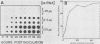Abstract
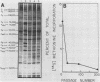
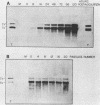
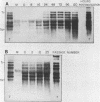
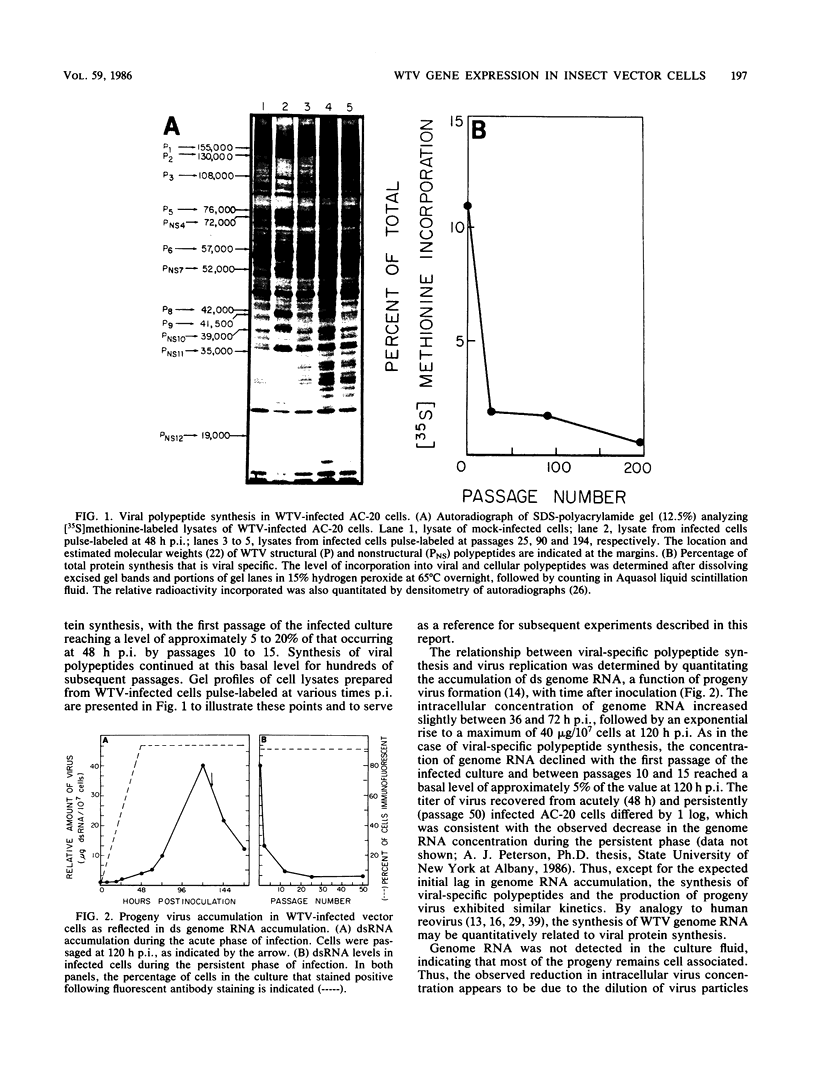
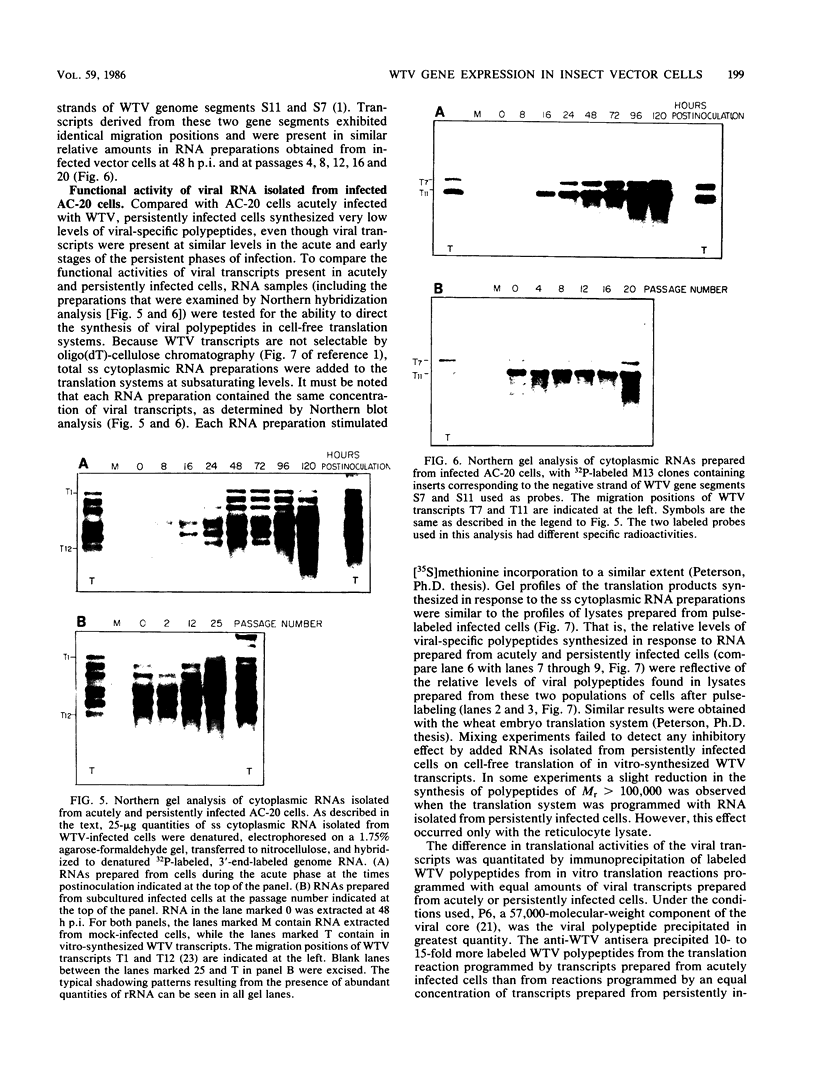
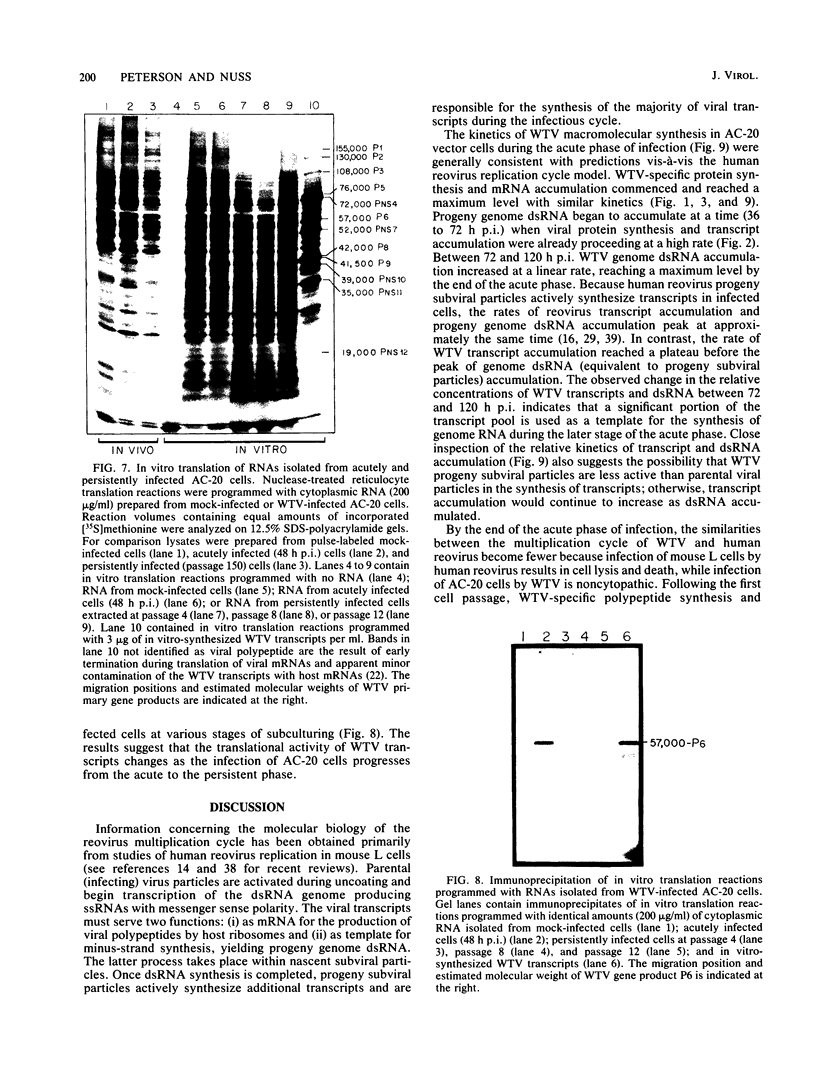
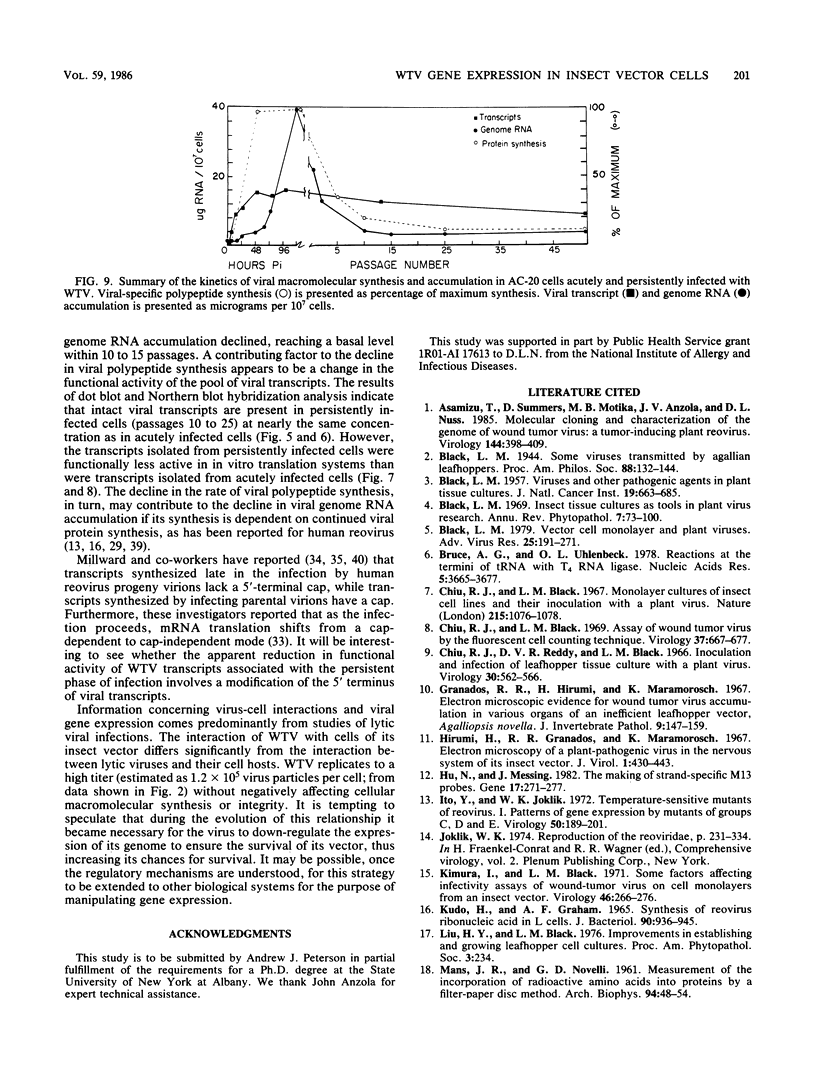
The interaction between a plant virus and its insect vector was studied at the molecular level by examining wound tumor virus (WTV) gene expression in cultured cells derived from its leafhopper vector. Infection of vector cells by WTV is noncytopathic and results in an acute phase (through day 5), followed by persistence beginning with the first cell passage. Viral-specific polypeptide synthesis and viral genome RNA accumulation increased to a maximum level during the first 5 days following inoculation and then decreased as infected cells were passaged (to 5 to 20% of the level observed during the acute phase by passages 10 to 15). In contrast, viral-specific mRNAs were present at approximately the same level in the acute phase and in the early stage (passage 10) of the persistent phase of infection. Although viral transcripts isolated at different times after inoculation exhibited identical electrophoretic migration patterns, they had different functional activities in cell-free translation systems. Transcripts isolated from persistently infected cells were inefficiently translated in vitro, reflecting the situation in infected cells. These results indicate that the decline in the level of viral polypeptide synthesis associated with the persistent phase of WTV infection is related to a change in the translational activity of viral transcripts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asamizu T., Summers D., Motika M. B., Anzola J. V., Nuss D. L. Molecular cloning and characterization of the genome of wound tumor virus: a tumor-inducing plant reovirus. Virology. 1985 Jul 30;144(2):398–409. doi: 10.1016/0042-6822(85)90281-8. [DOI] [PubMed] [Google Scholar]
- BLACK L. M. Viruses and other pathogenic agents in plant tissue cultures. J Natl Cancer Inst. 1957 Oct;19(4):663–685. [PubMed] [Google Scholar]
- Black L. M. Vector cell monolayers and plant viruses. Adv Virus Res. 1979;25:191–271. doi: 10.1016/s0065-3527(08)60571-0. [DOI] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 1978 Oct;5(10):3665–3677. doi: 10.1093/nar/5.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R. J., Black L. M. Assay of wound tumor virus by the fluorescent cell counting technique. Virology. 1969 Apr;37(4):667–677. doi: 10.1016/0042-6822(69)90285-2. [DOI] [PubMed] [Google Scholar]
- Chiu R. J., Black L. M. Monolayer cultures of insect cell lines and their inoculation with a plant virus. Nature. 1967 Sep 2;215(5105):1076–1078. doi: 10.1038/2151076a0. [DOI] [PubMed] [Google Scholar]
- Chiu R., Reddy D. V., Black L. M. Inoculation and infection of leafhopper tissue cultures with a plant virus. Virology. 1966 Nov;30(3):562–566. doi: 10.1016/0042-6822(66)90131-0. [DOI] [PubMed] [Google Scholar]
- Hirumi H., Granados R. R., Maramorosch K. Electron microscopy of a plant-pathogenic virus in the nervous system of its insect vector. J Virol. 1967 Apr;1(2):430–444. doi: 10.1128/jvi.1.2.430-444.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Ito Y., Joklik W. K. Temperature-sensitive mutants of reovirus. I. Patterns of gene expression by mutants of groups C, D, and E. Virology. 1972 Oct;50(1):189–201. doi: 10.1016/0042-6822(72)90359-5. [DOI] [PubMed] [Google Scholar]
- Kimura I., Black L. M. Some factors affecting infectivity assays of wound-tumor virus on cell monolayers from an insect vector. Virology. 1971 Nov;46(2):266–276. doi: 10.1016/0042-6822(71)90029-8. [DOI] [PubMed] [Google Scholar]
- Kudo H., Graham A. F. Synthesis of reovirus ribonucleic acid in L cells. J Bacteriol. 1965 Oct;90(4):936–945. doi: 10.1128/jb.90.4.936-945.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARAMOROSCH K. Arthropod transmission of plant viruses. Annu Rev Entomol. 1963;8:369–414. doi: 10.1146/annurev.en.08.010163.002101. [DOI] [PubMed] [Google Scholar]
- Nuss D. L. Molecular biology of wound tumor virus. Adv Virus Res. 1984;29:57–93. doi: 10.1016/s0065-3527(08)60405-4. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Peterson A. J. Expression of wound tumor virus gene products in vivo and in vitro. J Virol. 1980 May;34(2):532–541. doi: 10.1128/jvi.34.2.532-541.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L., Summers D. Variant dsRNAs associated with transmission-defective isolates of wound tumor virus represent terminally conserved remnants of genome segments. Virology. 1984 Mar;133(2):276–288. doi: 10.1016/0042-6822(84)90395-7. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Peterson A. J., Nuss D. L. Wound tumor virus polypeptide synthesis in productive noncytopathic infection of cultured insect vector cells. J Virol. 1985 Nov;56(2):620–624. doi: 10.1128/jvi.56.2.620-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Deletion mutations of the genome segments of wound tumor virus. Virology. 1974 Oct;61(2):458–473. doi: 10.1016/0042-6822(74)90282-7. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Increase of wound tumor virus in leafhoppers as assayed on vector cell monolayers. Virology. 1972 Nov;50(2):412–421. doi: 10.1016/0042-6822(72)90393-5. [DOI] [PubMed] [Google Scholar]
- SHIKATA E., ORENSKI S. W., HIRUMI H., MITSUHASHI J., MARAMOROSCH K. ELECTRON MICROGRAPHS OF WOUND-TUMOR VIRUS IN AN ANIMAL HOST AND IN A PLANT TUMOR. Virology. 1964 Jul;23:441–444. doi: 10.1016/0042-6822(64)90272-7. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Rada B. Reovirus-directed ribonucleic acid synthesis in infected L cells. J Virol. 1967 Feb;1(1):24–35. doi: 10.1128/jvi.1.1.24-35.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata E., Maramorosch K. Electron microscopic evidence for the systemic invasion of an insect host by a plant pathogenic virus. Virology. 1965 Dec;27(4):461–475. doi: 10.1016/0042-6822(65)90171-6. [DOI] [PubMed] [Google Scholar]
- Sinha R. C. Sequential infection and distribution of wound-tumor virus in the internal organs of a vector after ingestion of virus. Virology. 1965 Aug;26(4):673–686. doi: 10.1016/0042-6822(65)90330-2. [DOI] [PubMed] [Google Scholar]
- Skup D., Millward S. Reovirus-induced modification of cap-dependent translation in infected L cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):152–156. doi: 10.1073/pnas.77.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skup D., Millward S. mRNA capping enzymes are masked in reovirus progeny subviral particles. J Virol. 1980 May;34(2):490–496. doi: 10.1128/jvi.34.2.490-496.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skup D., Zarbl H., Millward S. Regulation of translation in L-cells infected with reovirus. J Mol Biol. 1981 Sep 5;151(1):35–55. doi: 10.1016/0022-2836(81)90220-5. [DOI] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Kudo H., Graham A. F. Selective inhibition of reovirus ribonucleic acid synthesis by cycloheximide. J Virol. 1967 Feb;1(1):36–44. doi: 10.1128/jvi.1.1.36-44.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbl H., Skup D., Millward S. Reovirus progeny subviral particles synthesize uncapped mRNA. J Virol. 1980 May;34(2):497–505. doi: 10.1128/jvi.34.2.497-505.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]