Abstract
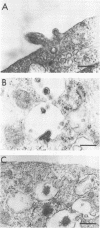
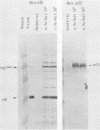
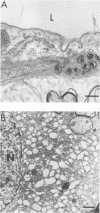
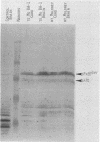
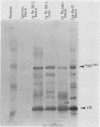
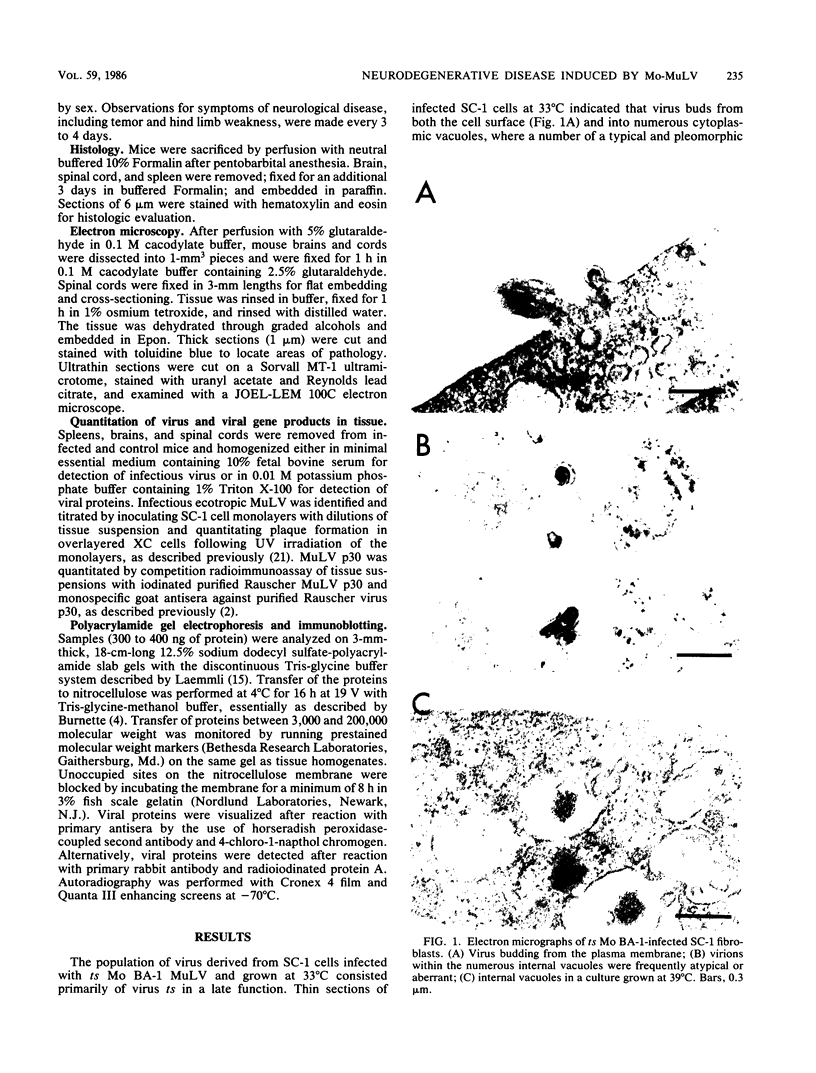
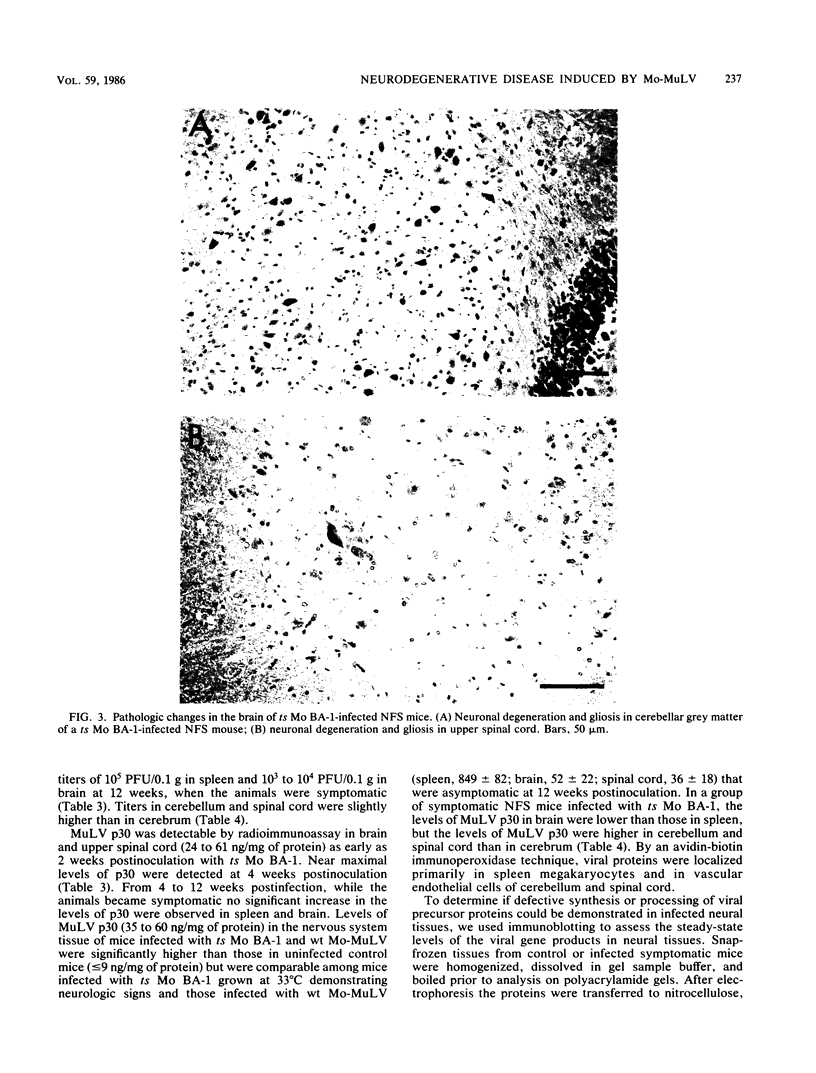
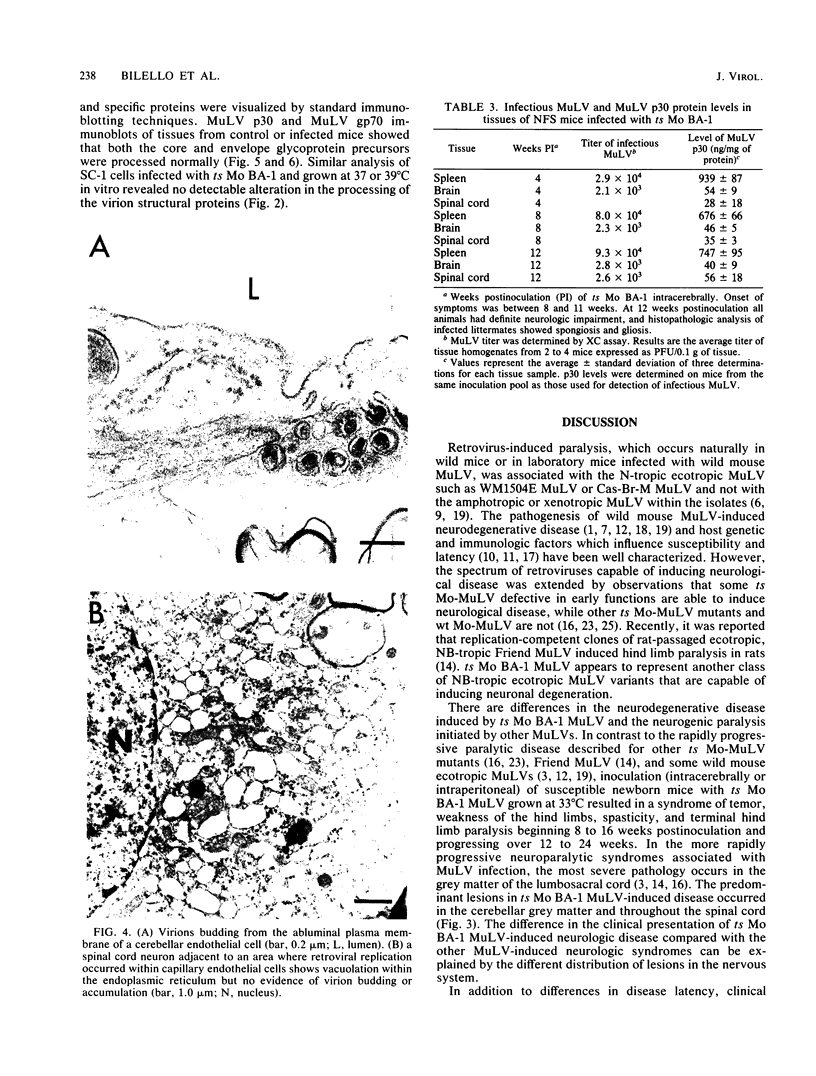
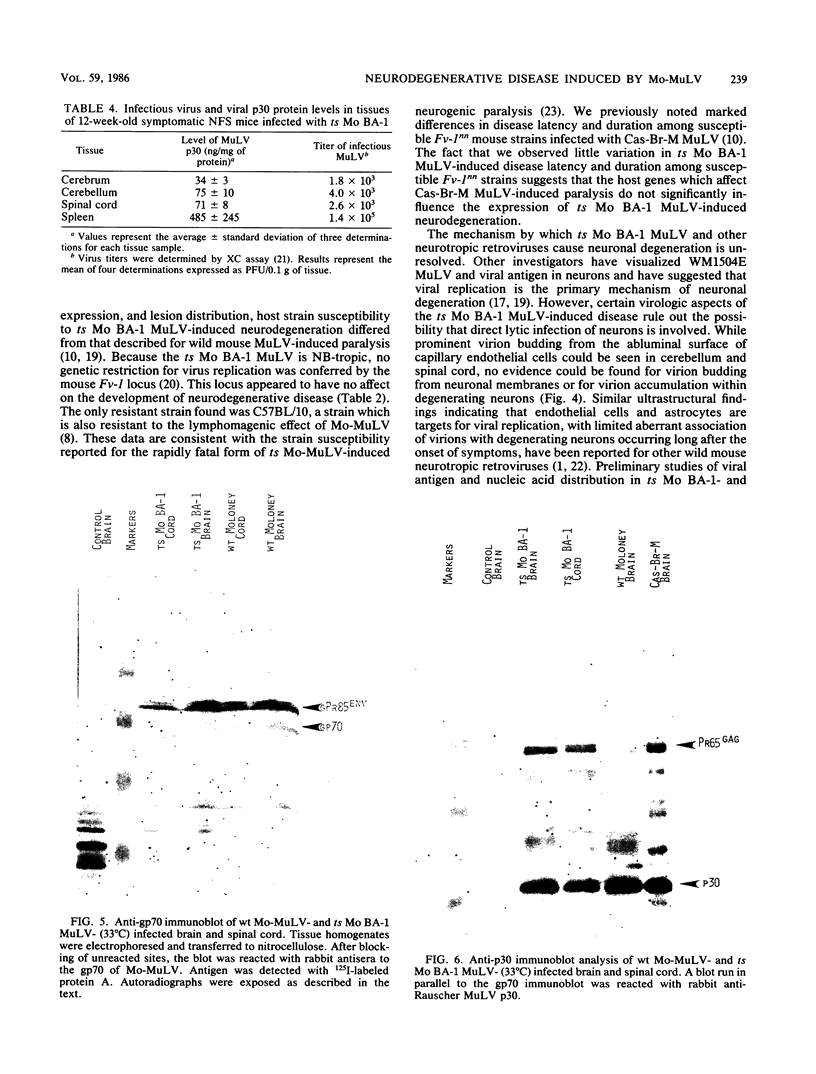
A progressive neurodegenerative disease occurred following infection of mice with a temperature-sensitive (ts) isolate of Moloney (Mo) murine leukemia virus (MuLV), ts Mo BA-1 MuLV. This NB-tropic ecotropic MuLV, which was ts for a late function, induced a syndrome of tremor, weakness of the hind limbs, and spasticity following infection of several strains of laboratory neonatal mice, including NFS, C3H/He, CBA, SJL, and BALB/c. The latent period of 8 to 16 weeks was considerably longer than that observed for the acute paralytic diseases observed following neonatal infection with other ts Mo-MuLV, rat-passaged Friend MuLV, and some wild mouse ecotropic MuLVs. Spongiform pathology without inflammation and degeneration of neurons devoid of budding virions occurred in the cerebellar grey matter, brain stem, and upper spinal cord; but lower spinal cord anterior horn cells were less obviously affected than in other MuLV-associated neuroparalytic syndromes. ts Mo BA-1 MuLV differed from other ts Mo-MuLV mutants that are capable of inducing a neuroparalytic syndrome in that while infected nervous system tissue contained high levels of MuLV p30 and gp70, no evidence of precursor accumulation or abnormal processing of MuLV p30 or gp70 could be demonstrated. The localization of virus within the nervous system suggests that direct neuronal infection may not be the etiologic mechanism in this MuLV-induced neurodegenerative disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J. M., Gardner M. B. Lower motor neuron degeneration associated with type C RNA virus infection in mice: neuropathological features. J Neuropathol Exp Neurol. 1974 Apr;33(2):285–307. doi: 10.1097/00005072-197404000-00007. [DOI] [PubMed] [Google Scholar]
- Bilello J. A., Strand M., August J. T. Murine sarcoma virus gene expression: transformants which express viral envelope glycoprotein in the absence of the major internal protein and infectious particles. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3234–3238. doi: 10.1073/pnas.71.8.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks B. R., Swarz J. R., Narayan O., Johnson R. T. Murine neurotropic retrovirus spongiform polioencephalomyelopathy: acceleration of disease by virus inoculum concentration. Infect Immun. 1979 Feb;23(2):540–544. doi: 10.1128/iai.23.2.540-544.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- DesGroseillers L., Barrette M., Jolicoeur P. Physical mapping of the paralysis-inducing determinant of a wild mouse ecotropic neurotropic retrovirus. J Virol. 1984 Nov;52(2):356–363. doi: 10.1128/jvi.52.2.356-363.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Henderson B. E., Officer J. E., Rongey R. W., Parker J. C., Oliver C., Estes J. D., Huebner R. J. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J Natl Cancer Inst. 1973 Oct;51(4):1243–1254. doi: 10.1093/jnci/51.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B. Type C viruses of wild mice: characterization and natural history of amphotropic, ecotropic, and xenotropic MuLv. Curr Top Microbiol Immunol. 1978;79:215–259. doi: 10.1007/978-3-642-66853-1_5. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht S., Pozo F., Debré P., Hurot M. A., Lacombe M. J., Levy J. P. Genetic control of sensitivity to Moloney-virus-induced leukemias in mice. I. Demonstration of multigenic control. Int J Cancer. 1978 May 15;21(5):626–634. doi: 10.1002/ijc.2910210513. [DOI] [PubMed] [Google Scholar]
- Hoffman P. M., Davidson W. F., Ruscetti S. K., Chused T. M., Morse H. C., 3rd Wild mouse ecotropic murine leukemia virus infection of inbred mice: dual-tropic virus expression precedes the onset of paralysis and lymphoma. J Virol. 1981 Aug;39(2):597–602. doi: 10.1128/jvi.39.2.597-602.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. M., Morse H. C., 3rd Host genetic determinants of neurological disease induced by Cas-Br-M murine leukemia virus. J Virol. 1985 Jan;53(1):40–43. doi: 10.1128/jvi.53.1.40-43.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. M., Robbins D. S., Morse H. C., 3rd Role of immunity in age-related resistance to paralysis after murine leukemia virus infection. J Virol. 1984 Dec;52(3):734–738. doi: 10.1128/jvi.52.3.734-738.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. M., Ruscetti S. K., Morse H. C., 3rd Pathogenesis of paralysis and lymphoma associated with a wild mouse retrovirus infection. Part 1. Age- and dose-related effects in susceptible laboratory mice. J Neuroimmunol. 1981 Sep;1(3):275–285. doi: 10.1016/0165-5728(81)90031-x. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Nicolaiew N., DesGroseillers L., Rassart E. Molecular cloning of infectious viral DNA from ecotropic neurotropic wild mouse retrovirus. J Virol. 1983 Mar;45(3):1159–1163. doi: 10.1128/jvi.45.3.1159-1163.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai K., Furuta T. Isolation of paralysis-inducing murine leukemia viruses from Friend virus passaged in rats. J Virol. 1984 Jun;50(3):970–973. doi: 10.1128/jvi.50.3.970-973.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCarter J. A., Ball J. K., Frei J. V. Lower limb paralysis induced in mice by a temperature-sensitive mutant of Moloney leukemia virus. J Natl Cancer Inst. 1977 Jul;59(1):179–183. doi: 10.1093/jnci/59.1.179. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Jensen F., Dixon F. J., Lampert P. W. Pathogenesis of the slow disease of the central nervous system associated with wild mouse virus. II. Role of virus and host gene products. Virology. 1980 Nov;107(1):180–193. doi: 10.1016/0042-6822(80)90283-4. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Jensen F., Elder J., Dixon F. J., Lampert P. W. Pathogenesis of the slow disease of the central nervous system associated with wild mouse virus. III. Role of input virus and MCF recombinants in disease. Virology. 1983 Jul 15;128(1):154–165. doi: 10.1016/0042-6822(83)90326-4. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Lampert P. W., Lee S., Dixon F. J. Pathogenesis of the slow disease of the central nervous system associated with WM 1504 E virus. I. Relationship of strain susceptibility and replication to disease. Am J Pathol. 1977 Jul;88(1):193–212. [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Swarz J. R., Brooks B. R., Johnson R. T. Spongiform polioencephalomyelopathy caused by a murine retrovirus. II. Ultrastructural localization of virus replication and spongiform changes in the central nervous system. Neuropathol Appl Neurobiol. 1981 Sep-Oct;7(5):365–380. doi: 10.1111/j.1365-2990.1981.tb00239.x. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Knupp C., Yuen P. H., Soong M. M., Zachary J. F., Tompkins W. A. ts1, a Paralytogenic mutant of Moloney murine leukemia virus TB, has an enhanced ability to replicate in the central nervous system and primary nerve cell culture. J Virol. 1985 Sep;55(3):760–767. doi: 10.1128/jvi.55.3.760-767.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., Soong M. M., MacLeod R., Gallick G. E., Yuen P. H. A group of temperature-sensitive mutants of Moloney leukemia virus which is defective in cleavage of env precursor polypeptide in infected cells also induces hind-limb paralysis in newborn CFW/D mice. Virology. 1983 Mar;125(2):513–518. doi: 10.1016/0042-6822(83)90225-8. [DOI] [PubMed] [Google Scholar]
- Yuen P. H., Malehorn D., Knupp C., Wong P. K. A 1.6-kilobase-pair fragment in the genome of the ts1 mutant of Moloney murine leukemia virus TB that is associated with temperature sensitivity, nonprocessing of Pr80env, and paralytogenesis. J Virol. 1985 May;54(2):364–373. doi: 10.1128/jvi.54.2.364-373.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen P. H., Malehorn D., Nau C., Soong M. M., Wong P. K. Molecular cloning of two paralytogenic, temperature-sensitive mutants, ts1 and ts7, and the parental wild-type Moloney murine leukemia virus. J Virol. 1985 Apr;54(1):178–185. doi: 10.1128/jvi.54.1.178-185.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]