Abstract
Rolipram, an inhibitor of phosphodiesterase 4 (PDE4) proteins that hydrolyze cAMP, increases axonal regeneration following spinal cord injury (SCI). Recent evidence indicates that rolipram also protects against a multitude of apoptotic signals, many of which are implicated in secondary cell death post-SCI. In the present study, we used immunohistochemistry and morphometry to determine potential spinal cord targets of rolipram and to test its protective potential in rats undergoing a cervical spinal cord contusive injury. We found that 3 PDE4 subtypes (PDE4A, B, D) were expressed by spinal cord oligodendrocytes. OX-42 immunopositive microglia only expressed the PDE4B subtype. Oligodendrocyte somata were quantified within the cervical ventrolateral funiculus, a white matter region critical for locomotion, at varying time points after SCI in rats receiving rolipram or vehicle treatments. We show that rolipram significantly attenuated oligodendrocyte death at 24 hours post-SCI continuing through 72 hours, the longest time point examined. These results demonstrate for the first time that spinal cord glial cells express PDE4 subtypes and that the PDE4 inhibitor rolipram protects oligodendrocytes from secondary cell death following a contusive SCI. They also indicate that further investigations into neuroprotection and axonal regeneration with rolipram are warranted for treating SCI.
Keywords: cAMP, Phosphodiesterase 4, White Matter, APC, OX-42, Microglia
Spinal cord injury (SCI) consists of an irreversible primary injury followed by a secondary injury cascade that promotes additional cell death further reducing the chance for functional recovery. The secondary injury is a manifestation of many processes including excitotoxicity [42], calcium overload [51], oxidative stress [1], and inflammation [23], all which lead to apoptosis and the unnecessary death of potentially viable cells. Recent evidence suggest that inhibition of phosphodiesterase 4 (PDE4), a protein family responsible for cAMP hydrolysis [31], with the drug rolipram [55] provides protection against a multitude of apoptotic insults including reduction of caspase-3 activity, a downstream mediator of multiple apoptotic cascades [10]. While earlier studies targeting PDE4 inhibition with rolipram have demonstrated its success in aiding axonal regeneration following SCI [17, 38, 44], the protective effects of this treatment are largely unknown.
Spared axon demyelination occurs in human and experimental SCI [15, 52]. A previous study using rolipram revealed increased numbers of oligodendrocyte-myelinated axons in the adult rat spinal cord white matter months after contusive SCI [44]. This could have been due to decreased oligodendrocyte death since they are highly vulnerable to secondary injury processes [11, 12]. In particular, excitotoxicity [22, 34] and tumor necrosis factor- α (TNF-α), a pro-inflammatory cytokine, [26, 50] are highly toxic to oligodendrocytes. Coincidentally, both have been implicated in augmenting PDE4 expression [16, 32]. Furthermore, rolipram decreased TNF-α production [21, 44, 57] as well as protected a cell line of immortalized, O-2A derived oligodendrocyte-like cells from excitotoxicity [58, 59], providing an additional benefit of rolipram treatment. Thus, in the present study we addressed whether rolipram prevents secondary death of oligodendrocytes in a rat model of contusive cervical SCI [39], the most frequent type of human SCI [6]. Using immunohistochemistry and morphometry, we show that oligodendrocytes and microglia co-express PDE4 subtypes providing two potential targets of rolipram. Moreover, we demonstrate the protective effect of rolipram on oligodendrocytes in the ventrolateral funiculs (VLF), a white matter region critical for locomotion [28, 29].
All methods were approved by the Institutional Animal Care and Use Committee at the University of Louisville. They were conducted to minimize pain and discomfort as well as in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and with the Policies on the Use of Animals and Humans in Neuroscience Research. Twenty-eight (4 normal and 24 injured) adult (228 g to 267 g) female Sprague Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) were housed individually throughout the experiment and maintained on a 12-hour light-dark cycle.
The rats were anesthetized with sodium pentobarbital (40–50 mg/kg, IP). The dorsal halves of the C3–C6 vertebrae were exposed and transverse vertebral process supports [40] were placed bilaterally at the C4–C5 vertebrae. Laminectomies were performed to expose the dura overlying the dorsal surfaces of the C5–C6 spinal cord segments between the C5 and C6 dorsal root entry zones. Contusive injuries of 180 ± 7 actual kilodynes were produced dorsal to ventral at the C5–C6 segments with a 3.7mm diameter tip and an Infinite Horizon Impactor [39, 49]. Two model 2002 (0.5 µl/hr) ALZET® mini-osmotic pumps (DURECT Corp., Cupertino, CA) were inserted subcutaneously and bilaterally over the ribs adjacent to the vertebral column following the injury. Animals were randomly assigned prior to surgery to a group of 12 rats that received rolipram (0.5 mg/kg/day, Sigma, St. Louis, MO) [44], dissolved in DMSO (Sigma) or to a control group of 12 rats that received only DMSO. The treatments were administered for the duration of the experiment. Muscle and skin incisions were closed with silk sutures and wound clips, respectively. Post-operative care included Gentozen™ (10 mg/kg, IM, Schering-Plough Animal Health, Omaha, NE) antibiotic, topical Bacitracin Zinc Ointment USP (E. Fougera & Co., Melville, NY), and 5% dextrose in lactated Ringer's solution (5 ml, SC, Baxter Healthcare Corp., Deerfield, IL). All rats were largely immobile after SCI. For veterinary care, lactated Ringer’s solution was injected subcutaneously plus Ensure® and cereal were provided to maintain hydration and attenuate bodyweight loss. Bladders were emptied at least once daily with gentle pressure and gastrointestinal function was monitored daily. One rat was excluded from the rolipram-treated group due to morbidity following injury.
All rats were anesthetized with sodium pentobarbital (120mg/kg, IP) 12, 24, or 72 hours post-SCI. Transcardial perfusions were performed with heparinized, oxygenated, and calcium-free Tyrodes solution, followed by 0.1M phosphate buffer, pH 7.4 (PB), containing 4% paraformaldehyde, and lastly with PB. Cervical spinal cords were removed and cryoprotected in PB containing 30% sucrose at 4°C for 3–4 days. They were sectioned at 20 µm in the transverse plane with a cryostat. Sections mounted onto charged microscope slides were stained with 0.5% cresyl violet (Sigma) [39] to view the morphology of the SCI site or immunostained [33] to visualize oligodendrocytes, microglia, PDE4 expression, and cAMP-dependent phosphorylated PKA substrates. Following rinses in Tris-buffer with 0.9% saline, pH 7.4 (TBS), sections were blocked with TBS containing 0.05% Triton X-100 (TBST) and 10% normal donkey serum (NDS, Jackson Immuno, West Grove, PA) for 1 hour at room temperature. They were next incubated overnight at 4°C with TBST containing 10% NDS and combinations of mouse anti-adenomatus polyposis coli (APC, 1:150, Calbiochem, San Diego, CA) to identify mature oligodendrocytes, mouse anti-OX-42 (1:200, BD Biosciences, San Jose, CA) to identify microglia, as well as rabbit anti-PDE4A(1:100, FabGennix Inc., Frisco, TX), rabbit anti-PDE4B(1:150, FabGennix Inc), rabbit anti-PDE4D (1:150, FabGennix Inc.), and rabbit anti-phospho-(Ser/Thr) PKA substrates (pPKA, 1:200, Cell Signaling Tech., Danvers, MA). Following rinses, sections were incubated for 1 hour with TBST and 5% NDS containing combinations of species-specific donkey IgG antibodies (Jackson ImmunoResearch Laboratories Inc) conjugated with flourescein isothiocyanate (FITC, 1:200) or cyanine 3 (Cy3, 1:200). Lastly, the sections were coverslipped with Mowiol mountant and stored at 4°C.
Confocal images of the VLF at each C5–C6 SCI site and at a similar location in normal rats (Supplementary Fig. 1A) were obtained using an Olympus laser confocal microscope and digitized with an Olympus Optical (Mellville, NY) laser Fluoview 500 software [33]. Adobe photoshop v9.02 (Adobe Systems Inc., San Jose, CA) was used to sharpen the images, adjust brightness and contrast, and compose the final images. Images of spinal cords from all rats were used to visualize PDE4 co-expression with oligodendrocytes. For PDE4 co-expression with microglia, images of sections from spinal cords at 3 days post-injury were used to ensure that activated microglia were present [24, 46]. To quantify the numbers of oligodendrocyte somata in each rat, randomly selected left or right side VLF in 2 [36] APC and pPKA immunostained images that were 200µm apart [7] at each C5–C6 SCI site and at a similar location in the normal rats were converted into black and white images then color inverted [27]. The total number of APC-immunopositive oligodendrocyte somata with pPKA-immunopositive nuclei in each section were quantified using ImageJ software (v.1.32j, National Institutes of Health) then converted to cells/cm2. APC-immunopositive cells within the gray matter were excluded from quantification [4]. The oligodendrocyte numbers found in both sections of each rat were averaged. After Levene’s test for equality of variances did not uncover significant differences, oligodendrocyte cell counts of the groups were compared using a 2×3 ANOVA followed by Tukey’s HSD post hoc t tests when appropriate with SPSS v.13.0 (SPSS, Chicago, IL) statistical software.
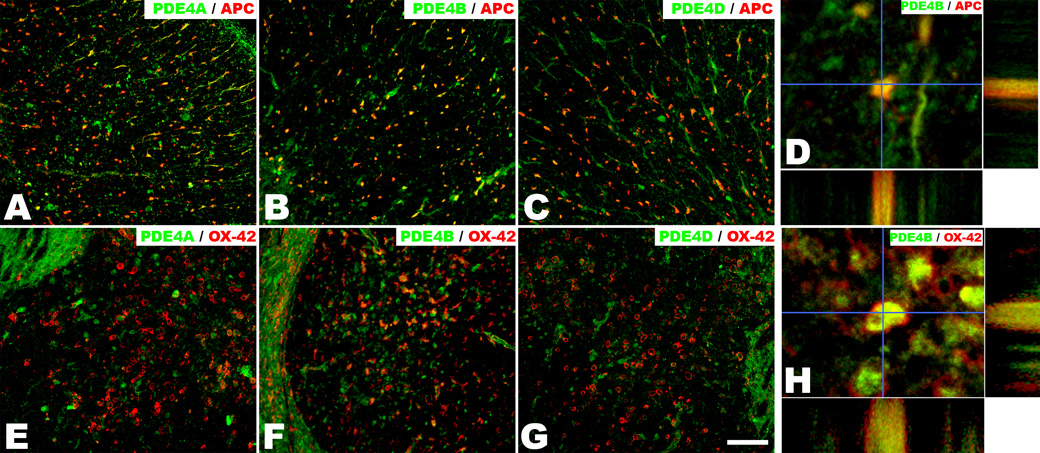
To locate potential spinal cord targets of rolipram treatment, we used double labeling with PDE4 sub-family-specific and glia-specific antibodies. Immunofluorescence revealed that APC-immunopositive oligodendrocytes throughout the cervical spinal cord white matter co-expressed all three PDE4 subtypes (PDE4A, B, D) (Figs. 1A–D). Additionally OX-42-immunopositive microglia at 3 days post-SCI, which are a major source of the pro-inflammatory cytokine TNF-α [2, 45], expressed only the PDE4B subtype (Figs. 1E–H). Coincidentally, PDE4 knockout studies revealed that lipopolysaccharide induced TNF-α production and secretion was dependent upon PDEB, not PDE4A or PDE4D [20, 21]. By maintaining and elevating their cAMP levels after SCI, rolipram may exert its effect directly in oligodendrocytes to reduce intrinsic apoptotic signaling cascades and / or indirectly by attenuating the inflammatory response of adjacent microglia, particularly the reduction of pro-inflammatory cytokine, TNF-α.
Figure 1.
Oligodendrocytes and microglia/macrophages in the adult rat cervical spinal cord VLF are co-labeled with PDE4 subtypes. Representative transverse sections of the 3-day injured adult rat C5–C6 spinal cord VLF immunostained in combination for PDE4A (A, E), PDE4B (B, F), or PDE4D (C,G) and APC (A–C) or OX-42 (E–G). APC-immunopositive oligodendrocytes were co-labeled with all three PDE4s, whereas OX-42-immunopositive microglia/macrophages only were co-labeled with PDE4B. Colocalization is confirmed in orthogonal views of PDE4B with APC (D) and OX-42 (H). Scale bar=100µm.
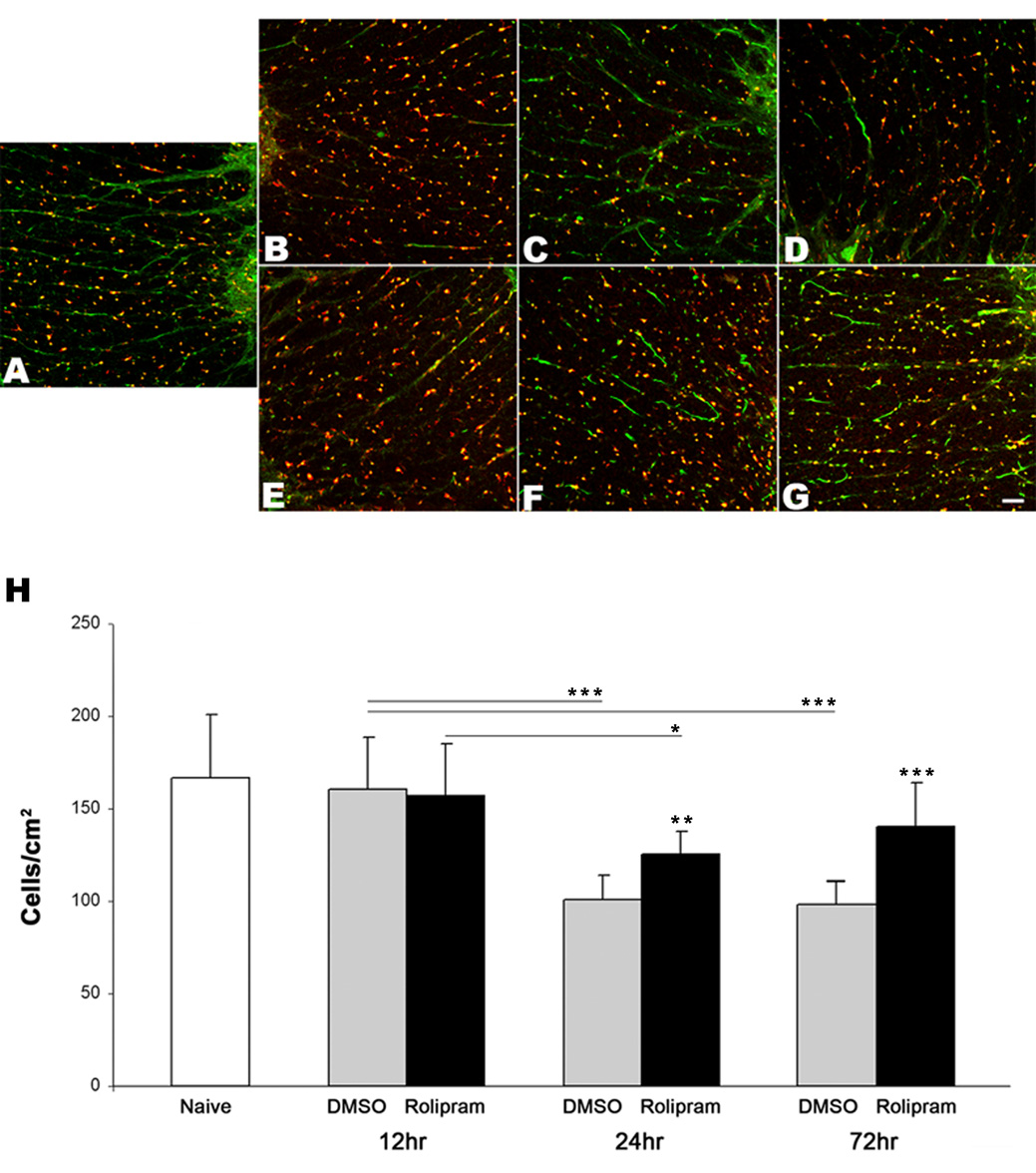
To determine the protective potential of rolipram on oligodendrocytes, we assessed their survival in the cervical spinal cord VLF at 12, 24, and 72 hours post-SCI (Fig. 2, Supplemental Fig. 1). At 12 hours post-SCI, both rolipram-treated rats (156.6 ± 28.4) and DMSO-treated rats (160.3 ± 28.7) had similar numbers of oligodendrocytes to each other and to those of normal rats (167.1 ± 34.0). This provides evidence that these cells had survived for 12 hours after the primary mechanical injury. There was a significant reduction of oligodendrocytes in both rolipram-treated rats (125.1 ± 12.6) and DMSO-treated rats (101.1 ± 13.1) at 24 hours post-SCI. However, rolipram treatment significantly attenuated the oligodendrocyte death compared to DMSO-treated rats at this time point and through 72 hours post-SCI (140.0 ± 24.1 vs. 98.6 ± 12.1). These data provide evidence that rolipram significantly protects oligodendrocytes from secondary injury following a contusive SCI.
Figure 2.
Oligodendrocytes are spared after SCI by rolipram treatment. Representative transverse sections show APC (red)-and PKA (green)-immunopositive oligodendrocytes in the C5–C6 spinal cord VLF of a normal rat (A) and of both DMSO-treated rats (B–D), and rolipram-treated rats (E–G) 12 (B, E), 24 (C, F), and 72 (D, G) hours post-contusive cervical SCI. Comparisons between the numbers of APC- and pPKA-immunopositive oligodendrocyte somata in the C5–C6 spinal cord VLF of normal rats (n=4) and injured rats (n=4 for each group at each time point except that n=3 for rolipram-treated rats at 72 hours) revealed a significant reduction in both treated groups at 24 and 72, but not at 12, hours post-SCI (H). In contrast to DMSO, rolipram treatment significantly attenuated this loss. Significantly more oligodendrocyte somata continued to be seen in rolipramtreated rats’ VLF at 72 hours post SCI compared to DMSO-treated rats. *p<0.05, **p<0.01, ***p<0.001. Error bars represent standard deviations. Scale bar=50µm.
Previous studies employing rolipram as a treatment have indicated its effectiveness in promoting regeneration following SCI through the inhibition of myelin associated glycoproteins [17, 38, 44]. The present study examined the use of rolipram treatment for oligodendrocyte protection following a contusive cervical SCI. It demonstrates that 1) all PDE4 subtypes are co-expressed by oligodendrocytes, 2) OX-42 positive microglia co-express only the PDE4B subtype, and that 3) rolipram attenuates secondary oligodendrocyte death.
Similar to previous findings in rats after contusive SCI [9, 11, 13, 25, 35, 47, 53], we report significant oligodendrocyte death at 24 hrs post-SCI. Previous literature using a similar model reported drastic reduction in spinal cord cAMP to ~60% that of normal levels at 24 hrs post-injury [44]. While the cause behind this reduction has yet to be fully delineated, it has been proposed that increased inflammation, particularly TNF-α mediated [3, 56], results in decreased cAMP [43]. This decrease could be due to changes in PDE4 expression or activity [20, 21, 32] since PDE4 expression levels were over 4- fold higher acutely post-SCI [8]. One possible mechanism of increased PDE4 expression could be through NF-κB activation after SCI [3, 41], a known downstream target of TNF-α [5] and promoter of PDE4 transcription [54].
In vitro analysis of excitotoxic oligodendrocyte death reveals a protective role of maintaining and / or elevating cAMP levels with rolipram and / or cAMP analogues [58, 59]. While it is unclear whether excitotoxicity has an effect on PDE4, it has been recently proposed that low concentrations of NMDA produce increased PDE4 protein expression and activity [16]. Consistent with this notion, experimental decreases in cAMP augment excitotoxic cell death [19, 58]. Moreover, TNF-α also exacerbates excitotoxicity [37].
Thus, it could be hypothesized that the PDE4-mediated reduction in cAMP as a result of inflammation and / or excitotoxicity increases the vulnerability of oligodendrocytes. To test this hypothesis, we administered the PDE4 inhibitor rolipram after contusive cervical SCI at a dose previously demonstrated to maintain basal levels of cAMP [44]. We found increased numbers of oligodendrocytes at 24 hours post-SCI persisting through 72 hours (the longest time point examined). This suggests that in addition to facilitating axonal regeneration [17] there is also a protective effect of maintaining cAMP levels.
A milieu of events occurs after SCI that lead to secondary cell death. In addition to inflammation and excitotoxicity, PDE4 may also be affected by other processes including increased oxidative stress [18] and p75ntr regulated cell death [48]. Likewise, oligodendrocytes are not the only cell types affected by apoptosis. Neurons are also vulnerable [14] and are thought to undergo similar events leading to apoptosis [13]. We observed expression of PDE4s by ventral horn motor neurons (data not shown). Whether rolipram plays a protective role on neurons following SCI warrants further investigation.
Our results demonstrate two potential targets of rolipram treatment, oligodendrocytes and microglia. Secondly, we provide evidence that rolipram treatment attenuates secondary death of oligodendrocytes within the VLF, a white matter region critical for locomotion. Additional protection might be obtained using larger doses of rolipram or combinatorial approaches, such as with neurotrophin-3 which when combined with cAMP elevating agents was shown to be beneficial in aiding axonal regeneration [30]. Also, further investigations into the mechanism(s) behind rolipram-mediated protection are essential for this, and newly developed PDE4 inhibitors, to effectively treat SCI.
Supplementary Material
Acute pathology of cervical SCI. Representative cresyl violet stained, transverse sections of uninjured naïve (A), 12 (B), 24 (C), and 72 (D) hour post SCI. Shaded region in (A) indicates VLF region. Scale bar=1mm.
Acknowledgements
This work was supported by NIH/NINDS NS40411 (SMO), NIH/NINDS NS047341 (MH), NIH/NCRR RR15576 (Core C: SMO, Project 5: MH), and the Kentucky Spinal Cord and Head Injury Research Trust (DSKM). We thank Christine Nunn, Julie Decker, James Massey, Dr. Erzsebet Szatmari, and George Harding for their expert guidance and assistance. We also thank Aaron Puckett and the University of Louisville Research Resources Center veterinarians and staff for their excellent assistance with veterinary care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson DK, Hall ED. Pathophysiology of spinal cord trauma. Ann Emerg Med. 1993;22:987–992. doi: 10.1016/s0196-0644(05)82739-8. [DOI] [PubMed] [Google Scholar]
- 2.Bartholdi D, Schwab ME. Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci. 1997;9:1422–1438. doi: 10.1111/j.1460-9568.1997.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 3.Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP. Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci. 1998;18:3251–3260. doi: 10.1523/JNEUROSCI.18-09-03251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM. Expression of the APC tumor suppressor protein in oligodendroglia. Glia. 1996;17:169–174. doi: 10.1002/(SICI)1098-1136(199606)17:2<169::AID-GLIA8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- 7.Cao Q, Xu XM, Devries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmel JB, Galante A, Soteropoulos P, Tolias P, Recce M, Young W, Hart RP. Gene expression profiling of acute spinal cord injury reveals spreading inflammatory signals and neuron loss. Physiol Genomics. 2001;7:201–213. doi: 10.1152/physiolgenomics.00074.2001. [DOI] [PubMed] [Google Scholar]
- 9.Casella GT, Bunge MB, Wood PM. Endothelial cell loss is not a major cause of neuronal and glial cell death following contusion injury of the spinal cord. Exp Neurol. 2006;202:8–20. doi: 10.1016/j.expneurol.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Chen RW, Williams AJ, Liao Z, Yao C, Tortella FC, Dave JR. Broad spectrum neuroprotection profile of phosphodiesterase inhibitors as related to modulation of cell-cycle elements and caspase-3 activation. Neurosci Lett. 2007;418:165–169. doi: 10.1016/j.neulet.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 11.Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 12.Emery E, Aldana P, Bunge MB, Puckett W, Srinivasan A, Keane RW, Bethea J, Levi AD. Apoptosis after traumatic human spinal cord injury. J Neurosurg. 1998;89:911–920. doi: 10.3171/jns.1998.89.6.0911. [DOI] [PubMed] [Google Scholar]
- 13.Grossman SD, Rosenberg LJ, Wrathall JR. Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp Neurol. 2001;168:273–282. doi: 10.1006/exnr.2001.7628. [DOI] [PubMed] [Google Scholar]
- 14.Grossman SD, Wolfe BB, Yasuda RP, Wrathall JR. Changes in NMDA receptor subunit expression in response to contusive spinal cord injury. J Neurochem. 2000;75:174–184. doi: 10.1046/j.1471-4159.2000.0750174.x. [DOI] [PubMed] [Google Scholar]
- 15.Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192:384–393. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 16.Hajjhussein H, Suvarna NU, Gremillion C, Chandler LJ, O'Donnell JM. Changes in NMDA receptor-induced cyclic nucleotide synthesis regulate the age-dependent increase in PDE4A expression in primary cortical cultures. Brain Res. 2007;1149:58–68. doi: 10.1016/j.brainres.2007.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannila SS, Filbin MT. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp Neurol. 2007 doi: 10.1016/j.expneurol.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill EV, Sheppard CL, Cheung YF, Gall I, Krause E, Houslay MD. Oxidative stress employs phosphatidyl inositol 3-kinase and ERK signalling pathways to activate cAMP phosphodiesterase-4D3 (PDE4D3) through multi-site phosphorylation at Ser239 and Ser579. Cell Signal. 2006;18:2056–2069. doi: 10.1016/j.cellsig.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Jaworski J, Mioduszewska B, Sanchez-Capelo A, Figiel I, Habas A, Gozdz A, Proszynski T, Hetman M, Mallet J, Kaczmarek L. Inducible cAMP early repressor, an endogenous antagonist of cAMP responsive element-binding protein, evokes neuronal apoptosis in vitro. J Neurosci. 2003;23:4519–4526. doi: 10.1523/JNEUROSCI.23-11-04519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci U S A. 2002;99:7628–7633. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin SL, Lan L, Zoudilova M, Conti M. Specific role of phosphodiesterase 4B in lipopolysaccharide-induced signaling in mouse macrophages. J Immunol. 2005;175:1523–1531. doi: 10.4049/jimmunol.175.3.1523. [DOI] [PubMed] [Google Scholar]
- 22.Karadottir R, Attwell D. Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience. 2007;145:1426–1438. doi: 10.1016/j.neuroscience.2006.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keane RW, Davis AR, Dietrich WD. Inflammatory and apoptotic signaling after spinal cord injury. J Neurotrauma. 2006;23:335–344. doi: 10.1089/neu.2006.23.335. [DOI] [PubMed] [Google Scholar]
- 24.Koshinaga M, Whittemore SR. The temporal and spatial activation of microglia in fiber tracts undergoing anterograde and retrograde degeneration following spinal cord lesion. J Neurotrauma. 1995;12:209–222. doi: 10.1089/neu.1995.12.209. [DOI] [PubMed] [Google Scholar]
- 25.Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louis JC, Magal E, Takayama S, Varon S. CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science. 1993;259:689–692. doi: 10.1126/science.8430320. [DOI] [PubMed] [Google Scholar]
- 27.Loy DN, Crawford CH, Darnall JB, Burke DA, Onifer SM, Whittemore SR. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J Comp Neurol. 2002;445:308–324. doi: 10.1002/cne.10168. [DOI] [PubMed] [Google Scholar]
- 28.Loy DN, Magnuson DS, Zhang YP, Onifer SM, Mills MD, Cao QL, Darnall JB, Fajardo LC, Burke DA, Whittemore SR. Functional redundancy of ventral spinal locomotor pathways. J Neurosci. 2002;22:315–323. doi: 10.1523/JNEUROSCI.22-01-00315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loy DN, Talbott JF, Onifer SM, Mills MD, Burke DA, Dennison JB, Fajardo LC, Magnuson DS, Whittemore SR. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp Neurol. 2002;177:575–580. doi: 10.1006/exnr.2002.7959. [DOI] [PubMed] [Google Scholar]
- 30.Lu P, Yang H, Jones LL, Filbin MT, Tuszynski MH. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Ma D, Wu P, Egan RW, Billah MM, Wang P. Phosphodiesterase 4B gene transcription is activated by lipopolysaccharide and inhibited by interleukin-10 in human monocytes. Mol Pharmacol. 1999;55:50–57. doi: 10.1124/mol.55.1.50. [DOI] [PubMed] [Google Scholar]
- 33.Massey JM, Amps J, Viapiano MS, Matthews RT, Wagoner MR, Whitaker CM, Alilain W, Yonkof AL, Khalyfa A, Cooper NG, Silver J, Onifer SM. Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp Neurol. 2007 doi: 10.1016/j.expneurol.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matute C, Alberdi E, Domercq M, Perez-Cerda F, Perez-Samartin A, Sanchez-Gomez MV. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 2001;24:224–230. doi: 10.1016/s0166-2236(00)01746-x. [DOI] [PubMed] [Google Scholar]
- 35.McEwen ML, Springer JE. A mapping study of caspase-3 activation following acute spinal cord contusion in rats. J Histochem Cytochem. 2005;53:809–819. doi: 10.1369/jhc.4A6467.2005. [DOI] [PubMed] [Google Scholar]
- 36.McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21:3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller BA, Sun F, Christensen RN, Ferguson AR, Bresnahan JC, Beattie MS. A sublethal dose of TNFalpha potentiates kainate-induced excitotoxicity in optic nerve oligodendrocytes. Neurochem Res. 2005;30:867–875. doi: 10.1007/s11064-005-6880-x. [DOI] [PubMed] [Google Scholar]
- 38.Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onifer SM, Nunn CD, Decker JA, Payne BN, Wagoner MR, Puckett AH, Massey JM, Armstrong J, Kaddumi EG, Fentress KG, Wells MJ, West RM, Calloway CC, Schnell JT, Whitaker CM, Burke DA, Hubscher CH. Loss and spontaneous recovery of forelimb evoked potentials in both the adult rat cuneate nucleus and somatosensory cortex following contusive cervical spinal cord injury. Exp Neurol. 2007 doi: 10.1016/j.expneurol.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onifer SM, Zhang YP, Burke DA, Brooks DL, Decker JA, McClure NJ, Floyd AR, Hall J, Proffitt BL, Shields CB, Magnuson DS. Adult rat forelimb dysfunction after dorsal cervical spinal cord injury. Exp Neurol. 2005;192:25–38. doi: 10.1016/j.expneurol.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Pang XP, Ross NS, Park M, Juillard GJ, Stanley TM, Hershman JM. Tumor necrosis factor-alpha activates nuclear factor kappa B and induces manganous superoxide dismutase and phosphodiesterase mRNA in human papillary thyroid carcinoma cells. J Biol Chem. 1992;267:12826–12830. [PubMed] [Google Scholar]
- 42.Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- 43.Patrizio M. Tumor necrosis factor reduces cAMP production in rat microglia. Glia. 2004;48:241–249. doi: 10.1002/glia.20074. [DOI] [PubMed] [Google Scholar]
- 44.Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 45.Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- 46.Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 47.Rabchevsky AG, Sullivan PG, Scheff SW. Temporal-spatial dynamics in oligodendrocyte and glial progenitor cell numbers throughout ventrolateral white matter following contusion spinal cord injury. Glia. 2007;55:831–843. doi: 10.1002/glia.20508. [DOI] [PubMed] [Google Scholar]
- 48.Sachs BD, Baillie GS, McCall JR, Passino MA, Schachtrup C, Wallace DA, Dunlop AJ, MacKenzie KF, Klussmann E, Lynch MJ, Sikorski SL, Nuriel T, Tsigelny I, Zhang J, Houslay MD, Chao MV, Akassoglou K. p75 neurotrophin receptor regulates tissue fibrosis through inhibition of plasminogen activation via a PDE4/cAMP/PKA pathway. J Cell Biol. 2007;177:1119–1132. doi: 10.1083/jcb.200701040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- 50.Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- 51.Stys PK. White matter injury mechanisms. Curr Mol Med. 2004;4:113–130. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- 52.Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486:373–383. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- 53.Tripathi R, McTigue DM. Prominent oligodendrocyte genesis along the border of spinal contusion lesions. Glia. 2007;55:698–711. doi: 10.1002/glia.20491. [DOI] [PubMed] [Google Scholar]
- 54.Vicini E, Conti M. Characterization of an intronic promoter of a cyclic adenosine 3'5'-monophosphate (cAMP)-specific phosphodiesterase gene that confers hormone and cAMP inducibility. Mol Endocrinol. 1997;11:839–850. doi: 10.1210/mend.11.7.9941. [DOI] [PubMed] [Google Scholar]
- 55.Wachtel H. Characteristic behavioural alterations in rats induced by rolipram and other selective adenosine cyclic 3', 5'-monophosphate phosphodiesterase inhibitors. Psychopharmacology (Berl) 1982;77:309–316. doi: 10.1007/BF00432761. [DOI] [PubMed] [Google Scholar]
- 56.Yan P, Liu N, Kim GM, Xu J, Xu J, Li Q, Hsu CY, Xu XM. Expression of the type 1 and type 2 receptors for tumor necrosis factor after traumatic spinal cord injury in adult rats. Exp Neurol. 2003;183:286–297. doi: 10.1016/s0014-4886(03)00135-3. [DOI] [PubMed] [Google Scholar]
- 57.Yoshikawa M, Suzumura A, Tamaru T, Takayanagi T, Sawada M. Effects of phosphodiesterase inhibitors on cytokine production by microglia. Mult Scler. 1999;5:126–133. doi: 10.1177/135245859900500210. [DOI] [PubMed] [Google Scholar]
- 58.Yoshioka A, Shimizu Y, Hirose G, Kitasato H, Pleasure D. Cyclic AMP-elevating agents prevent oligodendroglial excitotoxicity. J Neurochem. 1998;70:2416–2423. doi: 10.1046/j.1471-4159.1998.70062416.x. [DOI] [PubMed] [Google Scholar]
- 59.Yoshioka A, Yamaya Y, Saiki S, Kanemoto M, Hirose G, Pleasure D. Cyclic GMP/cyclic GMP-dependent protein kinase system prevents excitotoxicity in an immortalized oligodendroglial cell line. J Neurochem. 2000;74:633–640. doi: 10.1046/j.1471-4159.2000.740633.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Acute pathology of cervical SCI. Representative cresyl violet stained, transverse sections of uninjured naïve (A), 12 (B), 24 (C), and 72 (D) hour post SCI. Shaded region in (A) indicates VLF region. Scale bar=1mm.