Abstract
We have previously shown that in HEp-2 cells, multivesicular bodies (MVBs) processing internalized epidermal growth factor–epidermal growth factor receptor complexes mature and fuse directly with lysosomes in which the complexes are degraded. The MVBs do not fuse with a prelysosomal compartment enriched in mannose 6-phosphate receptor (M6PR) as has been described in other cell types. Here we show that the cation-independent M6PR does not become enriched in the endocytic pathway en route to the lysosome, but if a pulse of M6PR or an M6PR ligand, cathepsin D, is followed, a significant fraction of these proteins are routed from the trans-Golgi to MVBs. Accumulation of M6PR does not occur because when the ligand dissociates, the receptor rapidly leaves the MVB. At steady state, most M6PR are distributed within the trans-Golgi and trans-Golgi network and in vacuolar structures distributed in the peripheral cytoplasm. We suggest that these M6PR-rich vacuoles are on the return route from MVBs to the trans-Golgi network and that a separate stable M6PR-rich compartment equivalent to the late endosome/prelysosome stage does not exist on the endosome–lysosome pathway in these cells.
INTRODUCTION
Mannose 6-phosphate receptors (M6PRs) deliver newly synthesized acid hydrolases to the endocytic pathway and then return to the trans-Golgi network (TGN; Storrie et al., 1984; Brown et al., 1986; Duncan and Kornfeld, 1988; Kornfeld and Mellman, 1989). The route taken by M6PR-acid hydrolase complexes within the endocytic pathway has not yet been clearly defined but, from studies of the steady-state distribution of M6PR, it is generally believed that these receptors concentrate in a compartment on the endosome-to-lysosome pathway, which has been called the prelysosome or late endosome (Griffiths et al., 1988, 1990; Kornfeld and Mellman, 1989). This prelysosomal compartment labels poorly for recycling receptors such as transferrin receptor (TR) but can be labeled with fluid phase probes, which are efficiently delivered to the lysosome (Griffiths et al., 1988, 1990). In our previous studies in HEp-2 cells, we have followed the processing of epidermal growth factor–epidermal growth factor receptor (EGF-EGFR) complexes en route to the lysosome (Felder et al., 1990; Hopkins et al., 1990; Futter et al., 1996). After endocytosis, these complexes accumulate in multivesicular bodies (MVBs) which, when first formed, contain recycling receptors such as TR (Hopkins et al., 1990; van Deurs et al., 1993), and then undergo a maturation process whereby TRs are removed and EGF-EGFRs accumulate. The MVBs then fuse directly with preexisting lysosomes and EGF degradation begins (van Deurs et al., 1995; Futter et al., 1996). M6PR can be detected during the maturation of the MVBs in HEp-2 cells (van Deurs et al., 1993), but neither the mature MVB nor the lysosome are enriched in M6PR (Futter et al., 1996), raising the question of how M6PRs deliver acid hydrolases to the endocytic pathway in HEp-2 cells.
In these previous studies of the maturation of MVBs in HEp-2 cells (van Deurs et al., 1993, 1995; Futter et al., 1996), the traffic of M6PR has not been directly measured. Therefore, to determine whether the small amount of M6PR that can be detected in the maturing MVB (van Deurs et al., 1993 and the current study) represents a major trafficking route of this receptor, we have directly measured the traffic of a single cohort of cation-independent M6PR, or cathepsin D (CD) carried by M6PR, and have shown that a significant proportion of M6PR and its ligand is transferred to MVBs that are processing recycling TR and EGFR. We also show that the M6PRs rapidly flux through the maturing MVBs without accumulating in these vacuoles and that at steady state, M6PRs are concentrated in two sites (the TGN and in vacuoles in the peripheral cytoplasm), but neither site is on the processing pathway carrying EGFRs from the endosome to the lysosome.
MATERIALS AND METHODS
Reagents
For immunoprecipitation, polyclonal antibodies to the cytoplasmic domain of the cation-independent M6PR and to all biosynthetic forms of CD were generously provided by K. von Figura (Gottingen, Germany) and A. Hasilik (Munster, Germany), respectively. For immunofluorescence, a polyclonal antibody to the cation-independent M6PR was kindly provided by W.J. Brown (Cornell University, New York, NY). Monoclonal antibodies specific for the extracellular domain of the TR (B3/25), used for conjugation to colloidal gold, and the cytoplasmic tail of TR (H68.4), used for Western blotting, were kindly provided by I. Trowbridge (Salk Institute, San Diego, CA), and the monoclonal antibody to the extracellular domain of the EGFR (108) was kindly provided by J. Schlessinger (New York University Medical Center, New York, NY). The monoclonal anti-horseradish peroxidase (HRP) antibody was obtained from Stratech Scientific (Luton, United Kingdom). Donkey anti-mouse IgG and donkey anti-rabbit IgG antibodies, conjugated to either fluorescein isothiocyanate (FITC), rhodamine, or Cy5, were obtained from Stratech Scientific. Biotinylated EGF conjugated to streptavidin–Texas red (EGF-TxR) was obtained from Molecular Probes (Eugene, OR). Human iron-saturated transferrin (TF) was obtained from Sigma Chemical (Poole, United Kingdom).
FITC-conjugated TF (TF-FITC) was prepared as described in the study of Hopkins et al. (1994). Gold particles (10 nm) were prepared using the tannic acid method of Slot and Geuze (1985) and were stabilized with the monoclonal antibodies against TR (B3/25) or EGFR (108), as previously described (Hopkins and Trowbridge, 1983). Before use the gold complexes were washed by centrifugation (25,000 × g for 15 min) in a Beckman L8–55 ultracentrifuge (Beckman Instruments, Fullerton, CA) and used at a concentration that gave OD580 of 0.3–0.4.
Cell Maintenance and Transfection
HEp-2 (American Type Culture Collection CCL23, Rockville, MD) cells were grown in DMEM supplemented with 10% fetal calf serum and maintained at 37°C in a 5% CO2 atmosphere.
Transient expression of the chimera, sialyl transferase-HRP (ST-HRP; Stinchcombe et al., 1995) was performed as described by Connolly et al. (1994). After transfection the cells were plated onto coverslips for immunofluorescence and maintained in the above medium for 24 h.
Isolation of Endosomal Compartments
HEp-2 cells, preincubated overnight at 37°C with trans-35S label (25 μCi/ml, ICN Biomedicals, Thame, United Kingdom), were incubated with antibody-gold conjugates in Hanks’ balanced salt solution containing 20 mM HEPES and 0.5% bovine serum albumin (pH 7.4). The cells were then washed three times with lysis buffer (0.25 M sucrose, 10 mM triethanolamine, 1 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, pH 7.4), scraped off the dish in a minimum volume of lysis buffer using a rubber scraper, and lysed at 4°C by eight strokes in a ball-bearing homogenizer (0.008 ball bearing). At least 60% of the cells were lysed using this method. Cell debris was removed by centrifugation (800 × g for 10 min) and the resulting postnuclear supernatants (PNSs) were layered on 12-ml linear sucrose gradients (26–52% sucrose in 10 mM triethanolamine, 1.5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, pH 7.4), formed in Beckman ultraclear centrifugation tubes, and centrifuged in a SW40 swinging bucket rotor (200,000 × g for 15 h at 4°C) in a Beckman L8–55 ultracentrifuge. The endosome pellets were recovered from the bottom of the centrifugation tubes and analyzed by Western blotting for TR and EGFR and immunoprecipitation for M6PR. Immunoprecipitates were analyzed by SDS-PAGE and quantitated by excision and then counting of radioactive bands. Western blots were quantitated by densitometry.
Immunofluorescence
After incubation with FITC or TxR-labeled tracers, cells were fixed with 4% paraformaldehyde, quenched with 15 mM glycine, and permeabilized with 0.05% saponin, all in phosphate-buffered saline. Subsequent antibody incubations were performed in phosphate-buffered saline containing 1% bovine serum albumin and 0.01% saponin. For double labeling of EGF-TxR and M6PR, rabbit anti-M6PR antibody followed by anti-rabbit IgG-FITC was used. For triple labeling of internalized TF-FITC, ST-HRP, and M6PR, mouse anti-HRP, and rabbit anti-M6PR antibodies were used, followed by anti-rabbit IgG-rhodamine and anti-mouse IgG-Cy5. Laser scanning confocal microscopy was performed using an MRC 1024 confocal microscope (Bio-Rad Laboratories, Hemel Hempstead, United Kingdom) with an argon/krypton laser. Final images were merged using Photoshop (Adobe Systems, Mountain View, CA) and photographed using a Sapphire Slide Recorder (Management Graphics, Minneapolis, MN).
Detection of Newly Synthesized Proteins in MVBs
To measure the rate of appearance of newly synthesized proteins in MVBs, cells were pulsed with trans-35S label (125 μCi/ml) for 15 min at 37°C (for M6PR) or 15 min at 37°C, followed by a 20-min chase at 37°C (for CD) in methionine-free medium. The cells were then chased for 4 h at 20°C in DMEM containing 10% fetal calf serum and 20 mM HEPES. Anti-TR-gold was added in the last hour of the 20°C chase and then the cells were transferred to 37°C for various times in the continued presence of anti-TR-gold. Gold-loaded MVBs were isolated on a sucrose density gradient, as already described. M6PR and CD were immunoprecipitated from an aliquot of the PNS and from the isolated MVB fraction. Immunoprecipitates were analyzed by SDS-PAGE and quantitated by excision and counting of radioactive bands.
RESULTS
The Steady-State Distribution of M6PR in HEp-2 Cells
To quantitate the amount of M6PR in MVBs processing endocytosed TF and EGF, we took advantage of a gold-mediated density shift protocol that uses gold-conjugated antireceptor antibodies to load MVBs. The internalized gold conjugates increase the density of endocytic elements so that they can be purified by sucrose density gradient centrifugation. This protocol yields a highly purified fraction that electron microscopy shows to be composed primarily of MVBs (Beardmore et al., 1987; Futter et al., 1989, 1993). MVBs can be isolated using either anti-TR or anti-EGFR gold. Thus, when cells are loaded with gold at 20°C, a temperature that inhibits exit of EGFR from the TR recycling pathway, highly purified MVBs that contain both TR and EGFR are isolated no matter which gold conjugate is used (Table 1). Fractionation at 37°C for up to an hour using anti-EGFR-gold conjugates in the presence of EGF allows endosomal structures along the entire EGF processing pathway to the lysosome to be isolated and the amount of M6PR and TR, relative to EGFR, can be quantitated. The MVBs isolated from cells stimulated with EGF for 10 min at 37°C contain high levels of TR, relative to EGFR (Table 2) and thus are similar to those isolated from cells loaded at 20°C. However, with more prolonged incubations the level of TR in EGFR-containing MVBs falls by threefold. This fall is coincident with fusion of MVBs with lysosomes (Futter et al., 1996) and the onset of EGF degradation which, on continuous incubation at 37°C, occurs with a t1/2 of approximately 45 min.
Table 1.
Enrichment of TR and EGFR in MVBs isolated using either anti-EGFR-gold or anti-TR-gold
| Gold | Antigen | Yield % PNS | Fold enrichment |
|---|---|---|---|
| Anti-TR | TR | 22.9 | 122.2 |
| (B3/25) | EGFR | 29.1 | 155.1 |
| Anti-EGFR | TR | 12.3 | 65.5 |
| (108) | EGFR | 30.2 | 160.1 |
MVBs were isolated by loading radiolabeled HEp-2 cells with either anti-EGFR-gold or anti-TR-gold in the presence of saturating EGF (200 ng/ml) for 90 min at 20°C. Gold-loaded MVBs were then isolated as described in MATERIALS AND METHODS. The amounts of TR and EGFR in these isolated fractions were determined by Western blotting and expressed as a percentage of the total of each loaded on the fractionation gradient (% PNS). By measuring trichloroacetic acid precipitable 35S-radioactivity in the PNS and isolated MVB fractions, it was possible to determine fold enrichment of TR and EGFR in the anti-EGFR-gold– and anti-TR-gold–isolated MVB.
Table 2.
Quantitation of the steady-state levels of M6PR in isolated TR- and EGFR-containing endosomes
| Time (min) | EGFR % PNS | TR % PNS | M6PR % PNS | % TR/ % EGFR | % M6PR/ % EGFR |
|---|---|---|---|---|---|
| 10 | 18.1 ± 0.3 | 13.1 ± 0.4 | 3.5 ± 0.1 | 0.72 | 0.19 |
| 25 | 24.8 ± 0.1 | 5.3 ± 0.8 | 3.0 ± 0.2 | 0.22 | 0.12 |
| 45 | 11.2 ± 0.5 | 2.9 ± 1.1 | 2.0 ± 1.2 | 0.24 | 0.17 |
| 60 | 9.1 ± 0.7 | 2.4 ± 0.8 | 1.6 ± 0.2 | 0.25 | 0.17 |
| No gold | 0.1 | NDa | 0.2 | – | – |
Radiolabeled HEp-2 cells were preincubated with anti-EGFR-gold for 30 min at 37°C and then stimulated with saturating EGF (200 ng/ml) for up to 60 min at 37°C. Cells were then lysed and fractionated on sucrose density gradients as described in MATERIALS AND METHODS. The amounts of EGFR and TR in the isolated fractions were determined by Western blotting and for M6PR by immunoprecipitation. In the right hand panels, to correct for the differences in endosome isolation, the yields of TR and M6PR have been expressed as a proportion of the EGFR yield. When no anti-EGFR-gold conjugate is added to the fractionation procedure, ≤0.2% of M6PR or TR is recovered. Note that the yield of M6PR recovered in endosome fractions is low at all time points.
ND, not detectable.
The yield of M6PR in EGFR-containing MVBs is low at all time points (Table 2) and double immunofluorescence for M6PR and endocytosed EGF-TxR detects only low amounts of M6PR in EGF-containing compartments at any time point after EGF internalization (Figure 1). After a 10-min incubation at 37°C, EGF-TxR is found in brightly fluorescent vacuoles distributed throughout the peripheral cytoplasm (Figure 1A) and, at later time points, these vacuoles cluster in the juxtanuclear area (Figure 1, B–D). M6PR is also found in the juxtanuclear area in these cells, but double-label immunofluorescence clearly shows the two populations of EGF- and M6PR-containing elements to be largely distinct, although a small number of EGF-containing vacuoles also contain M6PRs. M6PR is also found concentrated in discrete punctate foci distributed throughout the cytoplasm (Figures 1 and 2) and we attempted, therefore, to relate these various distributions to both the endocytic pathway and the TGN. To locate the TGN, cells were transfected with a cDNA-encoding sialyl transferase in which the lumenal domain has been replaced by HRP (ST-HRP). This construct has previously been shown to localize to the trans-most cisternum of the Golgi complex and to the TGN (Stinchcombe et al., 1995). Immunofluorescence shows that M6PR in the juxtanuclear compartment is associated with ST-HRP-containing trans-Golgi elements (Figure 2). There is almost no colocalization between these ST-HRP-containing trans-Golgi elements and TF-FITC endocytosed for 1 h at 37°C, supporting our previous studies which showed that TR do not cycle through the TGN in HEp-2 cells (Connolly et al., 1994; Futter et al., 1995). Triple-label immunofluorescence shows that the M6PR-positive vacuoles in the peripheral cytoplasm do not contain ST-HRP and cannot be labeled with endocytosed TF-FITC, although small amounts of M6PR could occasionally be seen in vacuoles strongly positive for TR (Figure 2). Taken together, these data indicate that the vacuoles enriched in M6PR do not lie on the endocytic pathway to the lysosome followed by EGFR and are not part of the TGN.
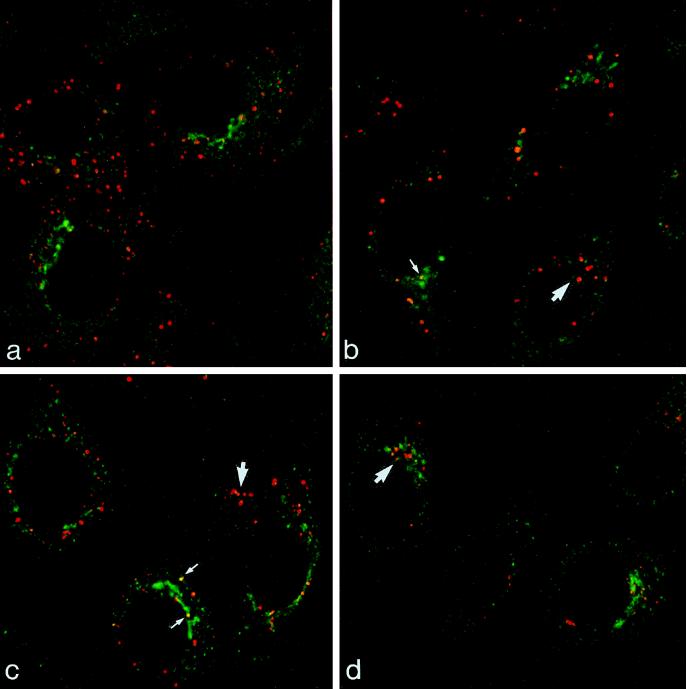
Figure 1.
Double label of M6PR and EGF en route to the lysosome. Cells were incubated with EGF-TxR (red) for 10–60 min at 37°C and were then immunolabeled for M6PR (green). After 10 min at 37°C, EGF-TxR is in vacuoles distributed throughout the peripheral cytoplasm (A). With further incubation at 37°C for 25 (B), 45 (C), and 60 min (D), EGF-TxR accumulates in the juxtanuclear region of the cell (large arrows). At all time points most of the elements labeled with EGF and M6PR are discrete and separate. Small arrows indicate the rare occasions of coincidence between EGF-TxR and M6PR.
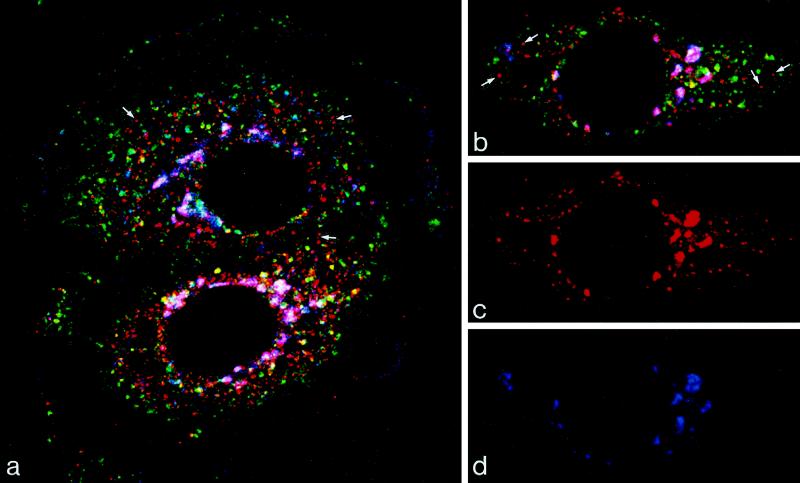
Figure 2.
Triple label showing M6PR in relation to markers of the trans-Golgi and endosome. Cells transiently transfected with ST-HRP were incubated with TF-FITC (green) for 60 min at 37°C and were then immunolabeled for M6PR (red) and HRP (blue). Merged images are shown in A and B, and single images of the cell shown in B are shown in C (M6PR) and D (ST-HRP). M6PR is found in the trans-Golgi region, in which trans-Golgi elements that also contain ST-HRP are stained magenta, and in vacuoles in the peripheral cytoplasm. The majority of the peripheral M6PR-positive vacuoles stain red (arrows) and so do not contain either ST-HRP or TF-FITC. Only a few M6PR-positive vacuoles contain TF (stained yellow), and TF-FITC is not detectable in the trans-Golgi.
Direct Measurement of the Transport of M6PR from the TGN to MVB
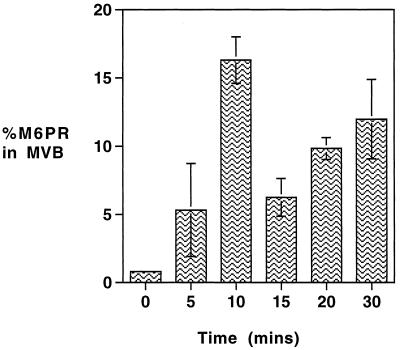
To follow the trafficking of M6PR through MVBs, a pulse of newly synthesized M6PR was allowed to accumulate within the Golgi complex at 20°C and then chased at 37°C. Thus, after radiolabeling for 15 min at 37°C with 35S-labeled amino acids, the cells were incubated for 4 h at 20°C, and during the last hour anti-TR-gold was added so that MVBs could be isolated. As shown in Figure 3, the level of radiolabeled M6PR detected in MVBs at the end of the 20°C incubation is <1% of the PNS, but a rapid increase in the amount of labeled M6PR recovered in isolated MVBs occurs on transfer to 37°C. This increase is detectable within 5 min and maximal by 10 min, when the amount of labeled M6PR recovered in isolated MVB is 20-fold higher than that at the end of the 20°C incubation. The content of M6PR in the MVBs then rapidly declines so that the level seen at 15 min is similar to that seen at 5 min (6% of PNS). With further incubation, it steadily increases again so that after 30 min at 37°C, the level within the MVB fraction is approximately 10-fold more than that seen at the end of the 20°C incubation. Thereafter, it declines and returns to steady-state levels (2.5% of PNS) in less than 90 min. The total yield of MVBs, as indicated by yield of TR (24–27% of total cellular receptor), did not change significantly over the time course of this experiment. These results demonstrate that the M6PR that accumulate in the Golgi at 20°C enter the TR-containing MVB as a discrete pulse (at 10 min) and then rapidly leave before a second, broader wave (at 30 min) enters and leaves.
Figure 3.
Trafficking of newly synthesized M6PR through MVBs. To measure the rate of appearance of newly synthesized M6PR in MVBs, HEp-2 cells were pulsed with 35S-labeled amino acids for 15 min at 37°C, washed, and then maintained at 20°C for 4 h. Anti-TR-gold was added for the last hour at 20°C before being transferred to 37°C for up to 30 min in the continued presence of anti-TR-gold. Gold-loaded MVBs were then isolated as described in MATERIALS AND METHODS. The amount of newly synthesized M6PR in the isolated MVB fractions was determined by immunoprecipitation and expressed as a percentage of the total loaded on the fractionation gradient (% PNS). Results are means ± SE of four observations.
Transport of Newly Synthesized CD to MVBs
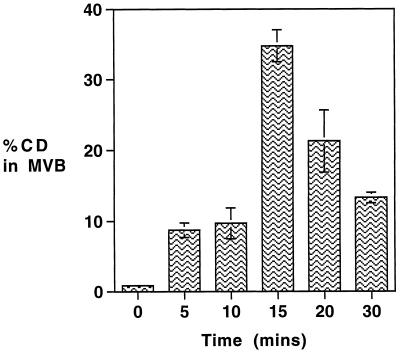
The processing of a lysosomal hydrolase, CD, which is known to be delivered to the endocytic pathway via an M6PR-mediated mechanism (Campbell and Rome, 1983) was followed using a similar incubation and fractionation protocol. To generate a sufficient signal for the pulse chase, it was necessary to extend the initial incubation with 35S-labeled amino acids to 35 min; this was followed by a 4-h, 20°C incubation (with anti-TR-gold added for the last hour at 20°C) before transfer to 37°C. As shown in Figure 4, on transfer to 37°C a significant proportion of newly synthesized CD, like M6PR, is delivered to the anti-TR-gold-loaded MVBs. The peak of the pulse after 15 min at 37°C shows a 40-fold increase over the level at the end of the 20°C incubation, when the amount of radiolabel in the MVB fraction is 30% of the PNS. Compared with the pattern of M6PR delivery to the MVBs at 37°C, the appearance of the CD pulse is delayed by approximately 5 min. This may reflect the more prolonged incubation at 37°C required to generate the pulse and the additional step required in the binding of the enzyme to M6PR. The yield of CD in MVBs does not decline as rapidly as that of M6PR, presumably because after dissociation from M6PR, CD is retained in the lumenal content of the maturing MVBs, whereas M6PR are rapidly removed. However, the yield of CD in isolated MVBs does subsequently decline, presumably as it enters the lysosome, and unlike the yield of M6PR, a second wave of newly synthesized CD is not seen to pass through the MVBs.
Figure 4.
Trafficking of newly synthesized CD through the MVBs. To measure the rate of appearance of newly synthesized CD in MVBs, HEp-2 cells were pulsed with 35S-labeled amino acids for 15 min at 37°C, washed, and incubated for 20 min at 37°C and then maintained at 20°C for 4 h. Anti-TR-gold was added for the last hour at 20°C before being transferred to 37°C for up to 30 min in the continued presence of anti-TR-gold. Gold-loaded MVBs were then isolated as described in MATERIALS AND METHODS. The amount of newly synthesized CD in the isolated MVB fractions was determined by immunoprecipitation and expressed as a percentage of the total loaded on the fractionation gradient (% PNS). Results are means ± SE of three observations.
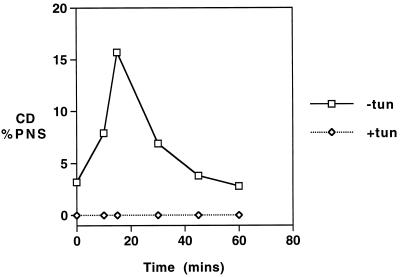
Inhibition of glycosylation by tunicamycin treatment can be used to determine whether traffic of acid hydrolases is dependent on the addition of mannose 6-phosphate and, hence, on association with M6PR (Kuliawat and Arvan, 1994). Although our unpublished results show that tunicamycin treatment causes an increase in the level of CD secretion, it completely prevents the appearance of CD in the MVB fraction (Figure 5).
Figure 5.
The effect of tunicamycin on trafficking of CD through MVBs. Cells were preincubated with tunicamycin (10 μg/ml) for 2 h at 37°C, and then pulsed with 35S-labeled amino acids for 15 min at 37°C, washed, and incubated for 20 min at 37°C and then maintained at 20°C for 4 h. Anti-TR-gold was added for the last hour at 20°C before transfer to 37°C for up to 60 min in the continued presence of anti-TR-gold. Gold-loaded MVBs were then isolated as described in MATERIALS AND METHODS. The amount of newly synthesized CD in the isolated MVB fractions was determined by immunoprecipitation and expressed as a percentage of the total loaded on the fractionation gradient (% PNS).
DISCUSSION
Our previous work on HEp-2 cells has shown that MVBs mature by removing recycling receptors such as TR and accumulating lysosomally directed proteins such as EGFR (Futter et al., 1996). The mature MVBs then fuse with preexisting lysosomes that contain active acid hydrolase and characteristic lysosomal membrane proteins (e.g., lamp-1). Our observations are at variance with previous models that suggested that MVBs fuse with a separate M6PR-rich compartment that is thought to identify a discrete late endosome or prelysosomal stage on the endocytic pathway (Gruenberg et al., 1989; Bomsel et al., 1990; Aniento et al., 1993). Here, we have taken advantage of a gold-mediated density shift technique that allows MVBs at all stages en route to the lysosome to be isolated to high purity (Beardmore et al., 1987; Futter et al., 1989, 1993). We show that MVBs containing internalized EGFR are rich in TR during the earlier stages of maturation (after 10 min of EGF stimulation at 37°C), but that these vacuoles do not become enriched in M6PR at any stage during their maturation. Double immunofluorescence of M6PR and endocytosed EGF-TxR confirm these results and show that the vacuoles in the juxtanuclear area that accumulate internalized EGF-TxR are distinct from elements that label strongly for M6PR and are also distributed in this area.
Despite the generally held belief that M6PR is concentrated in a prelysosomal compartment, there is evidence to suggest that M6PR can enter the endocytic pathway at the earlier stages that contain recycling TR (Ludwig et al., 1991; Rijnboutt et al., 1992). Therefore, to determine whether the small amounts of M6PR found in MVBs at steady state represent a component of the main acid hydrolase delivery process, we devised a method to measure the flux of M6PR through MVBs. Our results show that when a pulse of newly synthesized M6PR is released from the Golgi, a significant proportion is rapidly transferred to MVBs that contain TR and are thus at an early stage in their maturation. The pulse is detectable within the MVBs by 5 min at 37°C, is maximal by 10 min, and remains as a narrow pulse of radioactivity. In a previous study using a similar protocol, we showed that a pulse of newly synthesized TR could be detected in MVBs before it appeared on the cell surface (Futter et al., 1995). The kinetics with which TR were transferred from the Golgi to the MVB were almost identical to those we have observed for M6PR in the current study, indicating that the proportion of M6PR trafficking to MVBs does so directly rather than via the cell surface. We have shown in the current study that newly synthesized CD is also delivered to the MVB from the trans-Golgi, before becoming dissociated from the M6PR and delivered to lysosomes by the mature MVBs. In cells treated with tunicamycin, to prevent addition of mannose 6-phosphate to newly synthesized acid hydrolases, the transfer of CD to MVBs was abrogated. Instead there was an increase in the secretion of CD, which presumably occurs via a bulk flow signal-independent pathway to the cell surface. The time taken for the majority of CD to be released into the medium (more than 20 min) is significantly longer than the time taken for transfer to the MVBs (maximal at 15 min), providing further evidence that M6PR moves directly from the Golgi to MVBs. However, the second wave of M6PR that we have observed passing through MVBs is likely to have trafficked via the cell surface, because it peaks at 30 min and is less discrete than the first wave, consistent with its having traveled further.
Our data thus suggest that although the bulk of M6PRs reside in sialyl transferase-positive trans-Golgi elements and in peripheral vacuoles at steady state, there is a significant volume of M6PR traffic through TR and EGFR-containing MVBs. The maximum amount of newly synthesized M6PRs recovered in the MVB fraction in our studies is 16% of the total labeled receptor (compared with 2.5% at steady state). The amount of labeled M6PR in MVBs isolated at single time points is likely to be only a partial indication of the total amount of receptor traffic through the MVBs. Taken together with the nature of the fractionation system we have used, which yields a highly purified fraction and so does not allow the isolation of 100% of TR-containing MVBs, these data indicate that a significant proportion of newly synthesized M6PR traffic through the MVBs.
Our data suggest that the residence time for M6PR in the MVB is less than 15 min. Studies measuring return traffic of M6PR from endosomes to and through the TGN, as indicated by sialylation of the receptor, suggest that the time required for the full circuit to be traveled is more than 1 hour (Duncan and Kornfeld, 1988; Goda and Pfeffer, 1988). At steady state, the bulk of the M6PR must lie in the TGN or on the circuit between the MVB and the TGN, and it is probable, therefore, that the M6PR-rich vacuoles in the peripheral cytoplasm lie on this recycling stage of the circuit. It is possible that in other cell types the steady-state distributions of M6PR result from more rapid transit times through compartments such as the TGN, which in HEp-2 cells is clearly a compartment where through-put is relatively slow, and this would explain the different steady-state distributions seen in other cell types (Griffiths et al., 1988, 1990; Kornfeld and Mellman, 1989). These considerations clearly argue against the use of M6PR as a universal marker for a late endosome or prelysosome stage in endocytic pathways.
With the results presented in this article, our evidence on HEp-2 cells clearly suggests that multivesicular endosomes that initially contain recycling TR can acquire M6PR carrying acid hydrolases during their maturation and then lose the M6PR before they mature and fuse with lysosomal vacuoles, where the degradation of EGF takes place. It will now be of interest to measure the rate of flux of M6PR in the endocytotic pathway in cell types in which M6PR-rich compartments are believed to be located between the endosome and lysosome stages.
ACKNOWLEDGMENTS
This work was supported by a program grant from the Medical Research Council to C.R.H.
Footnotes
Abbreviations used: CD, cathepsin D; EGFR, epidermal growth factor receptor; EGF-TxR, EGF-Texas red; M6PR, mannose 6-phosphate receptor; MVB, multivesicular body; PNS, postnuclear supernatant; ST-HRP, sialyl transferase–horseradish peroxidase; TF-FITC, transferrin–fluorescein isothiocyanate; TGN, trans-Golgi network; TR, transferrin receptor.
REFERENCES
- Aniento F, Emans N, Griffiths G, Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol. 1993;123:1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardmore J, Howell KE, Miller K, Hopkins CR. Isolation of an endocytic compartment from A431 cells using a density modification procedure employing a receptor-specific monoclonal antibody complexed with colloidal gold. J Cell Sci. 1987;87:495–506. doi: 10.1242/jcs.87.4.495. [DOI] [PubMed] [Google Scholar]
- Bomsel M, Parton R, Kutznetsov SA, Schroer TA, Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990;62:719–731. doi: 10.1016/0092-8674(90)90117-w. [DOI] [PubMed] [Google Scholar]
- Brown WJ, Goodhouse J, Farquhar MG. Mannose 6-phosphate receptors for lysosomal enzymes cycle between the Golgi complex and endosomes. J Cell Biol. 1986;103:1235–1247. doi: 10.1083/jcb.103.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C, Rome LH. Coated vesicles from rat liver and calf brain contain lysosomal enzymes bound to mannose 6-phosphate receptors. J Biol Chem. 1983;258:13347–13352. [PubMed] [Google Scholar]
- Connolly CN, Futter CE, Gibson A, Hopkins CR, Cutler DF. Transport into and out of the Golgi complex studied by transfecting cells with cDNAs encoding horseradish peroxidase. J Cell Biol. 1994;127:641–652. doi: 10.1083/jcb.127.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Kornfeld S. Intracellular movement of two mannose 6-phosphate receptors: return to the Golgi apparatus. J Cell Biol. 1988;106:617–628. doi: 10.1083/jcb.106.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder S, Miller K, Moehren G, Ullrich A, Schlessinger J, Hopkins CR. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990;61:623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Futter CE, Connolly CN, Cutler DF, Hopkins CR. Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem. 1995;270:10999–11003. doi: 10.1074/jbc.270.18.10999. [DOI] [PubMed] [Google Scholar]
- Futter CE, Felder S, Schlessinger J, Ullrich A, Hopkins CR. Annexin I is phosphorylated in the multivesicular bodies during the processing of the epidermal growth factor receptor. J Cell Biol. 1993;120:77–83. doi: 10.1083/jcb.120.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Hopkins CR. Subfractionation of the endocytic pathway: isolation of compartments involved in the processing of internalized epidermal growth factor-receptor complexes. J Cell Sci. 1989;94:685–694. doi: 10.1242/jcs.94.4.685. [DOI] [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF–EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y, Pfeffer SR. Selective recycling of the mannose 6-phosphate/IGF-II receptor to the trans-Golgi network in vitro. Cell. 1988;55:309–320. doi: 10.1016/0092-8674(88)90054-2. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Matteoni R, Bach R, Hoflack B. Characterization of the cation-independent mannose-6-phosphate receptor-enriched prelysosomal compartment in NRK cells. J Cell Sci. 1990;95:441–461. doi: 10.1242/jcs.95.3.441. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Griffiths G, Howell KE. Characterization of the early endosome and putative endocytic carrier vesicles in vitro and with an assay of vesicle fusion in vitro. J Cell Biol. 1989;108:1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins CR, Gibson A, Shipman M, Miller K. Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature, 1990;346:335–339. doi: 10.1038/346335a0. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Gibson A, Shipman M, Strickland DK, Trowbridge IS. In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then recycled to the plasma membrane of the leading lamella. J Cell Biol. 1994;125:1265–1274. doi: 10.1083/jcb.125.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins CR, Trowbridge IS. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Kuliawat R, Arvan P. Distinct molecular mechanisms for protein sorting within immature secretory granules of pancreatic b cells. J Cell Biol. 1994;126:77–86. doi: 10.1083/jcb.126.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T, Griffiths G, Hoflack B. Distribution of newly synthesized lysosomal enzymes on the endocytic pathway of normal rat kidney cells. J Cell Biol. 1991;115:1561–1572. doi: 10.1083/jcb.115.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnboutt S, Stoorvogel W, Geuze HJ, Strous GJ. Identification of subcellular compartments involved in biosynthetic processing of cathepsin D. J Biol Chem. 1992;267:15665–15672. [PubMed] [Google Scholar]
- Slot JW, Geuze HJ. A new method for preparing gold probes for multiple labeling cytochemistry. Eur J Cell Biol. 1985;38:87–93. [PubMed] [Google Scholar]
- Stinchcombe JC, Nomoto H, Cutler DF, Hopkins CR. Anterograde and retrograde traffic between the rough endoplasmic reticulum and the Golgi complex. J Cell Biol. 1995;131:1387–1401. doi: 10.1083/jcb.131.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B, Pool RR, Sachdeva M, Maurey KM, Oliver C. Evidence for both prelysosomal and lysosomal intermediates in endocytic pathways. J Cell Biol. 1984;98:108–115. doi: 10.1083/jcb.98.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurs B, Holm PK, Kayser L, Sandvig K. Delivery to lysosomes in the human carcinoma cell line HEp-2 involves an actin filament-facilitated fusion between mature endosome and preexisting lysosomes. Eur J Cell Biol. 1995;66:309–323. [PubMed] [Google Scholar]
- van Deurs B, Holm PK, Kayser L, Sandvig K, Hansen SH. Multivesicular bodies in HEp-2 cells are maturing endosomes. Eur J Cell Biol. 1993;61:208–224. [PubMed] [Google Scholar]