Abstract
Tyrosine residues of middle-T and tyrosine phosphorylation are thought to be important in the transformation of cultured rodent cells by polyomavirus. Of the potential tyrosine sites in the carboxyl-terminal half of middle-T, tyrosines 297, 315, and 322 have been studied previously, whereas tyrosine 250 has not. Two mutant plasmids, XD121 and pT250, encode polyomavirus middle-T species in which the tyrosine 250 residue is affected. XD121 is a deletion mutant in which the region encoding tyrosine 250, together with three adjacent amino acids, is deleted, whereas pT250 is a point mutant in which the tyrosine 250 codon has been converted to a phenylalanine codon. The plasmids were handicapped in transforming ability, as judged by focus formation on a monolayer of Rat-1 cells. Both demonstrated a reduction in the number of foci produced and a lag in the time of appearance of foci when compared with wild-type plasmid. The importance of residue 250 in this phenotype was indicated by the observation that plasmids containing multiple mutations proximal to the tyrosine 250 codon were wild type in their transforming ability. Furthermore, a revertant of pT250 (pT250-w.t.), which utilized the alternative tyrosine codon of TAC, was shown to regain full transforming activity. A combined-mutant plasmid, pTH, encodes a middle-T species in which both tyrosines 250 and 315 are converted to phenylalanine. This plasmid was totally defective in the transformation of rodent cells in a focus formation assay; however, it did impart a small measure of anchorage-independent growth when the encoded protein was expressed in NIH 3T3 cells. The in vitro kinase activity and pp60c-src association of the mutant middle-T antigens were examined. These assays demonstrated a reduction in phosphate acceptor activity for the middle-T species encoded by pT250 and pTH. Quantitative kinase assays showed that all of the tyrosine-mutant middle-T species, encoded by pAS131 (containing the tyrosine 315 codon-to-phenylalanine codon mutation), pT250, and pTH, were able to enhance pp60c-src kinase activity but only at levels which were intermediate and which reflected their transforming abilities relative to wild type.
Full text
PDF




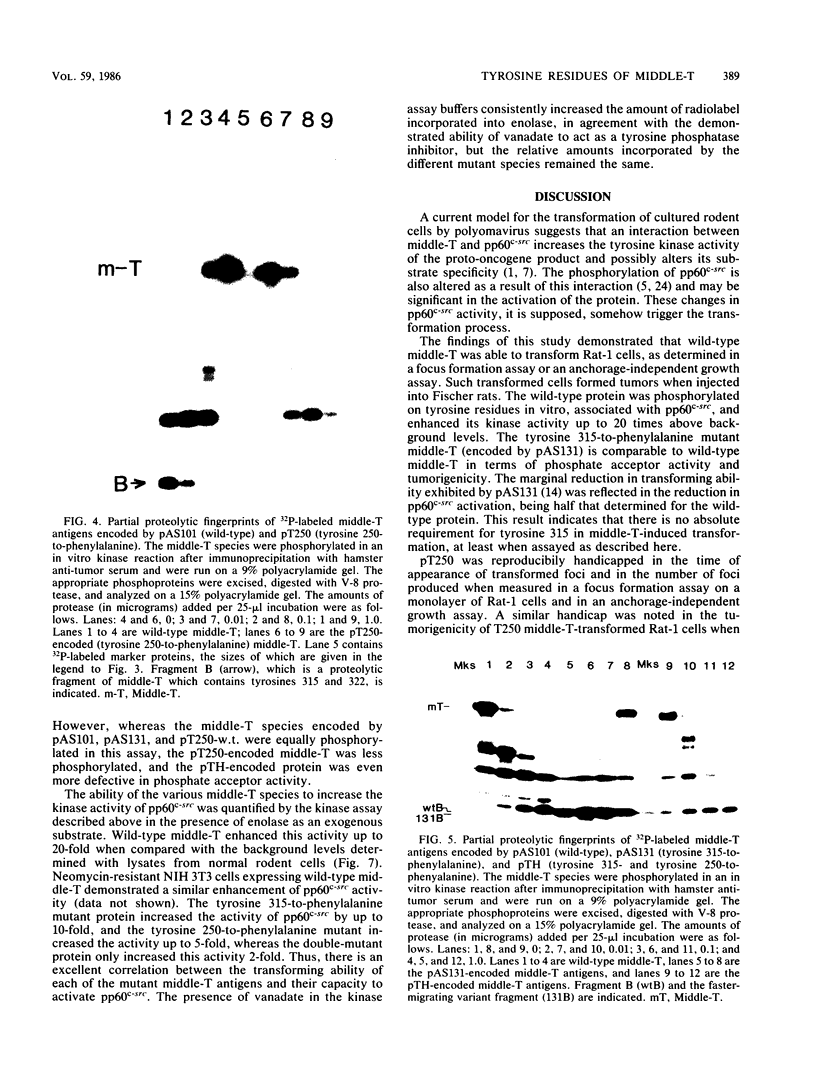


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Carmichael G., Schaffhausen B. S., Mandel G., Liang T. J., Benjamin T. L. Transformation by polyoma virus is drastically reduced by substitution of phenylalanine for tyrosine at residue 315 of middle-sized tumor antigen. Proc Natl Acad Sci U S A. 1984 Feb;81(3):679–683. doi: 10.1073/pnas.81.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Markland W., Markham A. F., Smith A. E. Mutations around the NG59 lesion indicate an active association of polyoma virus middle-T antigen with pp60c-src is required for cell transformation. EMBO J. 1986 Feb;5(2):325–334. doi: 10.1002/j.1460-2075.1986.tb04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. The complex of polyoma virus middle-T antigen and pp60c-src. EMBO J. 1984 Mar;3(3):585–591. doi: 10.1002/j.1460-2075.1984.tb01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Harvey R., Oostra B. A., Belsham G. J., Gillett P., Smith A. E. An antibody to a synthetic peptide recognizes polyomavirus middle-T antigen and reveals multiple in vitro tyrosine phosphorylation sites. Mol Cell Biol. 1984 Jul;4(7):1334–1342. doi: 10.1128/mcb.4.7.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Hutchinson M. A., Eckhart W. Polyoma middle-sized T antigen can be phosphorylated on tyrosine at multiple sites in vitro. EMBO J. 1984 Jan;3(1):73–79. doi: 10.1002/j.1460-2075.1984.tb01763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Oostra B. A., Ely B. K., Smith A. E. Deletion loop mutagenesis: a novel method for the construction of point mutations using deletion mutants. Nucleic Acids Res. 1982 Sep 11;10(17):5161–5171. doi: 10.1093/nar/10.17.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mes-Masson A. M., Schaffhausen B., Hassell J. A. The major site of tyrosine phosphorylation in polyomavirus middle T antigen is not required for transformation. J Virol. 1984 Nov;52(2):457–464. doi: 10.1128/jvi.52.2.457-464.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra B. A., Harvey R., Ely B. K., Markham A. F., Smith A. E. Transforming activity of polyoma virus middle-T antigen probed by site-directed mutagenesis. Nature. 1983 Aug 4;304(5925):456–459. doi: 10.1038/304456a0. [DOI] [PubMed] [Google Scholar]
- Ralston R., Bishop J. M. The product of the protooncogene c-src is modified during the cellular response to platelet-derived growth factor. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7845–7849. doi: 10.1073/pnas.82.23.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Phosphorylation of polyoma T antigens. Cell. 1979 Dec;18(4):935–946. doi: 10.1016/0092-8674(79)90206-x. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L. Comparison of phosphorylation of two polyoma virus middle T antigens in vivo and in vitro. J Virol. 1981 Oct;40(1):184–196. doi: 10.1128/jvi.40.1.184-196.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Fried M., Ito Y., Spurr N., Smith R. Is polyoma virus middle T antigen a protein kinase? Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):141–147. doi: 10.1101/sqb.1980.044.01.016. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Griffin B., Fried M. Protein kinase activity associated with polyoma virus middle T antigen in vitro. Cell. 1979 Dec;18(4):915–924. doi: 10.1016/0092-8674(79)90204-6. [DOI] [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemoto W., Jarvis-Morar M., Brugge J. S., Bolen J. B., Israel M. A. Tyrosine phosphorylation within the amino-terminal domain of pp60c-src molecules associated with polyoma virus middle-sized tumor antigen. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4568–4572. doi: 10.1073/pnas.82.14.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]