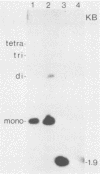
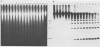
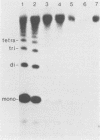
Abstract
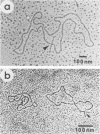
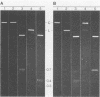
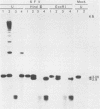
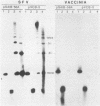
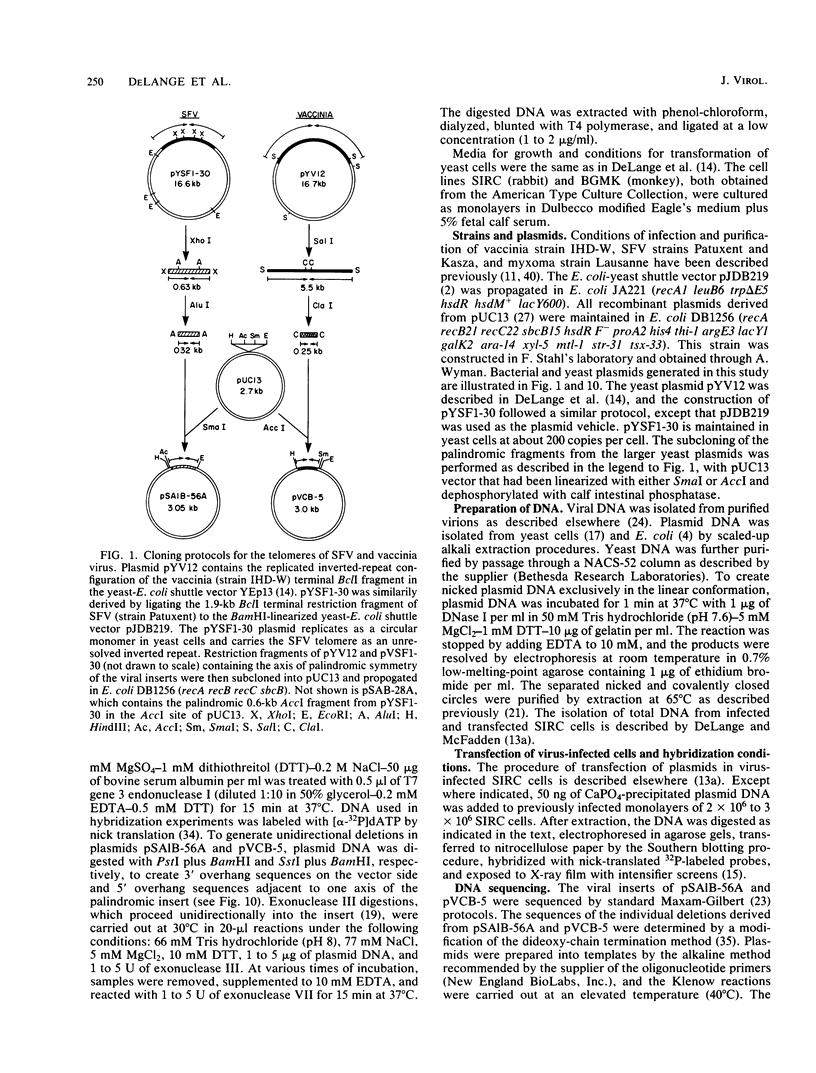
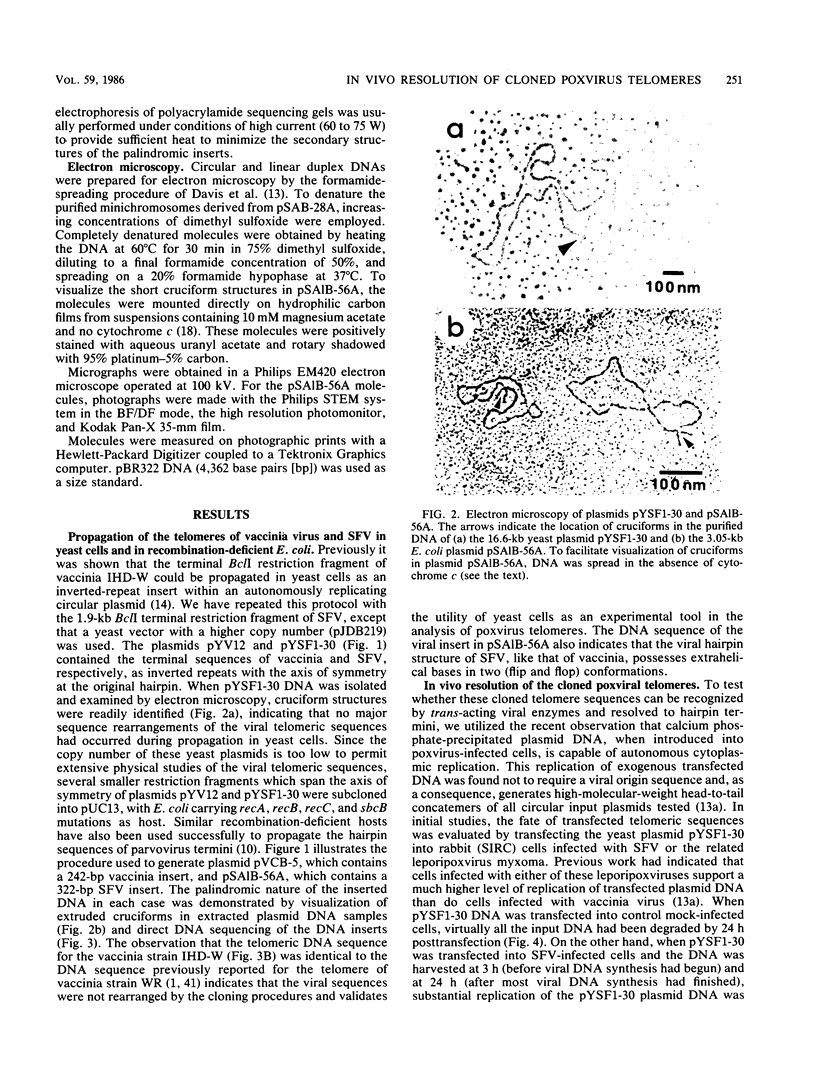
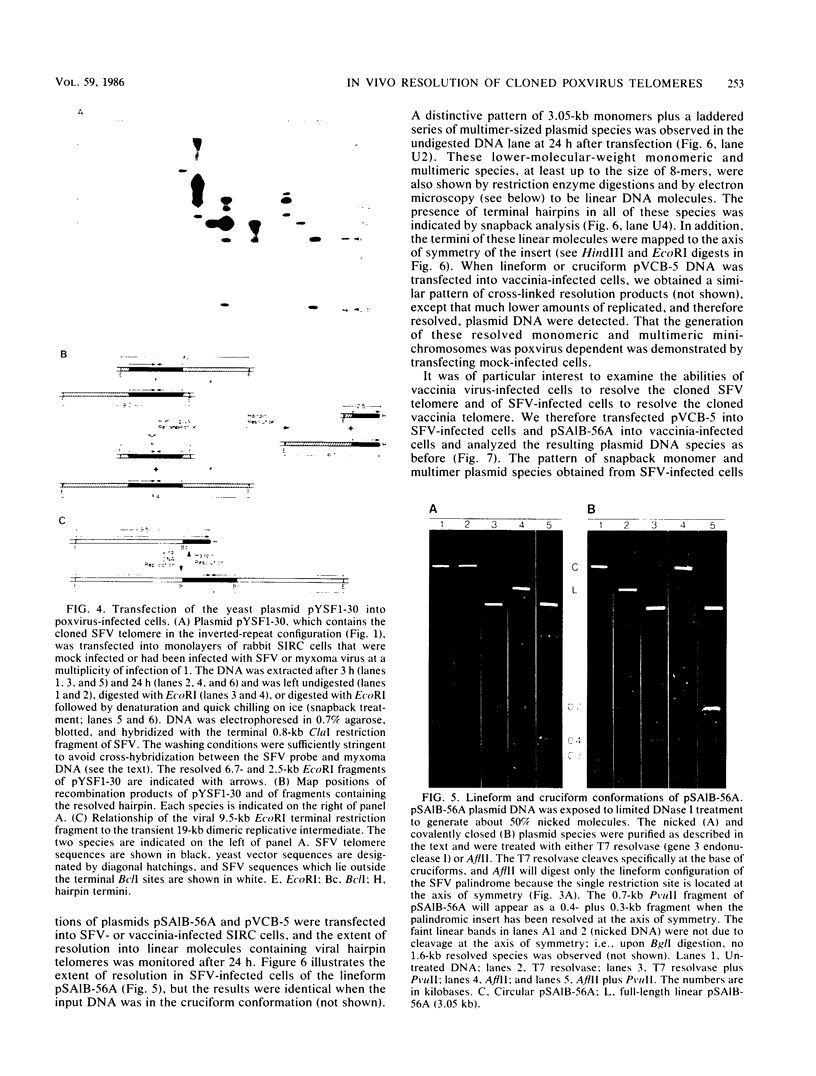
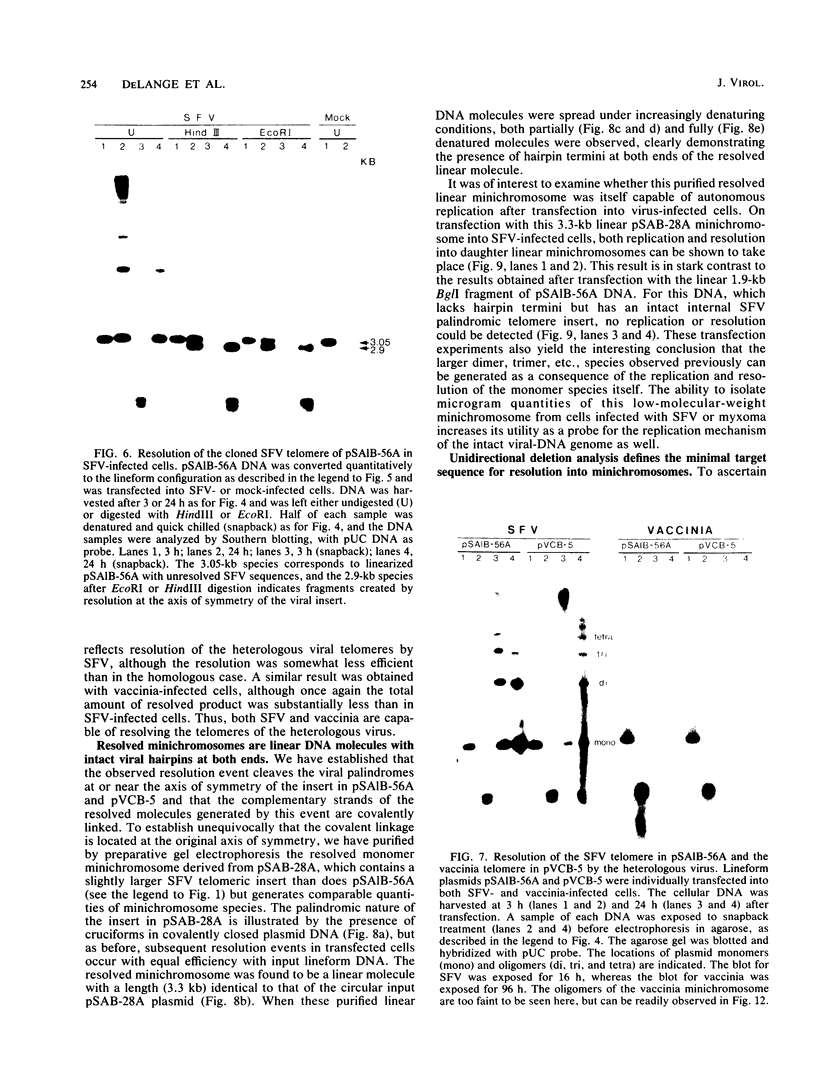
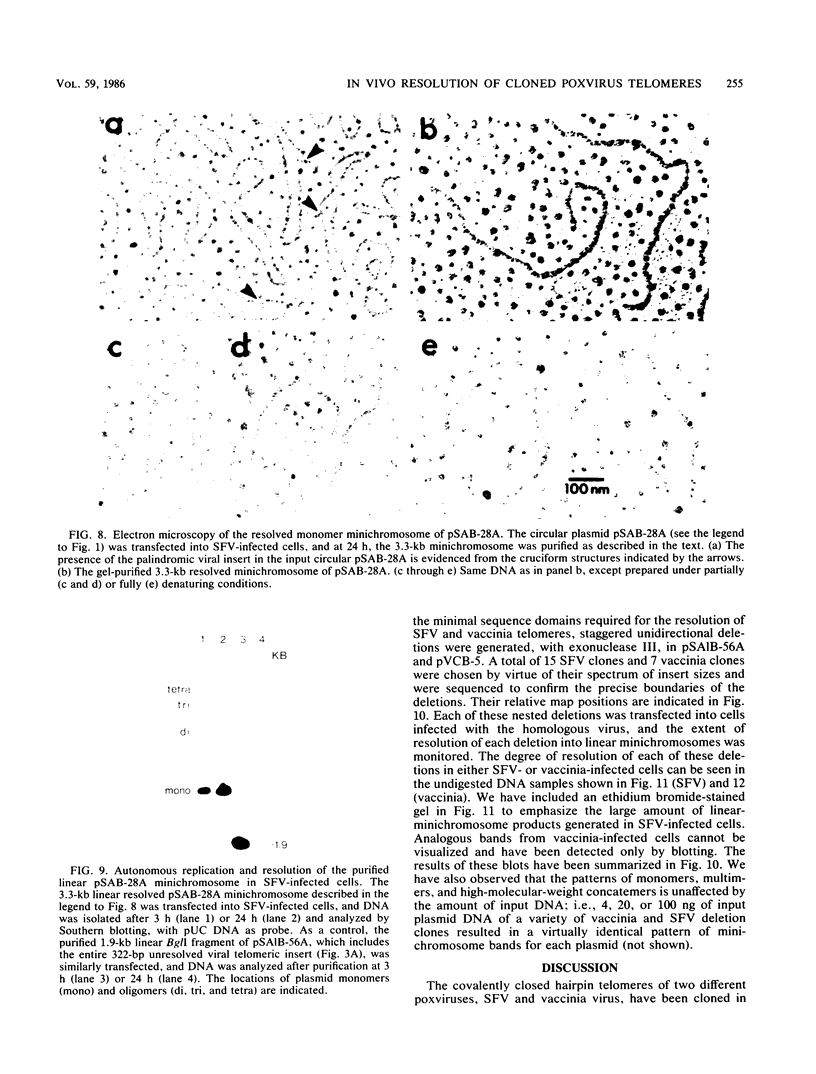
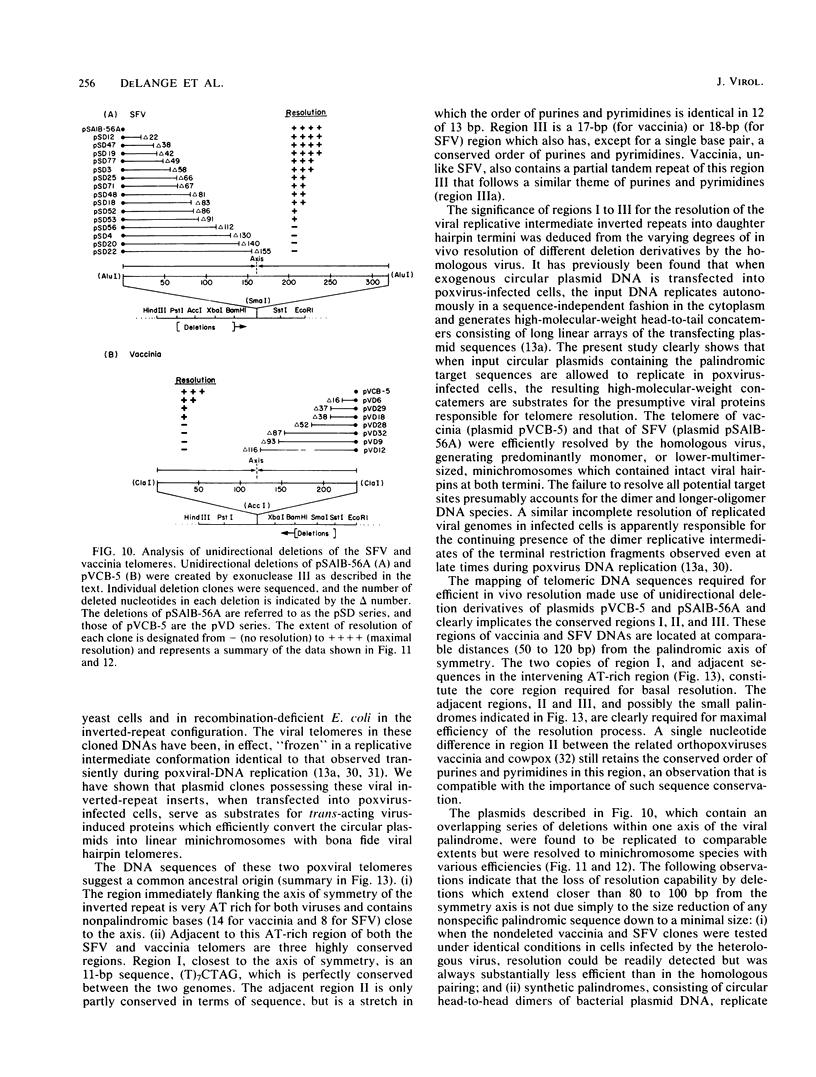
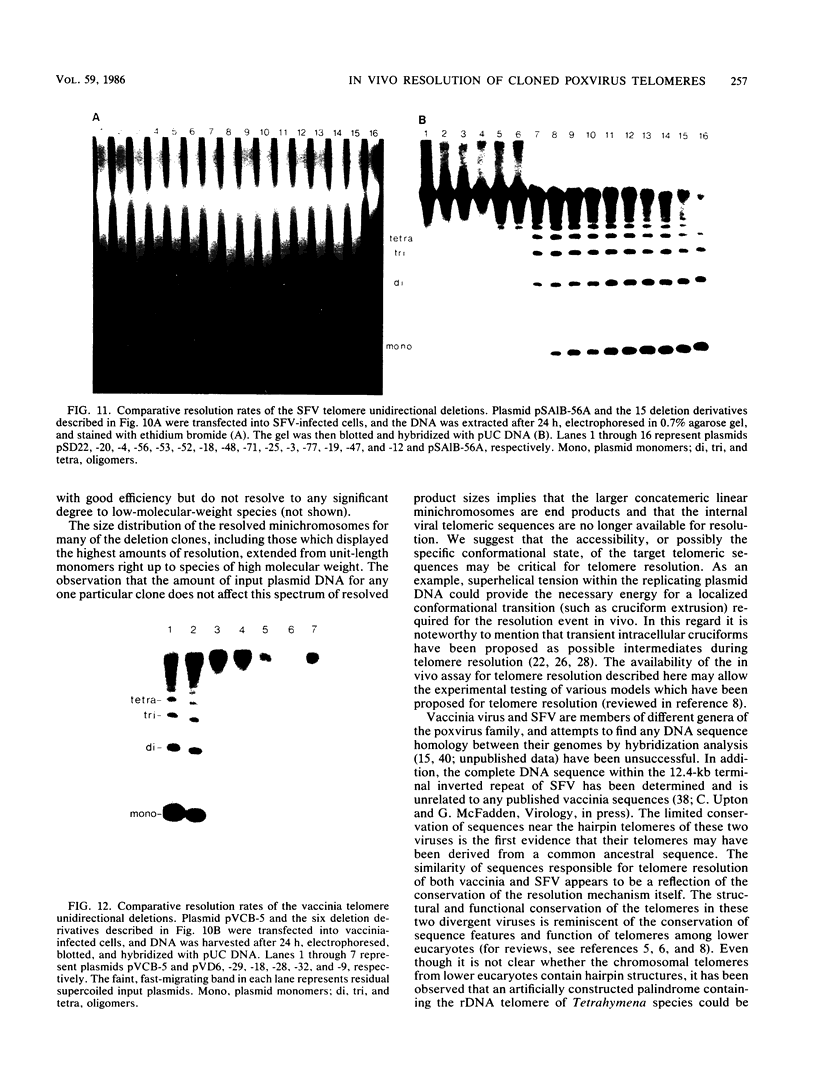
The covalently closed terminal hairpins of the linear duplex-DNA genomes of the orthopoxvirus vaccinia and the leporipoxvirus Shope fibroma virus (SFV) have been cloned as imperfect palindromes within circular plasmids in yeast cells and recombination-deficient Escherichia coli. The viral telomeres inserted within these recombinant plasmids are equivalent to the inverted-repeat structures detected as telomeric replicative intermediates during poxvirus replication in vivo. Although the telomeres of vaccinia and SFV show little sequence homology, the termini from both viral genomes exist as AT-rich terminal hairpins with extrahelical bases and alternate "flip-flop" configurations. Using an in vivo replication assay in which circular plasmid DNA was transfected into poxvirus-infected cells, we demonstrated the efficient replication and resolution of the cloned imperfect palindromes to bona fide hairpin termini. The resulting linear minichromosomes, which were readily purified from transfected cells, were shown by restriction enzyme mapping and by electron microscopy to have intact covalently closed hairpin termini at both ends. In addition, staggered unidirectional deletion derivatives of both the cloned vaccinia and SFV telomeric palindromes localized an approximately 200-base-pair DNA region in which the sequence organization was highly conserved and which was necessary for the resolution event. These data suggest a conserved mechanism of the resolution of poxvirus telomeres.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroudy B. M., Venkatesan S., Moss B. Incompletely base-paired flip-flop terminal loops link the two DNA strands of the vaccinia virus genome into one uninterrupted polynucleotide chain. Cell. 1982 Feb;28(2):315–324. doi: 10.1016/0092-8674(82)90349-x. [DOI] [PubMed] [Google Scholar]
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H., Gall J. G. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978 Mar 25;120(1):33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomeres: do the ends justify the means? Cell. 1984 May;37(1):7–8. doi: 10.1016/0092-8674(84)90295-2. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Boissy R., Astell C. R. An Escherichia coli recBCsbcBrecF host permits the deletion-resistant propagation of plasmid clones containing the 5'-terminal palindrome of minute virus of mice. Gene. 1985;35(1-2):179–185. doi: 10.1016/0378-1119(85)90170-2. [DOI] [PubMed] [Google Scholar]
- Dales S., Milovanovitch V., Pogo B. G., Weintraub S. B., Huima T., Wilton S., McFadden G. Biogenesis of vaccinia: isolation of conditional lethal mutants and electron microscopic characterization of their phenotypically expressed defects. Virology. 1978 Feb;84(2):403–428. doi: 10.1016/0042-6822(78)90258-1. [DOI] [PubMed] [Google Scholar]
- DeLange A. M., Futcher B., Morgan R., McFadden G. Cloning of the vaccinia virus telomere in a yeast plasmid vector. Gene. 1984 Jan;27(1):13–21. doi: 10.1016/0378-1119(84)90234-8. [DOI] [PubMed] [Google Scholar]
- DeLange A. M., McFadden G. Sequence-nonspecific replication of transfected plasmid DNA in poxvirus-infected cells. Proc Natl Acad Sci U S A. 1986 Feb;83(3):614–618. doi: 10.1073/pnas.83.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delange A. M., Macaulay C., Block W., Mueller T., McFadden G. Tumorigenic poxviruses: construction of the composite physical map of the Shope fibroma virus genome. J Virol. 1984 May;50(2):408–416. doi: 10.1128/jvi.50.2.408-416.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenish R. J., Newlon C. S. Isolation and characterization of yeast ring chromosome III by a method applicable to other circular DNAs. Gene. 1982 Jun;18(3):277–288. doi: 10.1016/0378-1119(82)90166-4. [DOI] [PubMed] [Google Scholar]
- Di Capua E., Engel A., Stasiak A., Koller T. Characterization of complexes between recA protein and duplex DNA by electron microscopy. J Mol Biol. 1982 May 5;157(1):87–103. doi: 10.1016/0022-2836(82)90514-9. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hsu P. L., Landy A. Resolution of synthetic att-site Holliday structures by the integrase protein of bacteriophage lambda. Nature. 1984 Oct 25;311(5988):721–726. doi: 10.1038/311721a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Lilley D. M., Kemper B. Cruciform-resolvase interactions in supercoiled DNA. Cell. 1984 Feb;36(2):413–422. doi: 10.1016/0092-8674(84)90234-4. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McFadden G., Dales S. Biogenesis of poxviruses: mirror-image deletions in vaccinia virus DNA. Cell. 1979 Sep;18(1):101–108. doi: 10.1016/0092-8674(79)90358-1. [DOI] [PubMed] [Google Scholar]
- McFadden G., Morgan A. R. DNA cruciform structures: implications for telomer replication in eukaryotes and instability of long palindromic DNA sequences in prokaryotes. J Theor Biol. 1982 Jul 21;97(2):343–349. doi: 10.1016/0022-5193(82)90112-6. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., Kemper B., Hays J., Weisberg R. A. T4 endonuclease VII cleaves holliday structures. Cell. 1982 Jun;29(2):357–365. doi: 10.1016/0092-8674(82)90152-0. [DOI] [PubMed] [Google Scholar]
- Moyer R. W., Graves R. L. The mechanism of cytoplasmic orthopoxvirus DNA replication. Cell. 1981 Dec;27(2 Pt 1):391–401. doi: 10.1016/0092-8674(81)90422-0. [DOI] [PubMed] [Google Scholar]
- Pickup D. J., Bastia D., Stone H. O., Joklik W. K. Sequence of terminal regions of cowpox virus DNA: arrangement of repeated and unique sequence elements. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7112–7116. doi: 10.1073/pnas.79.23.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard A. E., Cummings D. J. Replication of linear mitochondrial DNA from Paramecium: sequence and structure of the initiation-end crosslink. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7341–7345. doi: 10.1073/pnas.78.12.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Szostak J. W., Blackburn E. H. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982 May;29(1):245–255. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- Szostak J. W. Replication and resolution of telomeres in yeast. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1187–1194. doi: 10.1101/sqb.1983.047.01.134. [DOI] [PubMed] [Google Scholar]
- Upton C., McFadden G. DNA sequence homology between the terminal inverted repeats of Shope fibroma virus and an endogenous cellular plasmid species. Mol Cell Biol. 1986 Jan;6(1):265–276. doi: 10.1128/mcb.6.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. C., Körner A. Cleavage of cruciform DNA structures by an activity from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6445–6449. doi: 10.1073/pnas.82.19.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills A., Delange A. M., Gregson C., Macaulay C., McFadden G. Physical characterization and molecular cloning of the Shope fibroma virus DNA genome. Virology. 1983 Oct 30;130(2):403–414. doi: 10.1016/0042-6822(83)90095-8. [DOI] [PubMed] [Google Scholar]
- Winters E., Baroudy B. M., Moss B. Molecular cloning of the terminal hairpin of vaccinia virus DNA as an imperfect palindrome in an Escherichia coli plasmid. Gene. 1985;37(1-3):221–228. doi: 10.1016/0378-1119(85)90276-8. [DOI] [PubMed] [Google Scholar]
- Wittek R. Organization and expression of the poxvirus genome. Experientia. 1982 Mar 15;38(3):285–297. doi: 10.1007/BF01949349. [DOI] [PubMed] [Google Scholar]
- de Massy B., Studier F. W., Dorgai L., Appelbaum E., Weisberg R. A. Enzymes and sites of genetic recombination: studies with gene-3 endonuclease of phage T7 and with site-affinity mutants of phage lambda. Cold Spring Harb Symp Quant Biol. 1984;49:715–726. doi: 10.1101/sqb.1984.049.01.081. [DOI] [PubMed] [Google Scholar]