Abstract
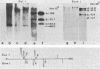
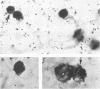
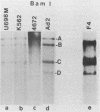
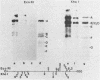
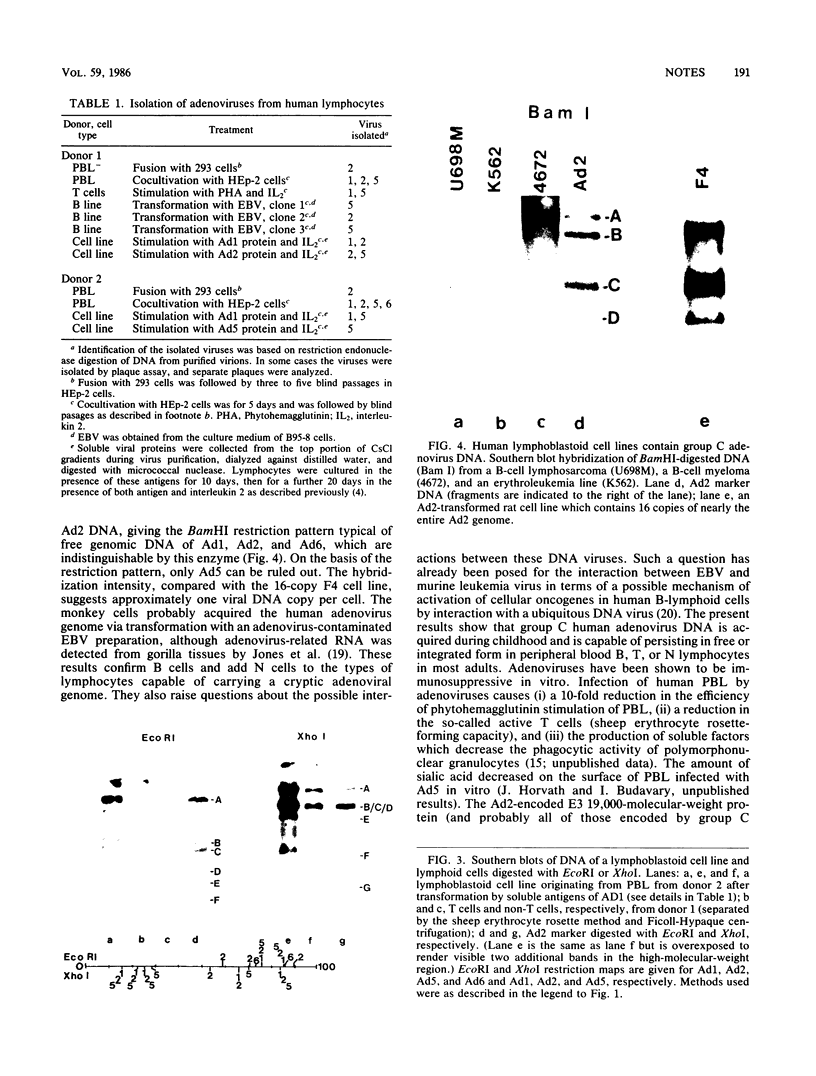
Human peripheral blood lymphocytes from healthy adults, cord blood lymphocytes, and lymphoblastoid cell lines were screened by hybridization for the presence of group C adenovirus DNA sequences. In 13 of 17 peripheral blood lymphocyte samples from adults, 1 of 10 cord blood samples, and seven of seven lymphoblastoid cell lines tested, results were positive for Group C adenovirus DNA (adenovirus 1 [Ad1], Ad2, Ad5, or Ad6). About 1 to 2% of the lymphocytes carried 50 to 100 viral genome copies per positive cell, as estimated by in situ hybridization. Infectious virus representing all members of group C were recovered, but cultivation in the presence of adenovirus antibody did not cure the cells of free viral genomes. Viral DNA was found in B, T, and N cells but only in 1 of 10 cord blood samples. The results suggest that group C adenovirus infections in childhood result in the persistence of the viral genome in circulating lymphocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andiman W. A., Jacobson R. I., Tucker G. Leukocyte-associated viremia with adenovirus type 2 in an infant with lower-respiratory-tract disease. N Engl J Med. 1977 Jul 14;297(2):100–101. doi: 10.1056/NEJM197707142970208. [DOI] [PubMed] [Google Scholar]
- Andiman W. A., Miller G. Persistent infection with adenovirus types 5 and 6 in lymphoid cells from humans and woolly monkeys. J Infect Dis. 1982 Jan;145(1):83–88. doi: 10.1093/infdis/145.1.83. [DOI] [PubMed] [Google Scholar]
- Arrand J. R., Walsh-Arrand J. E., Rymo L. Cytoplasmic RNA from normal and malignant human cells shows homology to the DNAs of Epstein-Barr virus and human adenoviruses. EMBO J. 1983;2(10):1673–1683. doi: 10.1002/j.1460-2075.1983.tb01642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak V., Fuks Z., Galilli N., Treves A. J. Selection and continuous growth of antigen-specific human T cells by antigen-treated monocytes. Eur J Immunol. 1983 Nov;13(11):952–956. doi: 10.1002/eji.1830131116. [DOI] [PubMed] [Google Scholar]
- Brown M., Weber J. Discrete subgenomic DNA fragments in incomplete particles of adenovirus type 2. J Gen Virol. 1982 Sep;62(Pt 1):81–89. doi: 10.1099/0022-1317-62-1-81. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985 Jul;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- EVANS A. S. Latent adenovirus infections of the human respiratory tract. Am J Hyg. 1958 May;67(3):256–266. doi: 10.1093/oxfordjournals.aje.a119932. [DOI] [PubMed] [Google Scholar]
- Faucon N., Chardonnet Y., Perrinet M. C., Sohier R. Superinfection with adenovirus of Burkitt's lymphoma cell lines. J Natl Cancer Inst. 1974 Aug;53(2):305–308. doi: 10.1093/jnci/53.2.305. [DOI] [PubMed] [Google Scholar]
- Faucon N., Desgranges C. Persistence of human adenovirus 5 in human cord blood lymphoblastoid cell lines transformed by Epstein-Barr virus. Infect Immun. 1980 Sep;29(3):1180–1184. doi: 10.1128/iai.29.3.1180-1184.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucon N., Ogier G., Chardonnet Y. Changes in human adenovirus 5 propagated in Burkitt's lymphoma cells. J Natl Cancer Inst. 1982 Dec;69(6):1215–1220. [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Green M., Wold W. S., Mackey J. K., Rigden P. Analysis of human tonsil and cancer DNAs and RNAs for DNA sequences of group C (serotypes 1, 2, 5, and 6) human adenoviruses. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6606–6610. doi: 10.1073/pnas.76.12.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Harris J. D., Traynor B., Ventura P., Peluso R., Brahic M. Visna DNA synthesis and the tempo of infection in vitro. Virology. 1982 Jun;119(2):399–410. doi: 10.1016/0042-6822(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Horváth J., Kulcsár G., Ugryumov J. P., Dás P., Nász I., Barinsky I. F., Simon G., Ongrádi J. Effect of adenovirus infection on human peripheral lymphocytes. Acta Microbiol Hung. 1983;30(3-4):203–209. [PubMed] [Google Scholar]
- Ibelgaufts H., Jones K. W., Maitland N., Shaw J. F. Adenovirus-related RNA sequences in human neurogenic tumours. Acta Neuropathol. 1982;56(2):113–117. doi: 10.1007/BF00690581. [DOI] [PubMed] [Google Scholar]
- Jones K. W., Kinross J., Maitland N., Norval M. Normal human tissues contain RNA and antigens related to infectious adenovirus type 2. Nature. 1979 Jan 25;277(5694):274–279. doi: 10.1038/277274a0. [DOI] [PubMed] [Google Scholar]
- Lasky R. D., Troy F. A. Possible DNA-RNA tumor virus interaction in human lymphomas: expression of retroviral proteins in Ramos lymphoma lines is enhanced after conversion with Epstein-Barr virus. Proc Natl Acad Sci U S A. 1984 Jan;81(1):33–37. doi: 10.1073/pnas.81.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikol J., Felten-Papaiconomou A., Ferchal F., Perol Y., Gautier B., Haguenau M., Pepin B. Inclusion-body myositis: clinicopathological studies and isolation of an adenovirus type 2 from muscle biopsy specimen. Ann Neurol. 1982 Jun;11(6):576–581. doi: 10.1002/ana.410110605. [DOI] [PubMed] [Google Scholar]
- Nász I., Kulcsár G., Dán P., Sallay K. A possible pathogenic role for virus-carrier lymphocytes. J Infect Dis. 1971 Aug;124(2):214–216. doi: 10.1093/infdis/124.2.214. [DOI] [PubMed] [Google Scholar]
- Schranz V., Kulcsár G., Dán P., Horváth J., Nász I., Barinsky I. F., Ugryumov E. P. Interaction of human lymphocytes and viruses in vitro. Acta Microbiol Acad Sci Hung. 1979;26(1):1–9. [PubMed] [Google Scholar]
- Trifajová J., Brûcková M., Rýc M., Lukásová M. Type 5 adenovirus isolated from urine of patient with Hodgkin's disease. J Hyg Epidemiol Microbiol Immunol. 1981;25(3):321–323. [PubMed] [Google Scholar]
- de Jong P. J., Valderrama G., Spigland I., Horwitz M. S. Adenovirus isolates from urine of patients with acquired immunodeficiency syndrome. Lancet. 1983 Jun 11;1(8337):1293–1296. doi: 10.1016/s0140-6736(83)92411-x. [DOI] [PubMed] [Google Scholar]
- van der Veen J., Lambriex M. Relationship of adenovirus to lymphocytes in naturally infected human tonsils and adenoids. Infect Immun. 1973 Apr;7(4):604–609. doi: 10.1128/iai.7.4.604-609.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]