observed when cells are arrested at Start in G1 by exposure to the mating pheromone α-factor. Duplication is completed after passage through Start, yielding side-by-side SPBs joined together by a complete bridge. Later, the SPBs separate and migrate apart to organize the mitotic spindle.
Cells with the temperature-sensitive-for-growth (ts) mps1–1 mutation fail early in SPB duplication at the nonpermissive temperature and form a monopolar spindle (Winey et al., 1991). Electron microscopy of these cells reveals a single, large SPB with an enlarged and very prominent half-bridge structure. Order-of-function experiments indicate that MPS1 is required for the transition from satellite-bearing to side-by-side SPBs. When mps1–1 cells are first arrested in G1 with satellite-bearing SPBs by α-factor treatment and then released from that block at the nonpermissive temperature, they fail in SPB duplication (Winey et al., 1991). Maintenance or stability of the satellite appears to be affected, because the existing satellite from α-factor arrest is lost. Mutations that affect SPB duplication later in the pathway have also been identified. In mps2 or ndc1 mutants, duplication proceeds much farther but produces a defective SPB that is not inserted into the nuclear envelope (Winey et al., 1991, 1993).
Mps1p is essential for successful SPB duplication in every cell cycle. In addition, this protein performs a nonessential function in the spindle assembly checkpoint. This checkpoint initiates a mitotic cell cycle arrest in response to SPB duplication failure or microtubule-depolymerizing drugs, preventing aberrant cell division (Hoyt et al., 1991; Li and Murray, 1991; Weiss and Winey, 1996). At the nonpermissive temperature, checkpoint function is lost and mps1–1 cells that have failed in SPB duplication cannot arrest. Instead, they carry out a monopolar mitosis with gross defects in chromosome segregation, leading to a drastic drop in viability (Winey et al., 1991). Unlike the role of Mps1p in SPB duplication, its checkpoint function is only required when spindle integrity has been compromised (Weiss and Winey, 1996). Strong overexpression of wild-type, epitope-tagged Mps1p causes mitotic arrest through ectopic activation of the checkpoint, and this arrest correlates with phosphorylation of the checkpoint protein Mad1p (Hardwick et al., 1996).
We have characterized a collection of ts alleles of the MPS1 gene hoping to learn more about the functions of the protein kinase it encodes. A total of 15 isolates were sequenced and found to define exactly six alleles of the gene. Each contains a single mutation in the protein kinase domain. Several conserved residues are affected, and these changes cause varying effects on the in vitro and in vivo enzymatic activity of epitope-tagged Mps1p. Most mps1 mutants display the single enlarged SPB observed previously (Winey et al., 1991), but the SPB morphology in mps1–737 cells is strikingly different. These cells produce a second, defective SPB similar to that reported for mps2 and ndc1 mutants (Winey et al., 1991, 1993). This genetic and molecular analysis of MPS1 is not only informative about the structure and function of protein kinases, but also reveals a previously unknown requirement for Mps1p in SPB duplication.
MATERIALS AND METHODS
Yeast Strains, Cell Culture, and Genetic Techniques
The yeast strains used in this study are listed in Table 1. Yeast culture and genetic and molecular techniques were as described by Ausubel et al. (1994) and Elble (1992). Escherichia coli DH5α (Sikorski and Hieter, 1989) was cultured and transformed as described by Ausubel et al. (1994). Temperature-sensitive mps1 isolates were successively outcrossed to S288c-derived wild-type strains (Table 1) to eliminate any irrelevant ts mutations present in these heavily mutagenized strains (generally, four to six outcrosses).
Table 1.
Yeast strain list
| Strain | Genotype | Source |
|---|---|---|
| 256 RM124 | α his4-519 ura2 leu1 ade1 arg4 | M. Culbertsona |
| 508 5007/2 | α his4-212 ade2-1 (ochre) | M. Culbertsona |
| 1640 R39 | a his4-209 HOL1 | M. Culbertsona |
| 1870 LM739-1B | a his4-504 leu2-3 ura3-52 | M. Culbertsona |
| AS102-5b | a mps1-585 lys2 leu2-3 | This studyb |
| AS106-8b | a mps1-773 tyr1 | This studyb |
| AS107-1a | a mps1-1169 tyr1 | This studyb |
| AS112-5b | a mps1-502 lys2-801 ura3-52 | This studyb |
| AS114-2a | a mps1-676 lys2-801 | This studyb |
| AS115-4c | a mps1-688 lys2-801 ura3-52 | This studyb |
| AS121-1a | a mps1-422 lys2 leu2-3,112 ura3-52 his3Δ200 trp1Δ1 | This studyc |
| AS123-4a | a mps1-3497 leu2-3 | This studyd |
| AS126-2b | α mps1-1237 ura3-52 leu2-3,112 his3Δ200 | Schutz et al. (1997) |
| AS126-5a | a mps1-1237 ura3-52 leu2-3,112 lys2 | This studyb |
| AS127-1a | α mps1-412 ura3-52 his3Δ200 leu2-3,112 trp1Δ1 | This studyc |
| AS127-1d | a mps1-412 ura3-52 his3Δ200 leu2-3,112 trp1Δ1 | This studyc |
| AS131-2d | a mps1-3796 ura3-52 his3 leu2-3,112 | This studyd |
| AS131-5b | α mps1-3796 ura3-52 his3 trp1Δ1 | This studyd |
| AS132-3a | a mps1-737 ura3-52 his3Δ200 leu2-3,112 | This studyb |
| AS132-8c | a mps1-737 ura3-52 his3Δ200 leu2-3,112 lys2 | This studyb |
| AS132-10a | α mps1-737 ura3-52 his3Δ200 leu2-3,112 lys2 | This studyb |
| AS181-2b | a mps1-6 ura3-52 leu2-3,112 trp1Δ1 | This studye |
| AS234-GFP | a mps1-737 spc42Δ1::LEU2 TRP1::SPC42-GFP leu2-3,112 trp1Δ1 his3 ura3-52 ade2 | This study |
| AS235-GFP | a mps1-3796 spc42Δ1::LEU2 TRP1::SPC42-GFP leu2-3,112 trp1Δ1 his3 ura3-52 ade2 | This study |
| DB5 | a/α mps1::HIS3/MPS1 ura3-52/ura3-52 leu2-3,112/leu2-3,112 | This study |
| DBY3704 | α mps1-3704 his4 | T. Huffakerd |
| FLY14A | a ura3-52 trp1 leu2 pep4-3 prb1-1122 prc1-407 bar1 | F. Lucaf |
| S288c | α gal2 | R.K. Mortimerg |
| Wx241-17a | α mps1-1 ura3-52 leu2-3 his3Δ200 | Schutz et al. (1997) |
University of Wisconsin, Madison, WI.
This mps1 allele originally isolated by E.A. Siewert, University of Colorado, Boulder, CO.
This mps1 allele originally isolated by J.V. Kilmartin, MRC, Cambridge, England.
This mps1 allele originally isolated by T. Huffaker, Cornell University, Ithaca, NY.
This mps1 allele originally isolated by D. Koshland, Carnegie Institute of Washington, Baltimore, MD.
University of Colorado, Boulder, CO.
University of California, Berkeley, CA.
Cell Synchronization and Release from Arrest
Cells were arrested in G1 with α-factor (7–10 μM) obtained by a custom peptide synthesis using F-MOC chemistry on a peptide synthesizer (model 488, Applied Biosystems, Foster City, CA). Efficiency of arrest was monitored by budding index determination. Arrest was deemed successful if greater than 90% of a briefly sonicated sample of 100–200 cells was unbudded, and was later confirmed by flow cytometric determination of DNA content. Cells were rinsed and released from the arrest into growth medium without α-factor that was equilibrated to the temperature being used for the release. Reentry into the cell cycle was monitored by budding index and flow cytometry.
Fine-Structure Mapping of Mutant Lesions
The mitotic recombination assay was performed essentially as described by Mannis and Mortimer (1964). Homoallelic or heteroallelic diploids were cultured overnight in YPD (rich) liquid medium, and approximately 107 cells were plated on YPD agar. Plates were then exposed to a 4-krad dose of x-irradiation in a Torrex 120D x-ray inspection system (EG&G Astrophysics Research, Princeton, NJ). Experiments were performed in duplicate, and nonirradiated controls were also performed for comparison of spontaneous and induced recombination. After x-ray exposure, plates were incubated at the nonpermissive temperature for 48 h. Mitotic recombination events were assessed by counting the colonies that appeared at the nonpermissive temperature.
The assay was calibrated using sequenced alleles of the HIS4 gene (Mathison and Culbertson, 1985). Cells mutant for HIS4 are unable to grow without supplemental histidine. Heteroallelic diploids were constructed that carried lesions 40 or 95 nucleotides apart (his4–209 and his4–212 alleles, or his4–519 and his4–504, respectively; Table 1). Cultures of these diploids were plated on medium lacking histidine and subjected to x-irradiation. Mutations only 40 nucleotides apart (his4–209 and his4–212) could be clearly resolved by this assay. This distance is similar to Mannis and Mortimer’s (1964) initial estimate of 45 nucleotides as the limit of resolution.
Gapped plasmid repair was performed as described (Rothstein, 1991), making use of various unique restriction sites in the pRS316-derived plasmid pMPS1ΔB. For some strains, the lesion was further mapped within the kinase domain by inducing recombination between the chromosome and an MPS1 plasmid truncated at the KpnI site in the kinase domain (Lauzéet al., 1995). x-Irradiation was performed as described above for the mitotic recombination assay.
Nucleic Acid Techniques
DNA was manipulated by standard techniques as described by Ausubel et al. (1994). Plasmid DNA was prepared from E. coli with Wizard prep kits (Promega, Madison, WI).
Plasmid Constructs.
An EcoRI–SalI restriction fragment containing the MPS1 gene was cloned into a centromeric, URA3-marked pRS316 vector (Sikorski and Hieter, 1989; Lauzéet al., 1995), and this clone was modified as follows to create pMPS1ΔB. The EcoRI–NotI fragment was removed from the polylinker, the ends filled in with Klenow, and the plasmid religated; this eliminated the BamHI site in the linker, making the site in the gene unique. Later this clone was modified by partial digestion with Asp718 (an isoschizimer of KpnI), followed by fill-in and religation to destroy the KpnI site. This clone, which now contained unique BamHI, KpnI, and MroI sites in the protein kinase domain, was named pMPS1ΔBK. DNA fragments containing the individual mps1 mutations were placed into this construct.
Fusions were made between the mps1 alleles and the Schistosoma japonicum glutathione-S-transferase (GST) gene in the vector pEG(KT) under the control of the inducible GAL1 promoter (Mitchell et al., 1993; Lauzéet al., 1995). For each mutant clone, a ∼3-kilobase (kb) HincII fragment containing mps1 was excised and inserted at the SmaI site of pEG(KT). This created GST::mps1 fusions containing all but the first two amino acids of the open reading frame.
Polymerase Chain Reaction (PCR) Amplification and DNA Sequencing.
For sequencing of the mutant lesions, yeast genomic DNA isolated by the method of Hoffman and Winston (1987) was used as template for standard PCR reactions performed with Taq polymerase (Promega) in a PTC-100 thermal cycler (MJ Research, Watertown, MA). Custom oligos synthesized by Life Technologies (Gaithersburg, MD) were used to amplify portions of the MPS1 gene encoding the kinase domain. The products of four independent amplifications were pooled and then sequenced on both strands, using the Sequenase PCR Product Sequencing Kit (United States Biochemical, Cleveland, OH) as directed by the supplier. For lesions that had been mapped to one-half of the kinase domain, only that region was sequenced; for the others, the entire kinase domain was sequenced. Once the lesions were identified, these regions were amplified, and the products of several independent reactions were pooled and cloned into an otherwise wild-type copy of MPS1 in the plasmid pMPS1ΔBK using unique BamHI, KpnI, and MroI restriction sites. These clones were then sequenced to confirm the presence of the mutant lesion and absence of PCR-derived errors. Sequencing of double-stranded plasmid was performed with a Sequenase 2.0 kit (United States Biochemical) using 1 pmol template and 2 pmol primer, annealed by coprecipitation in ethanol after alkaline denaturation of the template.
Primers
For amplification: BSK1 5′-GAA TCT TTT CAT TAT TG-3′, MPS D 5′-CGA AAA AAT AGA ACT TTT GGG-3′, MPS I 5′-CCA CAT CAT TGA GAT CAT C-3′, and MPS J 5′-GCA TAA ACG CAA ATC ACG C-3′. For sequencing: BKS1 5′-CAG TTT CCA AGG CCA AAA-3′, BSK1 5′-GAA TCT TTT CAT TAT TG-3′, MPS F 5′-CGG CAT TCC TAA TAA GG-3′, MPS H 5′-GTT TTA GTG AAA GGT ATC-3′, MPS K 5′-GTA AAT GAC TCC CAG TAC G-3′, and MPS L 5′-TCC CAA TTT GAG TTT CAC G-3′.
Cytological Techniques
Yeast cells were prepared for flow cytometry as described by Hutter and Eipel (1979) using the DNA stain propidium iodide (Sigma Chemical, St. Louis, MO). Stained cells were analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA) using the LYSYS software package to obtain and analyze data.
Yeast cells were prepared for thin sectioning as described by Byers and Goetsch (1974, 1975). After sectioning, some sections were stained a second time with uranyl acetate to increase contrast. Serial sections were viewed on a Philips CM10 electron microscope (Philips Electronic Instruments, Mahwah, NJ). Immunofluorescent staining of microtubules was performed as described by Kilmartin and Adams (1984) as modified by Jacobs et al. (1988), using the rat monoclonal antibody YOL1/34 (anti-α-tubulin; Accurate Chemical, Westbury, NY).
The yeast strain IAY18, which carries a SPC42 deletion allele (spc42Δ1::LEU2) and also a green fluorescent protein (GFP)-tagged allele of SPC42 integrated into the chromosome and present in three copies (TRP1::SPC42-GFP), was obtained from J. Kilmartin and I. Adams (Medical Research Council, Cambridge, England). The deletion and GFP alleles were subsequently crossed into all six mps1 mutant backgrounds. GFP fluorescence was used to observe SPBs in these cells after growth at the permissive and nonpermissive temperatures. Cells were observed live or after a 5-min fixation with formaldehyde and staining of DNA with 4,6-amidino-2-phenylindole (DAPI). Cells were viewed with a Zeiss Universal microscope (Carl Zeiss, Oberkochen, Germany). Images were obtained using a CCD camera (Empix Imaging, Mississauga, Canada), IMAGE-640 frame-grabber board (Matrox, Dorval, Canada), and MetaMorph software (Universal Imaging, West Chester, PA).
Protein Techniques and Kinase Assays
For expression of the GST-tagged fusion proteins in the wild-type yeast strain FLY14A (Table 1) transformed with tagged plasmids, cells were cultured in selective medium (for retention of the plasmid) containing 2% raffinose, a sugar that does not repress the GAL1 promoter as glucose would. Because protein synthesis or stability could potentially be thermolabile, all cultures were grown at 25°C. Galactose was added to the medium to a final concentration of 4% when the OD600 reached 0.5–0.8 to induce protein production, and cultures were induced for 6–8 h. For Western blots, cells were lysed in 1× Laemmli sample buffer (Ausubel et al., 1994) by vortexing with 0.45- to 0.52-mm glass beads for 5 min at 4°C. Lysates were then separated on 8% SDS-PAGE gels (Ausubel et al., 1994) and electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes. GST fusions were detected on Western blots with goat anti-GST primary antibody (Pharmacia Biotech, Piscataway, NJ) diluted 1:1000, alkaline phosphatase-conjugated secondary antibody, and the NBT/BCIP color reaction assay (Promega, Madison, WI).
For kinase assays with GST-Mps1p fusion proteins, cells were cultured and induced as described above. Cells were collected and washed once in cold water, and cell pellets were quick frozen in liquid nitrogen and stored at −70°C. For small-scale purification (for one or a few kinase assays, from up to 100 ml of culture) cells were disrupted by vortexing for 5 min at 4°C in 0.5 ml buffer B (containing protease and phosphatase inhibitors; described in Lauzéet al., 1995) with 300 μl of 0.45- to 0.52-mm glass beads. When a larger amount of protein was desired, cells were lysed in the same buffer by passage through a French pressure cell press (American Instrument, Silver Spring, MD). After lysis, the lysate was clarified by centrifuging for 10 min at 5,000–10,000 × g at 4°C, and the supernatant was incubated with glutathione-Sepharose (Pharmacia) for 1–2 h at 4°C. The resin was washed three times in buffer B, three times in buffer B1, and twice in nondetergent buffer (Lauzéet al., 1995). This material was used for kinase assays as described in Lauzéet al. (1995), with two modifications: assays were performed at 25°C or 35°C and were carried out for 10–15 min.
Kinase assay material was separated on 15% Anderson SDS-PAGE gels (Anderson et al., 1973). The top half of the gel was subjected to electrophoretic transfer onto a PVDF membrane, while the lower half was stained with Coomassie Brilliant Blue (Ausubel et al., 1994). Phosphorylation in both halves was scanned and quantitated with a Storm 860 phosphorimager (Molecular Dynamics, Sunnyvale, CA) with the ImageQuaNT analysis package. GST-Mps1 protein levels on the blot portion were then quantitated using 1:1000 anti-GST antibody and alkaline-phosphatase secondary antibody, with the alkaline phosphatase substrate Attophos (JBL Scientific, Santa Clara, CA) used for detection and analysis by the phosphorimager.
RESULTS
Six Alleles of MPS1 with Mutations in the Kinase Domain
Winey et al. (1991) isolated the mps1–1 allele in a cytological screen for mutants with defects in microtubule organization. Similar screens carried out in other laboratories have identified ts isolates that fail to complement the temperature sensitivity of mps1–1 and could represent new alleles of the gene. These were designated 6 (from D. Koshland, Carnegie Institute of Washington, Baltimore, MD); 412, 422 (J.V. Kilmartin, Medical Research Council, Cambridge, England); 3704, 3497, and 3796 (T. Huffaker, Cornell University, Ithaca, NY). A collection of ts yeast strains (Hartwell, 1967) was also screened in this laboratory for additional strains that could not complement mps1–1, yielding isolates 502, 585, 676, 688, 737, 773, 1169, and 1237 (Siewert, personal communication). Complete outcrossing and characterization of all new isolates was impractical due to their number and origin. Ten of the 14 strains with noncomplementing mutations were drawn from the same collection of ts strains (Hartwell, 1967; Siewert and Kilmartin isolates) and could therefore have been redundant.
To quickly determine the minimum number of alleles represented by these isolates, we employed an x-ray–induced mitotic recombination assay (Mannis and Mortimer, 1964) and assigned the strains to mitotic recombination groups (the mps1–6 isolate was not obtained until much later and was not included in the recombination analysis). For this assay, heteroallelic diploids and homoallelic control strains were constructed. Heteroallelic diploids carried two alleles of MPS1 that might or might not be different, and homoallelic controls carried two identical alleles derived from the same isolate. Diploids were x-irradiated to cause chromosome breakage, thereby inducing mitotic recombination events. Mitotic recombination could have given rise to two distinct outcomes: if the genetic lesions in question were located in different regions of MPS1, recombination or gene conversion in between the lesions could have reconstructed a wild-type copy of the gene, generating Ts+ (temperature-resistant) cells; if the lesions were identical or fell very close together in the gene, no Ts+ cells would have been generated.
Using this assay, a variety of mps1 isolate combinations were tested for their ability to recombine. Diploids were plated on rich medium, exposed to a 4-krad dose of x-irradiation, and incubateded at the nonpermissive temperature (see MATERIALS AND METHODS). As predicted, two distinct classes were found (Table 2). One class displayed obvious recombination (a low level of spontaneous recombination and a dramatic increase upon x-irradiation), indicating that the isolate pair in question was nonallelic. The other class displayed very little or no recombination, indicating that these isolate pairs were either allelic or contained two mutant lesions that were very close together. All isolate pairs that failed to recombine were placed into the same mitotic recombination group. The isolates fell into five recombination groups and therefore defined at least five different alleles of MPS1 (Table 3). In all cases, recombination results were internally consistent for all members of a group that were tested. Three groups contained multiple isolates, while two had only a single member. One representative of each group was chosen for complete outcrossing and further characterization (Table 3), and all isolates were later sequenced.
Table 2.
x-Ray-induced mitotic recombination matrix
| Isolate pairs | 1 | 412 | 422 | 502 | 585 | 676 | 688 | 737 | 1237 | 3497 | 3704 | 3796 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | − | + | + | + | + | + | + | + | + | + | + | + |
| 412 | − | + | − | + | − | + | + | + | + | + | ||
| 422 | − | + | − | + | + | − | ||||||
| 502 | − | + | − | + | + | − | ||||||
| 585 | − | + | − | + | + | |||||||
| 676 | − | + | + | − | ||||||||
| 688 | − | + | + | |||||||||
| 737 | − | + | + | + | + | |||||||
| 1237 | − | − | − | + | ||||||||
| 3497 | − | − | + | |||||||||
| 3704 | ||||||||||||
| 3796 | − |
Isolate numbers are listed at the left and across the top of the grid. Each position in the matrix indicates + for recombination or − for failure to recombine between the two isolates corresponding to that position. Failure to recombine was defined as the production of ≤ 4 Ts+ colonies following after x-irradiation, and the smallest number of Ts+ colonies generated for any nonallelic pair was 164 (from approximately 107 cells irradiated; see MATERIALS AND METHODS). Not every combination was tested; as recombination groups emerged, some redundant isolates were eliminated. Isolates 773 and 1169 are not listed in this table because they were placed in the same recombination group with 1 in the very first round of testing and not examined further. If no result is indicated, that particular isolate combination was not tested.
Table 3.
The mps1 mitotic recombination groups
| Group | Members | Nonpermissive temperature | Intragenic complementation |
|---|---|---|---|
| I | 1*, 773, 1169 | 30°C | |
| II | 412*, 585, 688 | 34°C | With 737 |
| III | 737* | 34°C | With 412, 3796 |
| IV | 422, 502, 676, 1237*, 3497, 3704 | 34°C | |
| V | 3796* | 34°C | With 737 |
| 6* | 30°C |
The 14 isolates were subdivided into five mitotic recombination groups. One isolate, mps1-6, was not examined by this method. The representative of each group that was chosen for further analysis is marked with an asterisk. When mps1-737 is combined with either mps1-412 or mps1-3796 in a diploid cell, partial intragenic complementation occurs. The diploid can now grow at 36-37°C, which is normally lethal for each allele alone. These diploids remain ts at 38°C.
Gapped plasmid repair was used to map the molecular lesions in these alleles to particular regions of the gene (Rothstein, 1991). Restriction enzymes were used to create small gaps in a plasmid carrying the wild-type MPS1 gene, and gapped constructs were then transformed into yeast strains carrying various mps1 mutations (see MATERIALS AND METHODS). In each case, gap repair data (our unpublished results) indicated that the molecular lesion occurred between the BamHI and MroI restriction sites in MPS1, which bracket the region encoding the protein kinase domain (Figure 1). In some cases, mutations were further localized within the kinase domain. The plasmid pMPS1-KpnIΔ (Lauzéet al., 1995) contains a deletion allele of the gene that is truncated at the KpnI site in the middle of the kinase domain, and cannot complement mps1 mutations. Mutant strains harboring this plasmid were x-irradiated to induce chromosome breakage, similar to the mitotic recombination assay described above, resulting in plasmid-to-chromosome conversion (see MATERIALS AND METHODS). Because of the KpnI deletion, the plasmid could only supply wild-type coding information for the 5′-half of the kinase domain. The mps1–412 allele was rescued by recombination, indicating that the lesion lies 5′ to the KpnI site. Both mps1–1 and mps1–1237 were not rescued, indicating that these alleles carry lesions 3′ to KpnI.
Figure 1.
Strategy for identification of mutant lesions. The region of MPS1 that encodes the protein kinase consensus domain lies near the C terminus of the gene. Gapped plasmid repair data indicate that all mutations occur within this 800-bp region bounded by the BamHI and MroI restriction sites. The positions of unique BamHI, KpnI, and MroI restriction sites are shown. Arrows indicate the position and orientation of the oligonucleotide primers used for genomic PCR and for DNA sequence analysis (see MATERIALS AND METHODS). The location of each mutant lesion is marked with an X.
The area identified by gap repair spanned approximately 800 base pairs (bp) and contained the entire kinase domain, and in some cases the location of the mutation was restricted to one-half of this region. A variety of primers were used for PCR amplification and DNA sequence analysis of the appropriate regions (see MATERIALS AND METHODS). To determine the exact mutation, sequence from the mutants was compared with the sequence generated using wild-type S288c (Table 1) genomic DNA as template. All members of each mitotic recombination group were sequenced in parallel. The mps1–6 isolate was obtained much later than the others and was never examined by gap repair. Instead, we proceeded directly to sequencing of the kinase domain to search for a mutation.
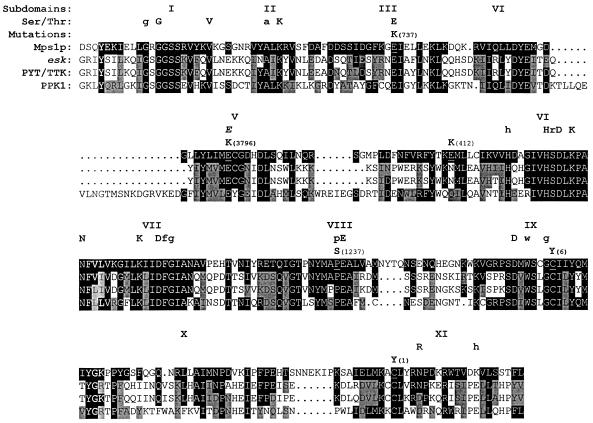
Every mutant strain contained a single base substitution in the region of MPS1 that encodes the protein kinase domain (Figure 1). Together, the five recombination groups (Table 3) and mps1–6 define exactly six alleles of the gene. In all cases, members of a mitotic recombination group shared identical changes. This result was somewhat surprising, considering that the largest recombination group (IV, with six members) was made up of isolates identified in three different laboratories and drawn from two independent ts mutant collections. Three types of amino acid substitutions were found (Figure 2): glutamate replaced by lysine (three alleles); cysteine replaced by tyrosine (two alleles); and proline replaced by serine (one allele). Several residues that are conserved among many protein kinases were affected, particularly the glutamate at position 486 (E486K; mps1–737) and proline at position 608 (P608S; mps1–1237). All mutations, with the exception of mps1–412, affected residues that are identical in Mps1p and its closest homologs, esk, PYT/TTK, and PPK1 (Figure 2).
Figure 2.
The six ts alleles each contain a single mutation in the kinase domain. The amino acid sequence of the Mps1p protein kinase domain is shown. Above the sequence, the 11 subdomains of the kinase domain and the residues conserved among the family of serine-threonine kinases are listed (Ser/Thr; see Hanks et al., 1988). Uppercase letters indicate highly conserved residues, and lowercase letters indicate less well conserved ones. The italicized E in subdomain V denotes a residue conserved in some dual-specificity kinases (Lindberg et al., 1992). The amino acid changes caused by each mps1 mutation and its corresponding allele number are noted above the Mps1p sequence, and the altered residue in Mps1p is underlined. Below the Mps1p sequence are those for the kinase domains of esk, PYT/TTK, and PPK1 (Douville et al., 1992; Lindberg et al., 1992; Kwart, personal communication). Identity between Mps1p and the other kinases is indicated by black boxes, and similarity is indicated by gray boxes. A homolog of MPS1 has also been found in Schizosaccharomyces pombe (He and Sazer, personal communication) but is not shown here. The sequences given here begin at the following amino acid numbers: Mps1p, position 437; esk, position 521; PYT/TTK, position 506; PPK1, position 144.
After sequencing, PCR products containing the individual mutations were placed into a pristine, unmutagenized copy of MPS1 in a centromeric vector, and the inserts were subsequently sequenced to confirm the presence of the correct single nucleotide change. The cloned mutant alleles were introduced into a mps1–1 mutant strain and into a strain carrying a null allele of MPS1 to confirm that these single base changes were sufficient to confer temperature sensitivity (Lauzéet al., 1995). Each mutant clone allowed growth of the cells up to approximately the correct temperature level, although each strain grew at a slightly higher temperature than expected. Centromeric plasmids may be present at two or three copies in a yeast cell, and this increased dosage of mutant alleles appeared to provide a slight growth advantage.
Phenotypic Analysis of mps1 Mutant Strains
Yeast strains carrying the ts alleles (Table 1) were examined carefully, and their behavior was compared with that of the prototypic allele, mps1–1. These studies were performed using one fully outcrossed representative from each mitotic recombination group (Table 3) and the mps1–6 allele. All proved to be recessive, and introduction of the wild-type MPS1 gene on a plasmid fully restored normal growth. During construction of the collection of heteroallelic diploids for mitotic recombination assays, partial intragenic complementation between some alleles became evident (Table 3). When a diploid cell carried the mps1–737 allele in combination with either mps1–412 or mps1–3796, this cell was able to grow at temperatures of 36–37°C, which normally were lethal for each allele alone and for all other heteroallelic diploids. Because this complementation was partial and diploids could not grow at 38°C, the mitotic recombination assay could still be performed. No other allele combinations exhibited this effect.
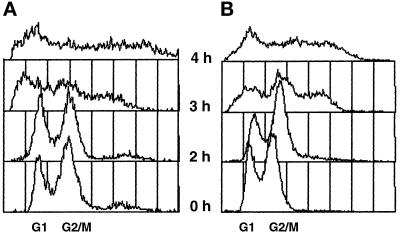
For each allele, several phenotypes were examined: cell cycle behavior at the nonpermissive temperature, as judged by flow cytometry and budding index determination; cell viability and formation of diploids after a brief shift to the nonpermissive temperature; and SPB morphology as determined by electron microscopy. In general, the behavior of the alleles was very similar. In all cases, when cells were shifted to the nonpermissive temperature, SPB duplication failed. Because MPS1 is required for the spindle assembly checkpoint (Weiss and Winey, 1996), there was no cell cycle arrest in response to the spindle defect, and cells proceeded through an aberrant mitosis. This event generated aploids, aneuploids, and cells of increased ploidy and produced a distinctive profile when the DNA content of cells in the culture was monitored by flow cytometry (Figure 3). Not surprisingly, this aberrant mitosis was usually a lethal event. For all alleles, at least 90% of cell viability was lost after a 4-h incubation at the nonpermissive temperature of 37°C. A characteristic feature of monopolar spindle mutants is that after brief exposure of a haploid culture to the nonpermissive temperature, surviving cells often recover with a diploid DNA content (Winey et al., 1991). This phenomenon, referred to as diploidization or endomitosis, is thought to occur because the monopolar spindle segregates all the chromosomes to that single pole, creating a diploid and an aploid cell (see Thomas and Botstein, 1986). In mps1–1 cultures, the few surviving cells are generally diploid (Winey et al., 1991). This behavior was observed for all alleles, but the recovery of diploids in mps1–737 cultures occurred at a lower frequency (Table 4).
Figure 3.
The mps1 mutant strains undergo aberrant mitosis, producing cells with increased ploidy. (A) mps1–6 cells (AS181–2b; Table 1) were exposed to the nonpermissive temperature and sampled for flow cytometry at 1-h intervals (2 h-4 h samples are shown). For comparison, a histogram for cells grown at the permissive temperature is shown (0 h). (B) The same experiment was performed with mps1–3796 cells (AS131–2d). Similar results were obtained for the remaining four alleles (our unpublished results). In these histograms, the x-axis is the relative DNA content determined by propidium iodide fluorescence, and the y-axis is the relative number of cells (see MATERIALS AND METHODS). The peaks corresponding to normal G1 and G2-M DNA content are indicated on the x-axis. Each sample represents 5,000 cells.
Table 4.
Survivors of a brief shift to the nonpermissive temperature recover as diploids
| Allele | Survivors tested | Haploids | Diploids |
|---|---|---|---|
| 1 | 5 | 0 | 5 |
| 6 | 10 | 0 | 10 |
| 412 | 5 | 0 | 5 |
| 737 | 24 | 9 | 15 |
| 1237 | 5 | 0 | 5 |
| 3796 | 5 | 0 | 5 |
Strains carrying the mps1-6 (AS181-2b), 1-412 (AS127-1d), 1-737 (AS132-8c), 1-1237 (AS126-5a), or 1-3796 (AS131-2d; Table 1) alleles were exposed to the nonpermissive temperature of 37°C for 4 h, and then plated for survivors at room temperature. Ploidy of survivors was determined by flow cytometry.
Unusual SPB Morphologies in mps1–737 Cells
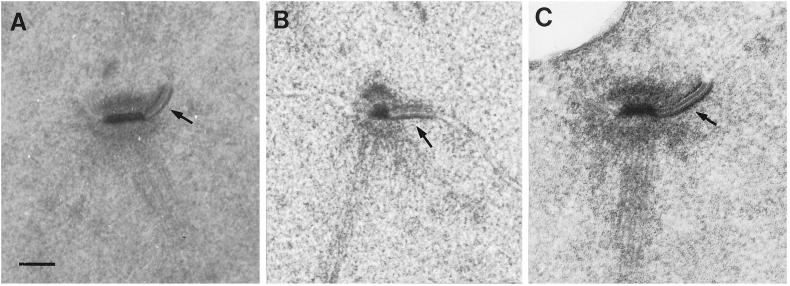
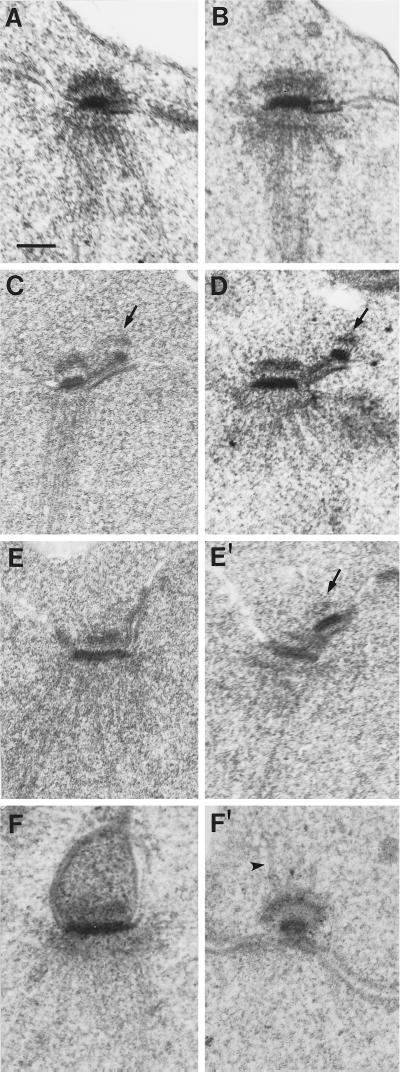
SPB phenotypes of the mps1 alleles were also examined by electron microscopy. In mps1–1 strains, a single SPB is seen by electron microscopy. It is enlarged compared with wild-type, bears a prominent and elongated half-bridge, and organizes a monopolar spindle (Winey et al., 1991). The same phenotype was seen in most other mps1 strains. Figure 4 shows examples, with the distinctive enlarged half-bridge indicated by an arrow. However, this was not the case with cells containing the mps1–737 mutation. A different phenotype was observed for this allele. The typical prominent half-bridge was generally not present, and sometimes a structure resembling a second SPB was observed. Examples of these structures are shown in Figure 5. This second SPB was found lying close to or separated from its sibling and did not appear to lie in the plane of the nuclear envelope, a morphology that is very similar to the defective SPB found in mps2 and ndc1 strains (Winey et al., 1991, 1993).
Figure 4.
Most mps1 alleles share the mps1–1 SPB phenotype. (A) In mps1–1 cells, a single SPB with very prominent half-bridge (arrow) is seen at the nonpermissive temperature (Winey et al., 1991). (B and C) Strains with the mps1–6 (B; AS181–2b) or mps1–3796 (C; AS131–2d) mutations were shifted to the nonpermissive temperature for 4 h, and their SPB phenotypes were examined by electron microscopy. Both strains exhibited the same SPB morphology seen in panel A: a single enlarged SPB with prominent half-bridge (arrows). This was also observed in mps1–412 and mps1–1237 strains (our unpublished results). Bar, 0.1 μm.
Figure 5.
Cells carrying the mps1–737 allele display an unusual SPB morphology. The mps1–737 strains AS132–3a or AS132–8c (Table 1) were exposed to the nonpermissive temperature for 3–4 h and examined by electron microscopy. In images A through D, the entire structure is seen in one thin section. The pairs of images in E and F represent two sections from the same nucleus, where relevant structures are seen in more than one section. A second SPB was found in four of 26 nuclei examined. (A and B) In most cells, a single SPB with a shorter and much less prominent half-bridge was found. (C and D) In each image, one complete SPB with enlarged half-bridge is present, and a second, structurally incomplete SPB (arrow) is perched at the end of the half-bridge on its cytoplasmic face. The second SPB has clear central and outer plaques. This morphology was observed in three cells. (E and E′) These images are two adjacent serial sections through the same nucleus. The intact SPB is seen in E, and the adjacent section (E′) reveals an aberrant SPB next to it (arrow). (F and F′) One normal SPB, which lies on an invagination of the nuclear envelope, is seen in F. In F′, the defective SPB is seen in another section from the same nucleus. This SPB has migrated away from its sibling to a distal location on the outer surface of the nuclear envelope, and microtubules can be seen emanating from its cytoplasmic face (arrowhead). This phenotype was observed once. Bar, 0.1 μm.
It was difficult to assess the significance of this phenotype because only a few examples were found. Examination of this phenotype in a large data set by electron microscopy would have been impractical and difficult. Therefore, we further investigated the presence of a possible second SPB using an indirect immunofluorescence approach. The second, defective SPB found in mps2 and ndc1 strains can nucleate microtubules on its cytoplasmic face (Winey et al., 1991, 1993). This property makes the structure easy to locate even when it has migrated far from the intact SPB, because examination of microtubule organization in these cells by indirect immunofluorescent staining of tubulin reveals two clear foci of staining, making this a more accurate method for assessment of SPB number than electron microscopy. In mps1–1 cells, only one focus of microtubule staining is seen (Winey et al., 1991). Since the mps1–737 second SPB had been seen bearing cytoplasmic microtubules on its outer plaque (see Figure 5F′), this method was used to count microtubule foci in mps1–737 cells before and after exposure to the nonpermissive temperature. At the nonpermissive temperature, a considerable number of cells exhibited a second focus of staining but showed no evidence of normal spindle formation (our unpublished observations). An mps1–3796 strain, which appeared by electron microscopy to contain only one SPB, was examined as a control and displayed only one focus of staining (our unpublished observations).
Because two SPBs that are close to one another cannot be unambiguously resolved by microtubule staining, a different technique was employed to more accurately quantify this phenotype. The Spc42p protein is a component of the SPB and localizes to the central plaque region (Rout and Kilmartin, 1991; Donaldson and Kilmartin, 1996). When the SPC42 gene is tagged with GFP and no wild-type allele is present, Spc42p-GFP can be seen as two bright dots at the spindle poles after SPB separation has occurred (Adams and Kilmartin, unpublished observation; see Figure 6). The TRP1::SPC42-GFP and spc42Δ1::LEU2 alleles were introduced into the six mps1 mutant backgrounds for this experiment. After exposure to the nonpermissive temperature, cells were fixed and stained with DAPI. GFP fluorescence was then visualized and the resulting SPB dots were counted. The numbers collected and phenotypes observed are shown in Figure 6. For five mps1 alleles, very few large budded cells displayed two dots of fluorescence at the nonpermissive temperature. Most cells contained a single SPB, which was usually found near the bud neck (as in Figure 6B). In contrast, nearly all mps1–737 cells contained a second SPB as evidenced by two foci of Spc42-GFP fluorescence, confirming the previous microtubule staining and electron microscopic results (Figure 6C). The two SPBs were often found fairly close to one another—in 64% of cells with two dots, both dots were located in the same cell body. Generally, the SPBs were found in the cell body that contained the majority of DAPI staining, although aploid bodies with only mitochondrial DNA staining that contained an SPB were observed (our unpublished results).
Figure 6.
mps1–737 cells contain two dots of Spc42p-GFP fluorescence, indicating that two SPBs are present. Strains that carry various mps1 alleles and the GFP-tagged allele of SPC42 were shifted to 37°C for 4 h, briefly fixed, and stained with DAPI to visualize DNA. DAPI and GFP fluorescence were then observed. Only large budded cells with evidence of DNA missegregation were scored. The number of dots of GFP fluorescence in a given cell reflects the number of SPBs. (A) At 25°C, large-budded cells display normal DNA segregation patterns (DAPI), and one SPB dot (Spc42p-GFP) localizes with each region of DAPI staining. Shown here are mps1–3796 and mps1–737 strains (AS235-GFP and AS234-GFP, respectively; Table 1). (B) In the mps1–3796 strain at 37°C, aberrant DNA segregation becomes apparent in large-budded cells. Only one SPB dot can be found in most cells, and it is often located close to the bud neck. Similar results were observed for all alleles except mps1–737. (C) When shifted to 37°C, mps1–737 cells behave differently. DNA segregation patterns become aberrant, but most cells clearly contain two distinct SPB dots. Both dots are generally found in the same cell body, and one is often located in a region that does not overlap with DAPI staining (arrow). (D) Number of SPB dots observed in all six mutant backgrounds.
The presence of two dots of Spc42p-GFP fluorescence in almost all mps1–737 cells indicated that they could proceed partially through SPB duplication and assemble a defective SPB. This unexpected phenotype suggests an additional, later requirement for MPS1 in the duplication process, but it was possible that the mps1–737 phenotype arose from an early structural mistake in satellite formation that precluded complete duplication. Cells with the mps1–1 mutation fail to form a satellite at the nonpermissive temperature and also fail to maintain a satellite deposited by pretreatment with α-factor (Winey et al., 1991). If a defect in satellite formation were responsible for the mps1–737 SPB morphology, then α-factor pretreatment to supply the cell with a normal satellite before exposure to the nonpermissive temperature should correct the defect. However, mps1–737 cells treated in this manner still failed in SPB duplication. Severe DNA missegregation was seen by flow cytometry, and aberrant microtubule arrays with no spindle or connection formed between the two foci of staining were again observed by immunofluorescence (our unpublished observations). These results are consistent with a later requirement for MPS1 function SPB duplication.
Characterization of the Mutant Proteins
An N-terminal GST epitope tag was used to monitor and purify mutant Mps1p proteins (Mitchell et al., 1993). The mutant alleles were tagged as described by Lauzéet al. (1995) by excision of a HincII fragment containing the gene from each centromeric mutant clone and placement into the GST vector (see MATERIALS AND METHODS). The resulting constructs contained all but the first two codons of the open reading frame, and overproduction of tagged fusion proteins could be induced by addition of galactose to the growth medium. The tagged proteins are referred to as GM- followed by WT (for the wild-type enzyme) or by the allele number. Previously, overexpression of GM-WT has been shown to cause a mitotic arrest by ectopic activation of the spindle assembly checkpoint (Hardwick et al., 1996). The overexpression phenotype of the tagged mutant proteins in the wild-type strain FLY14A (Table 1) was examined. When expression of each was induced with galactose at 25°C and cell cycle distribution of the induced cultures monitored by flow cytometry, some degree of G2/M delay or arrest was also observed (our unpublished observations). No such response to galactose was evident in cultures expressing GST from the pEG(KT) vector without insert. This indicated that the tagged mutant enzymes must possess sufficient in vivo kinase activity to activate the spindle assembly checkpoint in this experimental situation.
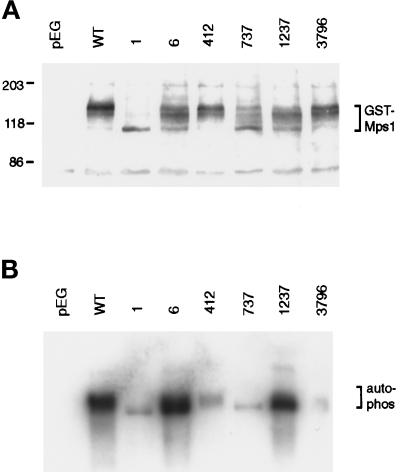
The GST-Mps1p fusion protein has a predicted molecular weight of 112 kDa. However, the wild-type–tagged protein has been shown to migrate at a higher apparent molecular weight on a Western blot as a broad and somewhat indistinct band (Lauzéet al., 1995). After treatment with protein phosphatases this band shifts down to the predicted molecular weight, suggesting that altered gel mobility is largely a result of phosphorylation. The observation that a catalytically inactive “kinase dead” form exhibits no band shift indicates that autophosphorylation is likely to be responsible (Lauzéet al., 1995). When the mutant proteins were overexpressed, we observed that gel mobility varied greatly between the different mutant proteins but was reproducible for any given mutant protein (Figure 7A). Some proteins, such as GM-412 and GM-3796, migrated similarly to GM-WT. Others migrated more quickly, with the most dramatic example being GM-1. This protein migrated as a single tight band at about the correct predicted molecular weight, similar to the migration of the catalytically inactivated protein (Lauzéet al., 1995). Other fusions displayed intermediate changes in gel mobility. As with the wild-type protein, mobility differences very likely arose from varying degrees of autophosphorylation, and so they may reflect different levels of kinase activity in the cell. The mutant proteins were generally present at lower levels than GM-WT.
Figure 7.
Autophosphorylation by GST-tagged mutant proteins varies greatly. Plasmids carrying the GST-tagged mps1 alleles were transformed into the wild-type strain FLY14A (Table 1), and expression of fusion proteins was induced as described (see MATERIALS AND METHODS). (A) GST fusions were detected on Western blots of whole cell lysates with anti-GST antibody. The control lane (pEG) shows cells carrying the GST vector without insert. The tagged wild-type protein (GM-WT) is phosphorylated, causing it to migrate above its predicted molecular weight of 112 kDa (Lauzéet al., 1995). (B) GST fusions were affinity-purified with glutathione-Sepharose and used for in vitro kinase assays at 25°C. Material was then separated on an SDS-PAGE gel and blotted. Proteins loadings are approximately equal across the gel, as determined by Western blot (our unpublished results).
Kinase Activity of the Mutant Proteins
The in vitro kinase activities of all six mutant proteins were compared with that of wild type. For this experiment, wild-type cells (FLY14A; Table 1) with vector carrying no insert, GM-WT, or GST-tagged mutant alleles were induced for 8 h to produce sufficient protein and then collected and lysed. Tagged proteins were purified and used for kinase assays (Figure 7B). In this experiment, approximately equal amounts of protein were present in each lane, as determined by Western blotting (see MATERIALS AND METHODS). Both autophosphorylation (shown) and activity against the exogenous substrate myelin basic protein (our unpublished results) were tested and behaved similarly. No kinase activity was detected from the control strain (pEG(KT)), whereas substantial activity could be attributed to GM-WT. The mutant proteins varied in their activity. Most appeared to be largely inactive in vitro. Only two proteins, GM-6 and GM-1237, demonstrated significant kinase activity. These two mutant enzymes were examined more closely.
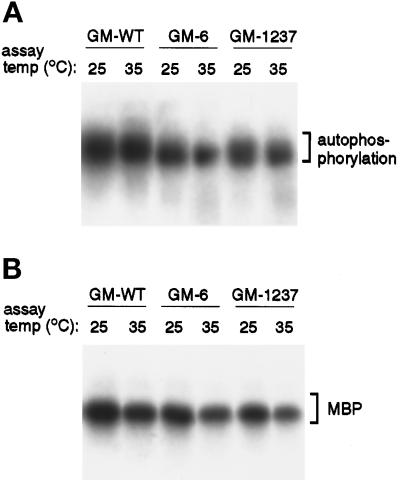
Because these mutations cause a ts phenotype in the cell, we investigated the possible thermolability of the GM-6 and GM-1237 mutant proteins in kinase assays performed at 35°C, a temperature that is nonpermissive for both corresponding mutant strains. When wild-type protein is assayed at this temperature, autophosphorylation signal is unchanged, although phosphorylation of MBP is somewhat reduced. To compare wild-type and mutant kinase activities, the three purified proteins were assayed in parallel at 25°C and 35°C (Figure 8). After separation of kinase assay material on SDS-PAGE gels, the portion of the gel containing GST-tagged Mps1p was Western blotted. Autophosphorylation and phosphorylation of MBP were quantitated, and similar Mps1p protein loadings were confirmed by Western blot using a chemifluorescent detection substrate (see MATERIALS AND METHODS). Two trends were apparent from the data (Figure 8). First, the activities of GM-6 and GM-1237 were consistently lower than that of GM-WT. Second, both of the mutant kinases clearly retained substantial activity at 35°C; they were not obviously thermolabile in this in vitro assay. For the corresponding mps1–6 and mps1–1237 mutant strains, the nonpermissive temperature is 34°C. This result suggests that thermolability of these mutant proteins may not arise from a general loss of kinase activity, but may involve interactions with other factors within the cell.
Figure 8.
In vitro activity of the GM-6 and GM-1237 mutant proteins is not thermolabile. Affinity-purified GM-WT, GM-6, and GM-1237 kinases were assayed in vitro at 25°C and 35°C, and then separated on an SDS-PAGE gel and blotted. This elevated assay temperature would be nonpermissive for growth of both mps1–6 and mps1–1237 mutant strains. (A) Autophosphorylation activity of the proteins at both assay temperatures. (B) Substrate-level phosphorylation of myelin basic protein (MBP). For each pair of samples (25°C and 35°C), protein loadings were approximately equal (determined by Western blot).
DISCUSSION
We have described the phenotypic and molecular characterization of six alleles of the MPS1 gene. Each allele contains a single point mutation in the region encoding the protein kinase domain, and these lesions affect the activity of the resulting mutant kinases in different ways. Five of the six alleles cause the same type of early SPB duplication failure. However, cells carrying the mps1–737 allele proceed farther in duplication before they fail. This phenotype suggests that the Mps1p kinase is required for multiple events in SPB duplication.
The Mutant Lesions
The fact that all six ts mutations occur in the C-terminal protein kinase domain indicates that Mps1p kinase activity is likely to be essential for its physiological role. This collection of alleles probably represents a variety of defects in ATP binding, substrate binding, and catalytic activity; however, most alleles produce the same SPB duplication phenotype, and all are similarly defective for the spindle assembly checkpoint. The location of all lesions in the kinase domain also suggests that the methods used to identify these alleles were somewhat limited. In all cases, temperature sensitivity and failure to complement the original mps1–1 allele were requirements for identification. Isolates that displayed some degree of intragenic complementation with mps1–1 could easily have been overlooked, along with any nonconditional mps1 mutants. In fact, point mutations in the N-terminal region have now been identified, and these mps1 mutants have no conditional phenotype. They are lethal in combination with a null allele of CIN8, but display no defects in SPB duplication (Geiser et al., 1997; Winey and Chen, unpublished observation).
The six mutations reported here also provide information about the correlation between Mps1p structure and function. Often ts mutations in protein kinases are located in structural transition regions that lie in between conserved motifs—a suitable location for mutations that cause instability and thermolability of the protein without entirely destroying catalytic activity [for examples, see Lorincz and Reed (1986); Carr et al. (1989); Hollingsworth et al. (1992)]. However, many of the mps1 lesions defy this paradigm. Of the six mutations, three affect residues located in conserved or invariant sequence motifs in the protein kinase catalytic domain (see Figure 2). The most drastic of these is alteration of the invariant glutamate residue at position 486 in subdomain III to a lysine, in mps1–737. Normally, this residue and Lys468 in subdomain II would be predicted to cooperate in formation of the docking site for Mg-ATP (Taylor et al., 1992). Such an extreme change might be predicted to abolish kinase activity, but this mutant protein can still support growth, although mps1–737 cells grow slowly even at the permissive temperature. Perhaps another glutamate residue nearby can compensate, possibly Glu488 or Glu491.
Another conserved residue affected is Pro608, which lies in the peptide-binding lobe of the protein in subdomain VIII and is altered to serine in the mps1–1237 allele. This proline residue is somewhat conserved among kinases in general, and identical in Mps1p, esk, PYT/TTK, and PPK1. The residues in subdomain VIII appear to be particularly important for enzyme–substrate interactions. In a variety of kinases, this region participates in the formation of two side chain pockets in the kinase structure that interact with substrate residues in the immediate vicinity of the amino acid that will be phosphorylated, contributing to substrate selectivity and orientation (Songyang et al., 1994, 1996). The alteration of Glu517 to lysine in mps1–3796 also affects a somewhat conserved residue, one that is found in many dual-specificity kinases (Lindberg et al., 1992). The remaining three mutations occur at positions that are not conserved among protein kinases, but two of these affect residues that are identical in esk, PYT/TTK, and PPK1.
These six mutations cause a variety of effects on the in vivo and in vitro activity of GST-Mps1p fusion proteins. All six mutant kinases must possess some degree of activity in vivo, because they support growth at the permissive temperature and can cause a G2-M bias in the cell cycle distribution of wild-type cells when they are overexpressed. Their gel mobility suggests that autophosphorylation activity varies greatly; however, the significance of autophosphorylation for protein function is not yet known. Kinase assay experiments with GST fusions show that in vitro activity also varies, but does not correlate well with gel mobility as an assessment of activity in vivo, with the exception that proteins with little or no gel shift are inactive in this in vitro assay. The observation that many mutant Mps1p proteins are inactive in vitro is not unexpected, since their folding may be compromised enough that they cannot withstand lysis and purification. Similar results have been obtained with mutant forms of the yeast cAMP-dependent protein kinase that are partially or even fully functional in the cell (Gibbs and Zoller, 1991).
The behavior of the GM-6 and GM-1237 mutant enzymes in vitro raises interesting questions about the nature of their temperature-sensitive defects. Both fusions display significant in vitro kinase activity, but this activity does not appear to be thermolabile when the assays are performed at a temperature that is nonpermissive for growth of the corresponding mutant strains. This suggests that the ts defect does not arise soley from temperature-induced denaturation or aggregation of the protein, but may involve a more complex mechanism, such as disruption of interactions with other proteins. These two mutations could affect the ability of the kinase to bind substrates, activators, or regulatory subunits. Such defects would not be apparent when purified GM-6 or GM-1237 is assayed; accessory proteins and relevant substrates would not be present, and regulatory signals such as phosphorylation would already have been delivered. Such a model is particularly appealing when information about substrate-binding motifs is taken into account. The proline-to-serine substitution in Mps1–1237p occurs very close to residues critical for binding of substrate residues that lie in the −2 or +1 positions relative to the phosphorylated amino acid, and the Mps1–6p substitution is near another group of residues also important for binding at the −2 position (Songyang et al., 1994, 1996). These two perturbations could weaken or disrupt binding of relevant substrates in a similar manner. If this mechanism is the source of temperature sensitivity, the mps1–6 and mps1–1237 alleles could prove useful genetically for identification of important interacting factors, particularly substrates.
Implications of the mps1–737 Phenotype
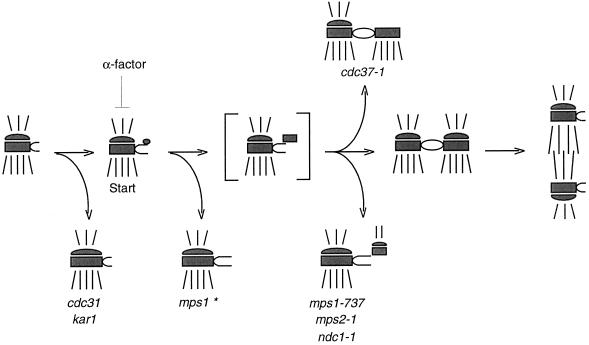
The mps1–737 allele is interesting because of the nature of its mutation (which drastically alters the highly conserved Glu486 residue), but even more so because of its phenotype. Most of the mps1 alleles described here closely resemble the original allele, mps1–1, and cause early failure in SPB duplication. In sharp contrast, cells carrying the mps1–737 allele are able to form a second, aberrant SPB. Why does this occur, and what does it indicate about the role of MPS1 in SPB duplication? This may not be a simplistic loss-of-function situation. We suggest that the Mps1p kinase is actually required for multiple events in SPB duplication (Figure 9), and that the mps1–737 allele represents a separation or alteration of those functions. Different mutations might affect activity of the kinase toward different substrates or its ability to respond correctly to regulatory signals, revealing phenotypic nuances. The Mps1–737p mutant enzyme may be competent to meet the previously identified early requirement for the kinase (Winey et al., 1991) but unable to carry out a later function. Failure to meet this later requirement would result in the mps1–737 phenotype. The assembly of an aberrant second SPB with central and outer plaques and cytoplasmic microtubules, which closely resembles the mps2 and ndc1 morphologies (Winey et al., 1991, 1993), is consistent with this type of previously unrecognized late requirement for MPS1. A later function for MPS1 in SPB duplication would not have been uncovered without this unique allele; no other genetic or pharmacological tools currently exist that reversibly block SPB duplication between the satellite-bearing and duplicated side-by-side stages.
Figure 9.
Proposed pathway for SPB duplication. In this schematic, the central and outer plaques of the SPB are depicted, with cytoplasmic and nuclear microtubules drawn above and below the SPB, respectively. The bracketed structure is a duplication intermediate that is inferred but has not been reported. Execution point experiments with mps1–1, mps2–1, and ndc1–1 mutants (Winey et al., 1991, 1993) indicate where in the pathway these mutations cause SPB duplication to fail. The mps1–1 mutation causes failure early on and is marked with an asterisk to indicate that four of the other five alleles share this SPB morphology. However, the mps1–737 mutation allows duplication to proceed farther before failure occurs and has been placed here at the same point in the pathway as mps2–1 and ndc1–1 because of their phenotypic similarity. Mutation of CDC37 also causes a later failure in SPB duplication, but generates a different, partially duplicated structure (Schutz et al., 1997). The two distinct mps1 mutant phenotypes suggest that this gene is required continuously or at multiple times during SPB duplication.
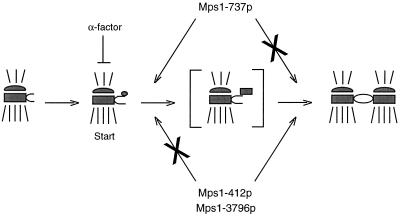
Multiple requirements could also provide a simple mechanism for the intragenic complementation observed when the mps1–737 allele is combined with either mps1–412 or mps1–3796 (Figure 10). It appears that Mps1–737p can supply only the earlier duplication function. Like mps1–737, mps1–412 and mps1–3796 could also be separation-of-function alleles that cannot meet the early requirement but would be competent to carry out the later SPB duplication function if given the opportunity. When combined in a diploid cell, Mps1–737p could cooperate with either Mps1–412p or Mps1–3796p, together supplying both functions and permitting growth at a higher temperature. A partial loss of Mps1p function may also occur under different circumstances. The Cdc37p protein is required to promote the activity of several protein kinases in yeast (Gerber et al., 1995; Dey et al., 1996; Schutz et al., 1997). Mutation of CDC37 causes a significant decrease in Mps1p kinase activity and results in a late failure in SPB duplication (Schutz et al., 1997). However, the partially duplicated SPB morphology seen is different than that observed for any other duplication mutants (Figure 9). The observation that different perturbations of Mps1p function correlate with different mutant SPB morphologies argues strongly that this kinase is required for multiple events during the duplication process, or possibly throughout a window of time in G1.
Figure 10.
A model for intragenic complementation between mps1 alleles. The intragenic complementation observed between mps1–737 and either the mps1–412 or mps1–3796 alleles could be a result of multiple requirements for Mps1p during SPB duplication. The mps1–737 gene product, Mps1–737p, may be competent to meet the early requirement reported previously (Winey et al., 1991) but apparently cannot perform an important later function. Mps1–412p and Mps1–3796p clearly fail to meet the early requirement, but it is possible that they are competent to perform the later function that is lost in mps1–737 cells. If so, the combination of two partially functional kinases in mps1–737/mps1–412 or mps1–737/mps1–3796 diploid cells could meet both requirements, allowing the cell to grow at a higher temperature. According to this model, the three remaining mps1 alleles would be defective for both functions and thus would not display intragenic complementation. All six mps1 alleles are defective for the spindle assembly checkpoint function (Weiss and Winey, 1996).
The differing mps1 mutant phenotypes also invite speculation about the reversibility of SPB duplication problems. Diploidization by surviving cells of most mutant strains after brief exposure to the nonpermissive temperature indicates that SPB duplication failure is irreversible in these cells. Presumably, a second pole can no longer be made, and the single, enlarged SPB is left to carry out mitosis alone, sometimes pulling all the chromosomes to that pole and creating a viable diploid. Cells with the mps1–737 mutation are able to recover as viable haploids more frequently than observed for other alleles. Does this indicate that their different SPB defects can be reversed? Cells carrying the mps2–1 or ndc1–1 mutations, which share the same SPB morphology as mps1–737, also recover from exposure to the nonpermissive temperature as a mixture of both haploids and diploids (Winey et al., 1991; Thomas and Botstein, 1986). This correlation suggests that perhaps the defective second SPB in these cells is not always a dead-end product, but in some cases can be remodeled or built upon to produce a fully functional SPB.
The continued analysis of strains with mutant alleles of MPS1 and other genes important for SPB duplication should yield further insight into this process. Furthermore, analysis of the regulation of MpsIp, and identification of relevant substrates in SPB duplication and spindle checkpoint control, should lead us to a deeper understanding of the duplication process and of the roles of this important kinase in the SPB cycle and in cell cycle control.
ACKNOWLEDGMENTS
We thank Ian Adams and John Kilmartin for the GFP-tagged allele of SPC42; Tim Huffaker, John Kilmartin, Doug Koshland, and Elizabeth Siewert for mps1 isolates; and Mike Culbertson for his4 strains. We also thank Andrea Castillo for critical reading of the manuscript; Tom Giddings and Estelle Steiner for technical advice and assistance; and the members of the Winey laboratory for their input. This work was initiated under an American Cancer Society grant (MV63940) and completed with support from the National Institutes of Health (NIH GM-51312). Additional support was provided by an American Cancer Society Junior Faculty Research Award (A70760) and the Pew Scholars Program in the Biomedical Sciences award (P0020SC) to M.W. A.R.S. was supported by an NIH training grant (GM-07135), National Science Foundation Predoctoral Fellowship, and Truman Scholarship.
Footnotes
Abbreviations used: GFP, green fluorescent protein; GST, glutathione-S-transferase; MBP, myelin basic protein; mps, monopolar spindle; SPB, spindle pole body; ts, temperature-sensitive for growth.
REFERENCES
- Anderson CW, Baum PR, Gesteland RF. Processing of adenovirus-2 induced proteins. J Virol. 1973;12:241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1994. [Google Scholar]
- Byers B, Goetsch L. Duplication of spindle plaques and integration of the yeast cell cycle. Cold Spring Harbor Symp Quant Biol. 1974;38:123–131. doi: 10.1101/sqb.1974.038.01.016. [DOI] [PubMed] [Google Scholar]
- Byers B, Goetsch L. Behavior of the spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae. J Bacteriol. 1975;124:511–523. doi: 10.1128/jb.124.1.511-523.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AM, MacNeill SA, Hayles J, Nurse P. Molecular cloning and sequence analysis of mutant alleles of the fission yeast cdc2+ protein kinase gene: implications for cdc2 protein structure and function. Mol Gen Genetics. 1989;218:41–49. doi: 10.1007/BF00330563. [DOI] [PubMed] [Google Scholar]
- Dey B, Lightbody JJ, Boschelli F. CDC37 is required for p60v-src activity in yeast. Mol Biol Cell. 1996;7:1405–1417. doi: 10.1091/mbc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson AD, Kilmartin JV. Spc42p: a phosphorylated component of the S cerevisiae spindle pole body (SPB) with an essential function during SPB duplication. J Cell Biol. 1996;132:887–901. doi: 10.1083/jcb.132.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douville EMJ, Afar DEH, Howell BW, Letwin K, Tannock L, Ben-david Y, Pawson T, Bell JC. Multiple cDNAs encoding the esk kinase predict transmembrane and intracellular enzyme isoforms. Mol Cell Biol. 1992;12:2681–2689. doi: 10.1128/mcb.12.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elble R. A simple and efficient procedure for transformation of yeasts. BioFeedBack. 1992;13:18–20. [PubMed] [Google Scholar]
- Geiser JR, Schott EJ, Kingsbury TJ, Cole NB, Totis LJ, Bhattacharyya G, He L, Hoyt MA. Saccharomyces cerevisiae genes required in the absence of the CIN8-encoded spindle motor act in functionally diverse mitotic pathways. Mol Biol Cell. 1997;8:1035–1050. doi: 10.1091/mbc.8.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber MR, Farrell A, Deshaies RJ, Herskowitz I, Morgan DO. Cdc37 is required for association of the protein kinase Cdc28 with G1 and mitotic cyclins. Proc Natl Acad Sci USA. 1995;92:4651–4655. doi: 10.1073/pnas.92.10.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs CS, Zoller MJ. Rational scanning mutagenesis of a protein kinase identifies functional regions involved in catalysis and substrate interactions. J Biol Chem. 1991;266:8923–8931. [PubMed] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hardwick KG, Weiss E, Luca FC, Winey M, Murray AW. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science. 1996;273:953–956. doi: 10.1126/science.273.5277.953. [DOI] [PubMed] [Google Scholar]
- Hartwell LH. Macromolecular synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967;93:1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CS, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Hogg D, Guidos C, Bailey D, Amendola A, Groves T, Davidson J, Schmandt R, Mills G. Cell cycle dependent regulation of the protein kinase TTK. Oncogene. 1994;9:89–96. [PubMed] [Google Scholar]
- Hollingsworth RE, Jr, Ostroff RM, Klein MB, Niswander LA, Sclafani RA. Molecular genetic studies of the Cdc7 protein kinase and induced mutagenesis in yeast. Genetics. 1992;132:53–62. doi: 10.1093/genetics/132.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt MA, Totis L, Roberts BT. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- Hutter KJ, Eipel HE. Microbial determination by flow cytometry. J Gen Microbiol. 1979;113:369–375. doi: 10.1099/00221287-113-2-369. [DOI] [PubMed] [Google Scholar]
- Jacobs CW, Adams AEM, Szaniszlo PJ, Pringle JR. Functions of microtubules in the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1988;107:1409–1426. doi: 10.1083/jcb.107.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin JV, Adams AEM. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984;98:922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzé E, Stoelcker BS, Luca FC, Weiss E, Schutz AR, Winey M. Yeast spindle pole body duplication gene MPS1 encodes an essential dual specificity protein kinase. EMBO J. 1995;14:1655–1663. doi: 10.1002/j.1460-2075.1995.tb07154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Murray AW. Feedback control in mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- Lindberg RA, Quinn AM, Hunter T. Dual specificity protein kinases: will any hydroxyl do? Trends Biochem Sci. 1992;17:114–119. doi: 10.1016/0968-0004(92)90248-8. [DOI] [PubMed] [Google Scholar]
- Lindberg RA, Fischer WH, Hunter T. Characterization of a human protein threonine kinase isolated by screening an expression library with antibodies to phosphotyrosine. Oncogene. 1993;8:351–359. [PubMed] [Google Scholar]
- Lorincz AT, Reed SI. Sequence analysis of temperature-sensitive mutations in the Saccharomyces cerevisiae gene CDC28. Mol Cell Biol. 1986;6:4099–4103. doi: 10.1128/mcb.6.11.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannis TR, Mortimer RK. Allelic mapping in yeast by x-ray induced mitotic reversion. Science. 1964;143:581–582. doi: 10.1126/science.143.3606.581. [DOI] [PubMed] [Google Scholar]
- Mathison L, Culbertson MR. Suppressible and nonsuppressible +1 G-C base pair insertions induced by ICR-170 at the his4 locus in Saccharomyces cerevisiae. Mol Cell Biol. 1985;5:2247–2256. doi: 10.1128/mcb.5.9.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DA, Marshall TK, Deschenes RJ. Vectors for the inducible expression of glutathione S-transferase fusion proteins in yeast. Yeast. 1993;9:715–723. doi: 10.1002/yea.320090705. [DOI] [PubMed] [Google Scholar]
- Mills GB, Schmandt R, McGill M, Amendola A, Hill M, Jacobs K, May C, Rodricks A, Campbell S, Hogg D. Expression of TTK, a novel human protein kinase, is associated with cell proliferation. J Biol Chem. 1992;267:16000–16006. [PubMed] [Google Scholar]
- Poch O, Schwob E, de Fraipont F, Camasses A, Bordonné R, Martin RP. RPK1, an essential yeast protein kinase involved in the regulation of the onset of mitosis, shows homology to mammalian dual-specificity kinases. Mol Gen Genet. 1994;243:641–653. doi: 10.1007/BF00279573. [DOI] [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- Rout MP, Kilmartin JV. Yeast spindle pole body components. Cold Spring Harbor Symp Quant Biol. 1991;61:687–692. doi: 10.1101/sqb.1991.056.01.077. [DOI] [PubMed] [Google Scholar]
- Schutz AR, Giddings TH, Jr, Steiner E, Winey M. The yeast CDC37 gene interacts with MPS1 and is required for proper execution of spindle pole body duplication. J Cell Biol. 1997;136:969–982. doi: 10.1083/jcb.136.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Blechner S, Hoagland N, Hoekstra MF, Piwnica-Worms H, Cantley LC. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr Biol. 1994;4:973–982. doi: 10.1016/s0960-9822(00)00221-9. [DOI] [PubMed] [Google Scholar]
- Songyang Z, et al. A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol Cell Biol. 1996;16:6486–6493. doi: 10.1128/mcb.16.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, Knighton DR, Zheng J, Ten Eyck LF, Sowadski JM. Structural framework for the protein kinase family. Annu Rev Cell Biol. 1992;8:429–462. doi: 10.1146/annurev.cb.08.110192.002241. [DOI] [PubMed] [Google Scholar]
- Thomas JH, Botstein D. A gene required for the separation of chromosomes on the spindle apparatus in yeast. Cell. 1986;44:65–76. doi: 10.1016/0092-8674(86)90485-x. [DOI] [PubMed] [Google Scholar]
- Weiss E, Winey M. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J Cell Biol. 1996;132:111–123. doi: 10.1083/jcb.132.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M, Byers B. Assembly and functions of the spindle pole body in budding yeast. Trends Genet. 1993;9:300–304. doi: 10.1016/0168-9525(93)90247-f. [DOI] [PubMed] [Google Scholar]
- Winey M, Goetsch L, Baum P, Byers B. MPS1 and MPS2: novel yeast genes defining distinct steps of spindle pole body duplication. J Cell Biol. 1991;114:745–754. doi: 10.1083/jcb.114.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M, Hoyt MA, Chan C, Goetsch L, Botstein D, Byers B. NDC1: a nuclear envelope component required for yeast spindle pole body duplication. J Cell Biol. 1993;122:743–751. doi: 10.1083/jcb.122.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]