Abstract
The higher molecular weight polycyclic aromatic hydrocarbons (PAHs) such as benzo(a)pyrene (BaP) are typically associated with genotoxicity, however, newer evidence suggests that these compounds may also act as endocrine system disruptors. We hypothesized that altered expression of the P450 enzyme aromatase genes could be a target for reproductive or developmental dysfunction caused by BaP exposure. Aromatase is at least partially responsible for estrogen homeostasis by converting androgens into estrogens. In fish, there are two isoforms of aromatase, a predominantly ovarian form, CYP19A1, and a brain form, CYP19A2. CYP19 mRNA expression was measured following BaP exposure (0, 10, 100 µg/L waterborne for 10 or 15 days) in Fundulus adults, juveniles and embryos by in situ hybridization. The CYP19A1 expression was significantly decreased after BaP exposure in the 3 month old Fundulus immature oocytes, but BaP did not affect CYP19A1 expression at any stage in adult oocytes. In embryo brains, BaP significantly decreased CYP19A2 compared to controls by 3.6-fold at 14 days post-fertilization. In adults, CYP19A2 expression was decreased significantly in the pituitary and hypothalamus (81% and 85% of controls, respectively). Promoter regions of Fundulus CYP19s were cloned, and putative response elements in the CYP19A1 and CYP19A2 promoters such as CRE, AhR and ERE may be involved in BaP-mediated changes in CYP19 expression. In order to compare the mechanism of BaP-mediated inhibition with that of a known aromatase inhibitor, fish were also exposed to fadrozole (20 and 100 µg/L). Fadrozole did not significantly decrease the mRNA expression in embryos or adult Fundulus. However, aromatase enzyme activity was significantly decreased in adult ovary and brain tissues. These studies provide a greater molecular understanding of the mechanisms of action of BaP and its potential to impact reproduction or development.
Keywords: Benzo(a)pyrene, Fundulus, Aromatase, CYP19
INTRODUCTION
The aromatase enzyme, encoded by the CYP19 gene, is responsible for the conversion of androgens (typically testosterone and androstenedione) to estrogens. It is well established that while mammals (excepting pigs) have a single form of this gene, fish have two distinct CYP19 genes (Simpson et al., 2002). Teleost CYP19A1 (CYP19a) is expressed predominantly in the ovary while CYP19A2 (CYP19b) is expressed primarily in the central nervous system. Estrogen homeostasis is integral to diverse physiological processes including reproduction, development, behavior, and carcinogenesis. Therefore, the potential for environmental contaminants to impact aromatase expression and, in turn, affect these downstream physiological processes has been a target of intense recent research and is the subject of two recent reviews (Sanderson, 2006; Cheshenko et al., 2008). Structurally diverse environmental contaminants including tributyltin (Cooke, 2002), methylmercury (Hinfray et al., 2006), triazole and imidazole fungicides (Zarn et al., 2003) have been suggested as aromatase inhibitors, while estrogenic compounds such as nonylphenol and ethynylestradiol induce aromatase expression (Kazeto et al., 2004; Contractor et al., 2004). The mechanisms by which these chemicals interact with aromatase, however, are diverse including inhibition or induction of message and/or protein levels or directly inhibiting enzyme activity. Furthermore, many reported effects are at the tissue level (e.g. whole brain or gonad), and not the specific regions or cell types which may be physiologically important in reproduction and development. The objective of our study was to investigate the potential for benzo(a)pyrene (BaP), a representative carcinogenic polycyclic aromatic hydrocarbon (PAH) and fadrozole, a therapeutically used non-steroidal aromatase inhibitor, to affect CYP19A1 and CYP19A2 mRNA expression in Fundulus heteroclitus. By using in situ hybridization and testing effects of exposure at different developmental stages, we aimed to highlight some of the stage and cell-type specific effects of these two compounds on CYP19 mRNA expression.
PAHs are ubiqitous environmental contaminants derived from incomplete combustion of carbon. In the United States, PAHs as a mixture (#7), BaP (#9) and benzo(b)fluroanthene (#10) are recognized in the top ten on CERCLA’s Priority List of Hazardous Substances (http://www.atsdr.cdc.gov/cercla/05list.html). In contrast to halogenated hydrocarbons such as PCBs and chlorinated pesticides which have shown downward trends in US sediment cores, trends in sediment PAH concentrations have been increasing particularly near urban land use (van Metre & Mahler, 2005). Benzo(a)pyrene is the most studied high molecular weight PAH. BaP induces aryl hydrocarbon receptor (AhR)-mediated CYP1 enzymes which, in turn, metabolize BaP to mutagenic and carcinogenic metabolites (ATSDR, 1995; Piskorska-Pliszczynska et al., 1986). While their role in carcinogenesis is accepted, PAHs also have been suggested as endocrine disrupting compounds (EDCs) by negatively impacting reproduction. In various in vitro and in vivo fish systems PAH exposure has resulted in decreased: egg output, ovarian somatic index, 17β-estradiol levels, and vitellogenin production (Hoffman & Oris, 2006; Monteiro et al., 2000; Nicolas, 1999; Thomas, 1990; Afonso et al., 1997). Three studies have specifically investigated BaP’s effects on fish CYP19A1 and CYP19A2 mRNA expression using RT-PCR with mixed results. In zebrafish (Danio rerio) exposed up to 3 µg/L BaP for 56 days, CYP19A2 mRNA was increased but no effect on CYP19A1 was observed (Hoffman & Oris, 2006). Similarly, CYP19A2 induction was found when 17 dpf juvenile zebrafish were exposed for 3 days to 10 µM BaP (2.5 mg/L) (Kazeto et al., 2004). In contrast, in our previous studies with Fundulus exposed to waterborne BaP up to 10 µg/L for 15 days, we did not see a significant effect on expression of either CYP19 message (Patel et al., 2006). In each of these cases mRNA was extracted from either whole tissues or organisms and very sensitive PCR methods were used.
Previous studies exposing fish to fadrozole have reported aromatase inhibition correlated with reduced fecundity and decreased plasma estrogen and vitellogenin concentrations in females (Ankley et al., 2002; Panter et al., 2004), gonadal masculation (Uchida et al., 2004; Fenske & Segner, 2004) and decreased courtship behaviors in males (Hallgren et al., 2006). Exposure of fadrozole to salmon ovarian and brain tissue reduced estradiol production in vitro and these types of experiments have been suggested as a rapid screening method for other environmental aromatase inhibitors (Afonso et al., 1997; Lee et al., 2006). While fadrozole is a well established competitive inhibitor of aromatase enzyme activity, its effects at the CYP19 message level are less clear (Villeneuve et al., 2006; Villeneuve et al., 2007). To our knowledge, none of the studies in fish have addressed cellular expression of CYP19 mRNAs following fadrozole exposure.
The utility of Fundulus as a “premier teleost model in environmental biology” has been highlighted in a recent review (Burnett et al., 2007). Fundulus are estuarine fish broadly distributed along the Atlantic coastline, but they maintain very small home ranges (Lotrich, 1975). Their reproductive and developmental biology has been well established (Taylor, 1999; Armstrong & Child, 1965). They have also been extensively used in both laboratory and field settings to further understand molecular mechanisms of environmental toxicants including, but not limited to: PAHs (Wassenberg & Di Giulio, 2004; Meyer et al., 2003; Willett et al., 1995; Wang et al., 2006; Stine et al., 2004), PCBs (Greytak et al., 2005; Greytak & Callard, 2007; Powell et al., 2000), dioxins (Prince & Cooper, 1995), metals (Zhou et al., 2000; Roling et al., 2007) and effluents (Dube & MacLatchy, 2001).
As introduced above, effects of both BaP and fadrozole on CYP19 expression in fish have shown mixed results. In previous studies in our laboratory, we found no statistically significant effect of BaP on CYP19A1 or CYP19A2 message by RT-PCR of whole brain or gonads. However, BaP exposure caused an inhibition of ovarian aromatase activity while it induced female brain activities (Patel et al., 2006). To more rigorously define constitutive CYP19 mRNA expression in Fundulus embryos, juvenile and adult fish, we developed in situ hybridization probes for both CYP19A1 and CYP19A2 mRNA (Dong & Willett, 2008). In this study, we have found that the PAH, BaP, alters local expression of CYP19 mRNA but in an age- and tissue-dependent way. In contrast, fadrozole inhibition was exclusively detected at the enzyme level. Putative response elements in the Fundulus CYP19A1 and CYP19A2 promoters were identified that may be playing a role in the BaP-mediated disregulation of the CYP19 genes.
MATERIALS AND METHODS
Fish source, care and handling
A parental population of F. heteroclitus collected from an uncontaminated site at the New River inlet near Beaufort, NC was raised under the University Institutional Animal Care and Use Committee approved conditions. Sexually mature fish were bred and kept in salt water (20–25 parts per thousand [ppt]). The fish were maintained at 14:10 light-dark cycle in summer and 10:14 light-dark cycle in the winter. Adult fish were fed twice daily with tropical flake fish food (Tetramin, Tetra Werke, Germany) and live brine shrimp. First generation offspring, from wild parents, were used for the studies described here.
Embryo exposure
Pooled oocytes and sperm stripped from parental population of around 50 fish were mixed together for in vitro fertilization. Fertilized eggs were randomly sorted into seven treatment groups, namely untreated, control dimethylsulfoxide (DMSO), (0.1 µg/ml), BaP (10 and 100 µg/L), and aromatase inhibitor fadrozole (1, 5 and 10 µg/L). Fadrozole (4-(5,6,7,8-tetrahydroimidazole[1,5-a]pyridin-5-yl)-benzonitrile monohydrochloride; CGS16949A) was kindly provided by Novartis Pharma AG, (Basel, Switzerland). Eggs were placed in 10 ml of water in clean glass bottle (n=10 embryos/bottle) and exposure began at approximately 4.5-hpf (hours postfertilization) until 10 dpf (days postfertilization). 1-Phenyl-2-thiourea (PTU, 0.003%) was added to the culture water from 5 to 10 dpf to prevent pigmentation of Fundulus embryos. At 10 dpf, eggs were removed from the exposure and placed in a culture plate on top of a piece of damp filter paper where they continued to develop until 14 dpf when they were collected for in situ hybridization.
Immature and adult exposure
Fish were exposed in 5 or 6-L tanks containing two male and two female for adult fish or four 3 month old fish (1.5 L water per adult fish, 1.25 L water per 3 month old fish). Fish were kept in the tanks 7 days for acclimatization prior to the beginning of the exposure. The Fundulus (n = 6–16/treatment) were exposed to the following treatment for 15 days (complete reproductive cycle): control (100 µl/L ethanol), BaP (10 µg/L (40 nM) and 100 µg/L (400 nM)) (Sigma, St Louis, MO, stock solution 1 mg/ml in 100% ethanol) or fadrozole (20 and 100 µg/L). Stock solutions of fadrozole were prepared in ethanol at concentrations of 20 and 100 mg/ml. The ethanol concentration in all treatments was 0.01%. Exposure conditions were 17.5–20.5°C, 14:10 light-dark period, and 20–25 ppt. Fish were fed Tetramin flakes twice daily. The tanks were checked twice every 24 h, and water was changed (85–100% static water renewal) daily at 15:00. On the 15th day of exposure, the fish were anesthetized by placing them into salt water that contained 3-aminobenzoic acid ethyl ester, MS222 (Sigma). The 3 month old fish were then weighed, measured, and fixed in 1 ml Bouin’s solution prior to paraffin embedding, sectioning and in situ hybridization. Adult fish were weighed and measured, and dissected to retrieve the gonads and brains which were also weighed. Gonads and brains were fixed in 4% (w/v) paraformaldehyde for paraffin sectioning or whole mount in situ hybridization from the BaP-treated fish and half of the fadrozole-treated fish. Brains and gonads from the other half of the fadrozole-treated fish were placed into phosphate buffer (10 mM potassium phosphate, 100 mM potassium chloride, 1 mM EDTA, pH 7.4) that equaled 4 times the organ weight. The tissues were homogenized and centrifuged for 10 minutes at 10,000 × g at 4°C. The protein concentration was quantitated by the Bradford assay, and samples were stored at −80°C. These tissues were used for aromatase enzyme activity determination.
Whole mount in situ hybridization
Fundulus embryos were fixed in 4% (w/v) paraformaldehyde in phosphate saline solution (pH 7.4) overnight, stored in −20°C freezer after the membranes were removed by watchmaker's forceps. Whole mount in situ hybridization was carried out according to (Dong & Willett, 2008). Embryos or adult brains were hybridized with an antisense probe of 717 base pairs for Fundulus CYP19A2, of which the cDNA was cloned previously (Patel et al., 2006). Following hybridization overnight at 65°C, embryos or adult brains were washed with 2× SSC (300 mM NaCl, 30 mM sodium citrate, pH 7.0) and 0.2× SSC twice for 30 min, respectively. After blocking with 2% blocking reagent (Roche, Mannheim, Germany), embryos or brains were incubated overnight with 4000× diluted anti-DIG antibody conjugated with alkaline phosphatase (Roche) at 4°C. The color reaction was carried out by incubation with BM-purple substrate (Roche).
Paraffin sectioning and in situ hybridization
Fundulus gonads were fixed in 4% (w/v) paraformaldehyde in phosphate saline solution (pH 7.4) overnight, followed by dehydration in increasing gradients of ethanol (70%, 80%, 90%, 95%, and 100% twice). After cleared in Clearify™ (American Master Tech Scientific, Inc., Lodi, CA), tissues were embedded in molten paraffin (Paraplast embedding media paraplast X-tra, Sigma). Sections of 7 µm thickness were made using a microtome (OLYMPUS CUT 4055, Olympus American Inc., San Jose, CA). The sections were cleared with a gradient of ethanol (100%-70%) and hybridized with a 1660 nt antisense probe of Fundulus CYP19A1. Following hybridization overnight at 50°C, sections were washed with 2× SSC and 0.2× SSC twice for 20 min, respectively. After blocking with 2% blocking reagent (Roche), sections were incubated overnight with 4000× diluted anti-DIG antibody conjugated with alkaline phosphatase (Roche) at 4°C. The color reaction was carried out by incubation with BM-purple substrate (Roche).
Expression quantification
CYP19 expression was recorded with a digital camera (OPTRONICS) and light microscope (BX40, Olympus). For quantifying the intensity of CYP19A1 or CYP19A2 expression, stained regions were outlined by means of KODAK 1D Image Analysis Software by creating regions of interest, (ROIs) and calculating mean intensity ( = sum intensity divided by ROI pixel area). For quantification of CYP19A2 mRNA expression in the embryonic Fundulus, conventional image software (Photoshop 7.0; Adobe) was used. The expression area was circled on the Photoshop image at the same magnification, and the expression area was divided by the embryo’s total head area.
Aromatase assay
Aromatase activity was quantified by the (Lephart & Simpson, 2002) tritiated water release assay as described by (Patel et al., 2006) with minor modifications. Briefly, tissue homogenates were incubated in a shaking water bath for 3 h at 28°C/100 rpm with substrate, 3.0 nM (for gonad) or 30 nM (for brain). The substrate 3H-androst-4-ene-3, 17-dione (specific activity 25.9 Ci/mmol (Perkin-Elmer Life Sciences, Boston, MA, USA)) was in the presence of a reaction buffer (10 mM potassium phosphate (dibasic), 100 mM potassium chloride, 1 mM EDTA, 1 mM dithiothreitol, 1 mM NADPH, 10 mM glucose-6-phosphate and 1 U/mL glucose-6-phosphate dehydrogenase), in 200 µL total volume of reaction. Reactions were terminated, and extracted as previously described, and 50 µL of the 3H2O was quantified by counting in 200 µL of premixed scintillation cocktail (Ultima Gold TMXR, Packard Biosciences, Boston, MA) with a liquid scintillation analyzer (Topcount™ microplate scintillation & Luminescence counter, Packard Meriden, CT). The specificity of the assay was determined by using 20 µM 4-hydroxyandrostenedione (Sigma), an irreversible inhibitor of the catalytic activity of aromatase. Aromatase activity (pmol/h/mg) was calculated by correcting for the tritium estimated in the blank tubes (containing substrate but no protein, dpm range 77–101), the dilution factor = 3, the 3H distribution of the [1β − 3H] androstenedione (75% at the 1β-position and 25% at the 1α-position), protein concentration (150 µg for brain and 500 µg for gonad) and the specific activity of the substrate (dpm/mass; 14,302 ± 935).
Statistics
Results are presented as mean ± SE. Data was tested for homogeneity of variance with the Bartlett’s test. If variances were not significantly different, experimental significance was determined by one-way ANOVA followed by Neuman-Keulls posthoc test (p < 0.05). When data failed the Bartlett’s test, the non-parametric test, Kruskal-Wallis and Dunn’s Multiple comparison test was used (p <0.05).
Promoter region cloning
In order to clone the promoter regions of both CYP19A1 and CYP19A2 reverse primers were designed based on the previously published CYP19 cDNA sequences (Patel et al., 2006). Primers were synthesized by Invitrogen. Primers 1 and 2 were specific to the CYP19A1 gene: Primer 1: 5’-GCTACGCTGAGATTGCTTTGAACCTCC-3’; Primer 2: 5’-CTTCGGGTTTTGAGGAGCTACG-3’. Primers 3–6 were complementary to the CYP19A2 gene: Primer 3: 5’- GGTCAGCAGAGGAGAAGAGAGCGAAC-3’; Primer 4: 5’-GAGTGGAGGGAGTTCTGCAGCT-3’; Primer 5: 5’-CAGGTGTGTGACAGTAAAGTTTGTCAG-3’; Primer 6: 5’-GTGGCACAAGAAATCTGTAAGGAGGC-3’. (See also Supplementary Figure for primer locations). An adult female Fundulus liver GenomeWalker library (Universal GenomeWalker Kit; Clontech, Mountain View, CA) was constructed according to the manufacturer’s protocol in order to use a PCR-based method for the isolation of the 5’-termini of the two Fundulus CYP19 genes. For CYP19A1, the first PCR was carried out using Primer 1 (and the kit’s adapter-specific primer) followed by a nested second amplification with Primer 2 using the library generated from DraI, EcoRV, PvuII, or StuI restriction-digested genomic DNA. For CYP19A2, Primers 5 and 6 were used with the same library for the nested PCR amplification of the 5’-flanking region of CYP19A2. To isolate amplicons corresponding to the entire 5’-flanking region, PCRs were carried out with the following sets of primer pairs: Primers 2 and 7 (Primer 7: 5’-GAATTCCTGAAATGGTTTTCAG-3’), and Primers 4 and 8 (Primer 8: 5’-TAGTAAAGAAGGTCATGGAC-3’), for CYP19A1 and CYP19A2, respectively. The putative transcription initiation sites for each promoter region were determined using the Neural Promoter Predictions Program (http://www.fruitfly.org/cgibin/seqtools/promoter.pl). Potential transcription factor binding sites were identified by MatInspector (http://www.genomatix.de) and TFSEARCH (http://www.cbrc.jp).
RESULTS
Effects of BaP and fadrozole on CYP19A2 mRNA expression in Fundulus embryos
In 14 dpf Fundulus embryos, CYP19A2 expression was detected by in situ hybridization in the brain especially the hypothalamus and adrenal-like cells in the kidney. BaP strongly decreased expression of CYP19A2 gene in the brain but had no effect on interrenal expression (Fig. 1, note adrenal cells not in same focal plane as brains, expression best shown in panel 1E). When the expression area in brains was traced and quantitated, 10 and 100 µg/L BaP significantly decreased CYP19A2 expression by 3.61- and 3.66-fold compared to controls, respectively (Table 1). In contrast, embryos treated with 10 µg/L fadrozole for ten days had no change in CYP19A2 expression (Fig.1E and Table 1). We found very weak expression of CYP19A2 in the hypothalamus and almost no expression in the pituitary in the 3 month old fish brain by in situ hybridization (Dong & Willett, 2008), so in this study we did not investigate BaP or fadrozole effects in 3 month old brains.
Figure 1. Effect of BaP exposure on CYP19A2 expression in the brain of developing embryos.
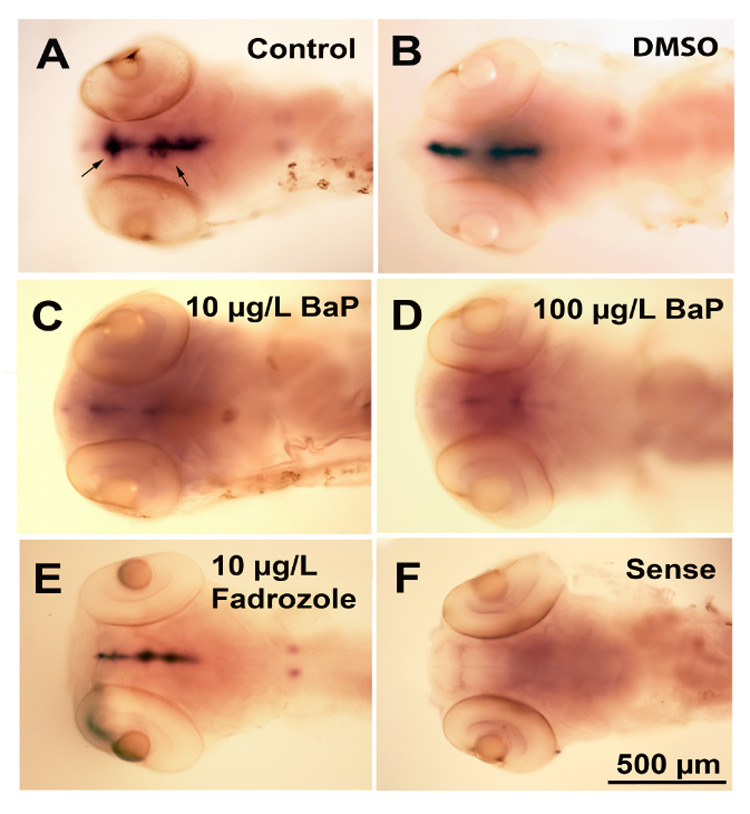
Embryos were untreated or exposed to DMSO, BaP (10 or 100 µg/L) or fadrozole (10 µg/L) from 4.5 hpf to 10 days. On days 5–10 PTU was added to decrease pigment and facilitate whole mount in situ hybridization at 14 dpf. Arrow shows CYP19A2 expression. Panel F indicates nonspecific binding using sense probe. (n= 5 embryos/treatment).
Table 1.
Quantitative CYP19A2 expression in the brain/head area of BaP- or fadrozole-exposed 14 dpf Fundulus embryos.
| Control | DMSO | BaP 10 µg/L | BaP 100 µg/L | Fadrozole 10 µg/L |
|---|---|---|---|---|
| 0.20 ± 0.02a | 0.18 ± 0.02 a | 0.06 ± 0.01 b | 0.05 ± 0.01 b | 0.16 ± 0.03 a |
The CYP19A2 expression area was quantified with image analysis and was expressed in pixels/head area.
Differences in letters signify significantly different (Mean ± SEM, n = 5 embryos/treatment, ANOVA, p ≤ 0.05) values.
Effects of BaP and fadrozole on CYP19A2 mRNA expression in adult Fundulus
CYP19A2 mRNA is constitutively expressed in pituitary, hypothalamus, ventral telencephalon and olfactory bulb in adult Fundulus (Dong & Willett, 2008). Furthermore, CYP19A2 expression between the genders was not significantly different except in the olfactory bulb. In this study, we did not find any differential constitutive expression in any of the brain regions between males and females. Adult Fundulus exposure for 15 days to BaP significantly downregulated CYP19A2 expression by approximately 15–20% in the hypothalamus and pituitary compared to controls, but decreases found in the olfactory bulb and ventral telencephalon were not significant (Fig. 2 and Table 2). Fadrozole exposure did not significantly alter CYP19A2 expression in adult brain regions.
Figure 2. Effect of BaP- or fadrozole-exposure on CYP19A2 expression in the adult Fundulus brain.
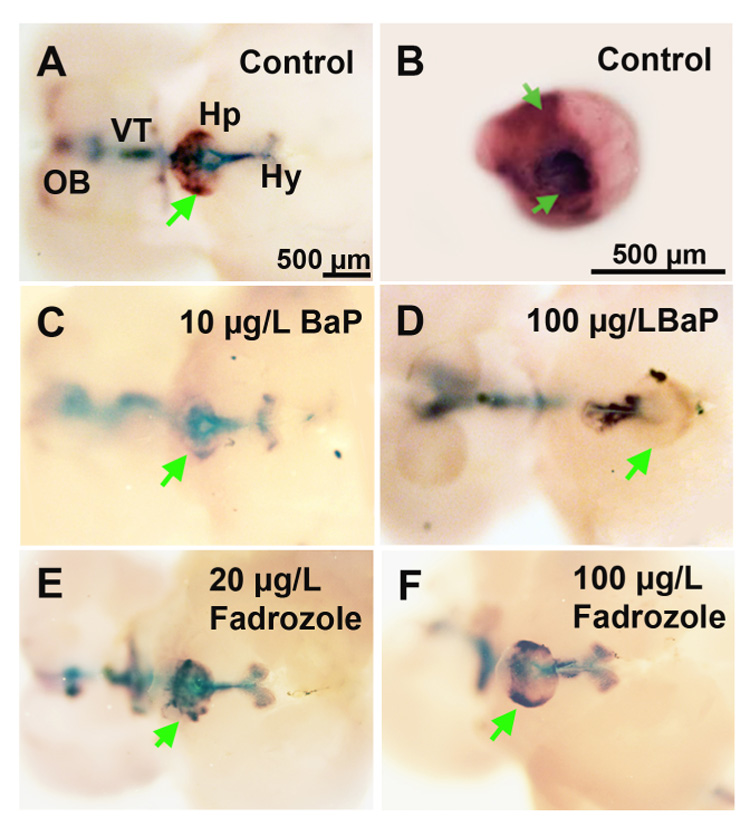
The CYP19A2 transcripts were found in the hypothalamus (Hy), pituitary (Hp), ventral telencephalon (VT), and extending to the olfactory bulb (OB). A and B are Control; C and D are 10 and 100 µg/L BaP treated fish; E and F are 20 and 100 µg/L fadrozole. B shows pituitary region dissected from A. Arrows indicate expression in the pituitary. (n = 3–8 fish/sex/treatment; females shown)
Table 2.
Quantitative CYP19A2 expression (×104) in brain regions in control, BaP or fadrozole-exposed adult Fundulus.
| n | HY | HP | VT | OB | |
|---|---|---|---|---|---|
| Control | 14 | 5.37 ± 0.10 a | 5.79 ± 0.05 a | 4.82 ± 0.11 a | 4.07 ± 0.14 a |
| 10 µg/L BaP | 6 | 4.75 ± 0.42 a | 4.76 ± 0.24 b | 4.20 ± 0.25 a | 3.95 ± 0.22 a |
| 100 µg/L BaP | 6 | 4.37 ± 0.32 b | 4.94 ± 0.21 b | 4.16 ± 0.17 a | 3.73 ± 0.32 a |
| 100 µg/L Fadrozole | 7 | 5.05 ± 0.30 a | 5.41 ± 0.12 a | 4.58 ± 0.29 a | 3.86 ± 0.25 a |
The mean intensity of CYP19A2 expression was quantified with KODAK 1D image analysis software. Fish were exposed for 15 days to ethanol control, BaP (10 and 100 µg/L) or fadrozole (100 µg/L). HY: hypothalamus; VT: ventral telencephalon; OB: olfactory bulb; HP: pituitary. There was no statistical difference between sexes so mean intensities were combined for males and females.
Differences in letters are significantly different with in a region (Mean ± SEM, Kruskal-Wallis, p ≤ 0.05) values.
Effects of BaP on CYP19A1 gene expression in Fundulus
CYP19A1 mRNA is not detectable with in situ hybridization in any of the embryo stages that we investigated. However, CYP19A1 mRNA is expressed in the ooplasm of early stage I oocytes (primary growth stage) as strong and evenly distributed signals in the ovaries of 3 month old Fundulus. The effects of BaP and fadrozole on CYP19A1 gene expression in juvenile fish are shown in Fig. 3. CYP19A1 expression was clearly decreased in the early stage I oocytes by BaP but not in the fadrozole treated group (Fig. 3). To quantitate the CYP19A1 expression, early stage I oocytes were divided by size into stage Ia1 (diameter less than 0.07 mm) and stage Ia2 (diameter about 0.1 mm). CYP19A1 expression in the BaP exposed groups was approximately 71% and 72% of controls, respectively, in the 10 and 100 µg/L group in the stage Ia1. In the stage Ia2, CYP19A1 mRNA expression was significantly decreased by 46% and 42% by 10 and 100 µg/L BaP, while fadrozole had no effect on CYP19A1 at either oocyte stage (Table 3)
Figure 3. Effect of BaP exposure on CYP19A1 mRNA expression in the ovary of 3 month old Fundulus.

Fish were sectioned and visualized by section in situ hybridization after exposure to DMSO (control), BaP (10 or 100 µg/L) or fadrozole (100 µg/L). B, D, F and H are magnification of A, C, E and G, respectively. The CYP19A1 expression was found in ooplasm of oocytes in the control and fadrozole treated group as strong and evenly distributed signals, but the signal was decreased in the BaP (10 and 100 µg/L) treated group (n= 4–5 fish/treatment).
Table 3.
Quantitative CYP19A1 expression (×104) in control and BaP exposed 3 month old Fundulus ovaries.
| Stage Ia (0–70 µm) | Stage Ia (71–150 µm) | ||||||
|---|---|---|---|---|---|---|---|
| Con | B 10 | B 100 | F 100 | Con | B 10 | B 100 | F 100 |
| 4.54 ± 0.28 | 3.21 ± 0.25 | 3.29 ± 0.27 | 4.18 ± 0.42 | 3.95 ± 0.29 | 2.13 ± 0.11* | 2.29 ± 0.16* | 3.75 ± 0.41 |
The mean intensity of CYP19A1 expression was quantified in the early stage I (stage Ia oocytes 0–70 µm in size and stage Ia oocytes 71–150 µm in size) of ooplasm of oocyte with KODAK 1D image analysis software after section in situ hybridization.
Mean ± SEM, n = 4–5 fish/treatment, Kruskal-Wallis
p ≤ 0.05 versus control.
In adult Fundulus, constitutive expression of CYP19A1 mRNA also was detected in the ooplasm of early stage I oocytes (Fig. 4B1, B2, B3 and B4). After early stage I, normal expression of CYP19A1 mRNA expression moves to the vitellogenic follicle then to the vitelline envelope of membrane. Neither BaP nor fadrozole affected CYP19A1 expression in adult Fundulus (Fig. 4).
Figure 4. Effect of BaP- or fadrozole-exposure on CYP19A1 mRNA expression in the ovary of adult Fundulus.
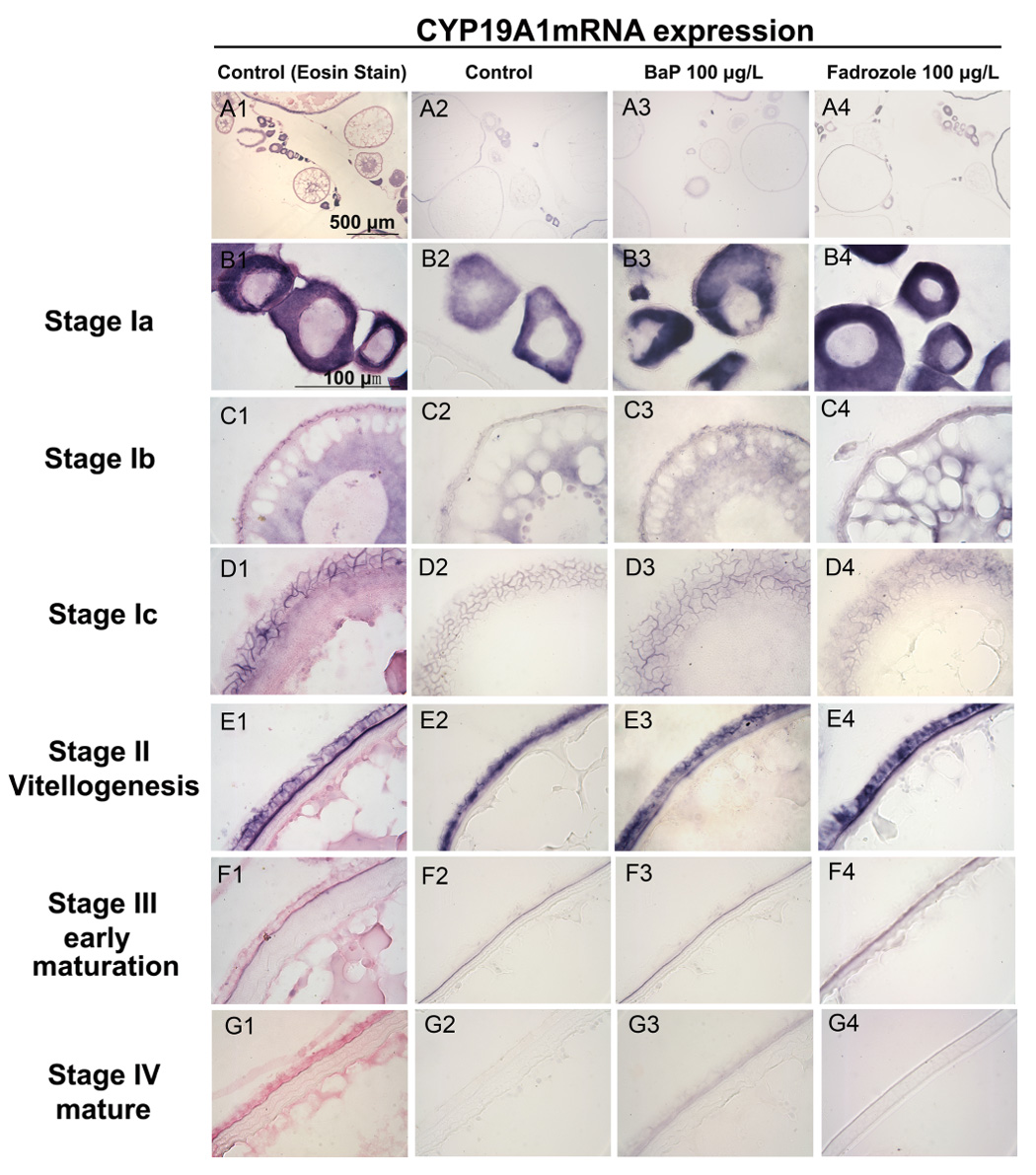
Fish were sectioned and visualized by section in situ hybridization after exposure to DMSO (control, A1-G1 and A2 -G2), BaP (100 µg/L, A3-G3) or fadrozole (100 µg/L, A4-G4). A1-G1 shows CYP19A1 mRNA expression (purple color) and Eosin contrast stain (pink color). Expression of CYP19A1 mRNA was detected in B: early stage I (primary growth stage, B1, B2, B3 and B4) in the ooplasma of oocyte; C: mid stage I (Stage Ib) in the membrane and ooplasma (previtellogenesis follicle stage, C1, C2, C3 and C4); D: stage Ic in the follicular epithelium (D1, D2, D3 and D4); E: Stage II (vitellogenesis) in the follicular epithelium of membrane (E1, E2, E3 and E4); F: Stage III (early maturational follicles) in the vitelline envelope of oocytes (F1, F2, F3 and F4); and G: No signal was detected after stage IV (maturational follicles, G1, G2, G3 and G4) oocytes. No significant effect was detected between control, BaP or fadrozole-treated groups in each stage. (n= 4–5 fish/treatment).
Effects of BaP and fadrozole on adult Fundulus GSI
BaP did not affect gonad somatic index (GSI) in either male or female fish compared to controls (Table 4). Fadrozole exposure increased male adult GSI at the highest dose, but had no effect on female GSI following the 15 day waterborne exposure.
Table 4.
Gonad somatic index (×102) of BaP- or fadrozole treated adult fish.
| Sex | Control | BaP 100 µg/L | Fadrozole 20 µg/L | Fadrozole 100 µg/L |
|---|---|---|---|---|
| Female | 4.13 ± 0.66 (13)a | 5.20 ± 1.05 (7) a | 5.64 ± 0.58 (3) a | 6.23 ± 1.97 (4) a |
| Male | 1.83 ± 0.24 (9) b | 1.53 ± 0.13 (8) b | 2.56 ± 0.50 (4) bc | 3.53 ± 1.43 (3) c |
Ovary or testis weight divided by whole fish weight. Different letters within a sex are significantly different (ANOVA, p ≤ 0.05, Mean ± SEM (n)).
Effects of fadrozole on adult Fundulus brain and ovary enzyme activity
Exposure to fadrozole (20 and 100 µg/L) for 15 days did not significantly affect adult fish or mean brain weight, size or survival (data not shown). While fadrozole treatment did not alter CYP19A1 or CYP19A2 mRNA expression, in vivo exposure to 20 and 100 µg/L fadrozole inhibited ovarian aromatase activity by 80% and 73%, respectively (p < 0.05, ANOVA) (Fig. 5A). Activity was not detectable in testis (data not shown). Fadrozole exposure decreased female brain activity 9.7- and 4.3-fold compared to control treated animals (Fig 5B). As expected when 2.5 µg/L fadrozole was added in vitro to control brain preparations, aromatase activity was also decreased (6.5-fold). However, male brain aromatase activity was not statistically affected by either in vivo or in vitro fadrozole exposure partially because of high replicate variability (Fig. 5C). Aromatase activity was decreased by 99% in the presence of 4-hydroxyandrostenedione, an irreversible inhibitor, validating the activity to be due to aromatase enzyme (data not shown).
Figure 5. Aromatase activity in Fundulus.

Fundulus were exposed to waterborne fadrozole (20 or 100 µg/L) for 15 days. Ethanol (180 µL) was used as the solvent control. Ovary or brain homogenate (500 or 150 µg protein, respectively) was incubated with 3 nM (or 30 nM) 3H-androstenedione for 3 h at 28°C. Aromatase activity was measured as the release of tritiated water and reported as pmol/mg/h. Each bar represents the mean ± SEM of 7–8 fish per treatment. As a positive control 2.5 µg/L fadrozole was added to control tissues during the course of the reaction and results are the bars labeled F in vitro. (A) Ovarian activities. (B) Female brain activities. (C) Male brain activities. (ANOVA, * p ≤ 0.05).
Isolation of the cDNAs encoding Fundulus CYP19s
To further understand the regulation of the Fundulus CYP19A1 and CYP19A2 genes, we isolated and sequenced both promoters (Table 5 and supplementary figure). The CYP19A1 promoter was 525 nt to the putative start site while the CYP19A2 promoter was 1257 nt long. The putative transcription factor binding sites in CYP19A1 included: a consensus TATA box, steroidogenic factor-1 (SF1/Ad4BP), cAMP responsive element (CRE), estrogen responsive element (ERE), aryl hydrocarbon responsive element (AhR/Arnt), peroxisomal proliferator-activated receptor/retinoid X receptor (PPAR/RXR) element, nuclear factor kappa β (NF-kappaβ), and two GATA binding factor sites (Supplementary Figure and Table 5, GenBank Accession no. EU045309). In the CYP19A2 5’-flanking sequence, the putative transcription factor binding sites were: a TATA box, SF1/Ad4BP, progesterone receptor (PR), AhR/Arnt, ERE, two androgen receptor (AR), and a GATA-binding factor 3 site (Supplementary Figure and Table 5, GenBank, Accession no. EU045310).
Table 5.
Putative response elements and their locations in the CYP19A1 and CYP19A2 promoter regions.
| Gene | Response Element | Location |
|---|---|---|
| CYP19A1 | GATA-1 | −396 to −384 |
| GATA-1 | −373 to −361 | |
| NF-kappaB | −360 to −349 | |
| PPAR | −312 to −290 | |
| AHR | −264 to −240 | |
| ER | −221 to −203 | |
| SF1/Ad4BP | −188 to −181 | |
| CRE | −124 to −104 | |
| TATA box | −100 to −84 | |
| CYP19A2 | GATA-3 | −1213 to −1201 |
| AR | −1063 to −1045 | |
| CAR/RXR | −773 to −763 | |
| AR | −705 to −688 | |
| NF-kappaB | −522 to −510 | |
| ERE/ER | −498 to −480 | |
| AHR/ARNT | −419 to −413 | |
| PR | −324 to −306 | |
| SF1/Ad4BP | −182 to −177 | |
| TATA box | −176 to −160 | |
Locations numbered back from atg (+1) start site. GenBank Accession nos. EU045309 and EU045310, respectively. PCR primer locations, putative response elements and transcription initiation sites are depicted in supplemental figure.
DISCUSSION
BaP decreased CYP19A1 expression in the ovary of immature but not adult Fundulus
We have previously investigated the effects of BaP on both Fundulus CYP19A1 and CYP19A2 mRNA expression using quantitative RT-PCR. Because whole brains or gonads were homogenized and there was a lot of interindividual variability, we were unable to measure a significant treatment-related effect on mRNA expression (Patel et al., 2006). Similarly, when zebrafish were exposed to up to 3 µg/L BaP for 56 days there was no detectable effect on CYP19A1 expression in mid-vitellogenic oocytes by PCR (Hoffman & Oris, 2006). To better understand the tissue and cellular expression of CYP19A1 mRNA, we have more recently developed in situ hybridization probes and have described constitutive expression of this gene in Fundulus during development (Dong & Willett, 2008). CYP19A1 expression was not detectable by in situ hybridization until fish were 3 months old when it is expressed strongly and evenly throughout the ooplasm of early stage I oocytes. BaP exposure did significantly decrease this expression by about 50% in immature stage I oocytes, but had no effect on adult oocyte expression where CYP19A1 is expressed in the follicular membrane and vitelline envelop in more mature oocytes. Follicular growth in Fundulus ovaries is asynchronous (Selman & Wallace, 1983), and during the reproductive season there are oocytes present at each stage of oocyte maturation. While we saw no effect on CYP19A1 message expression in adult oocytes, we have reported a ≥ 2.5-fold decrease in ovarian aromatase activity following a similar 15 day 10 µg/L waterborne BaP exposure (Patel et al., 2006).
What is the potential significance of the decreased CYP19A1 expression in immature oocytes in juvenile Fundulus? One could hypothesize that decreased circulating estrogen concentrations would increase the time it takes for fish to reach reproductive maturity. However, this would be difficult to test experimentally in Fundulus especially if continuous exposure to BaP was necessary to sustain the inhibition of CYP19A1 expression. Furthermore, research to date on this life-stage of Fundulus (i.e. non-adult) and associated normal steroid levels or reproductive development is very limited.
In contrast, adult Fundulus oogenesis and steroidogenesis have been elegantly described (Wallace & Selman, 1978; Wallace & Selman, 1980; Selman & Wallace, 1983; Petrino et al., 1989b; Petrino et al., 1989a; Petrino et al., 1990; Hsiao et al., 1996; Cerda et al., 1998; Cochran et al., 1988; Bradford & Taylor, 1987). Petrino and coworkers established that Fundulus prematurational follicles contain the enzymes (cholesterol side chain cleavage, Δ5-3β-hydroxysteroid dehydrogenase and aromatase) necessary for conversion of cholesterol to 17α-hydroxy,20β-dihydroprogesterone, testosterone and estrogen (Petrino et al., 1989b). Furthermore, follicle cells attached to the vitelline envelope but not thecal/surface epithelial cells were capable of producing all three steroids when stimulated with either Fundulus pituitary extracts, 25-hydroxycholesterol or pregnenolone (Petrino et al., 1989a).
Our CYP19A1 in situ hybridization results support this earlier work with respect to localization of expression, however, BaP exposure did not reduce CYP19A1 expression in any stage of adult oocyte development. This result presents at least two questions: 1) Why are 3 month old stage I oocytes more sensitive to inhibition than adult stage I oocytes, and 2) Why is aromatase enzyme activity significantly inhibited by 10 µg/L BaP (Patel et al., 2006) but CYP19A1 message is not altered even with exposures up to 100 µg/L? Answers to these questions are speculation at this point and surely deserve more investigation, and many direct or indirect hormonal or metabolic mechanisms may be involved. For example, the age related differences could possibly be related to discrepancies in absorption, distribution, metabolism or excretion of BaP between reproductively immature and adult fish. On one hand, younger Fundulus may not metabolize and excrete BaP as quickly allowing for higher concentrations to accumulate in the immature oocytes, in turn, allowing BaP to have a direct effect on CYP19A expression. However, the opposite result could be predicted based on Monteverdi and Di Giulio’s work wherein previtellogenic oocytes (less than 0.5 mm) accumulated low to non-detectable amounts of 14C-BaP following maternal exposure. In contrast, mid vitellogenic oocytes (1.1–1.4 mm) had the highest accumulation of BaP (Monteverdi & Di Giulio, 2000) suggesting more mature adult eggs would have higher BaP concentrations associated with vitellogenin uptake.
Similarly, a number of hypotheses could explain the differences in message and enzyme inhibition. Inhibition at the message level may be mediated through promoter response elements in the CYP19A1 gene. The Fundulus CYP19A1 promoter is similar to zebrafish (Tong & Chung, 2003; Kazeto et al., 2001) and goldfish (Tchoudakova et al., 2001) wherein there are putative AhR, SF1 and CRE binding sites in the 5’-flanking region. Normal expression of CYP19A1 is believed to be transcriptionally modulated through gonadotropin induced increases in cAMP (Petrino et al., 1990) which in turn stimulates the CYP19 CRE (reviewed in (Cheshenko et al., 2008). The Ad4BP/SF-1 site has also been suggested as being involved in transcriptional regulation of CYP19A1 in Nile tilapia ovarian follicles (Yoshiura et al., 2003). However, in vitro studies of zebrafish CYP19A1 SF1 and ERE putative sites have suggested that these elements are not responsible for estrogen-mediated effects on CYP19A1 expression (Cheshenko et al., 2007). In fact, a number of studies have shown that CYP19A1, in contrast to CYP19A2, expression is not inducible by estrogens (Kishida & Callard, 2002; Kazeto et al., 2004; Sawyer et al., 2006). With respect to the inhibition of CYP19A1 expression by BaP, a hypothesis has been suggested (Patel et al., 2006; Hoffman & Oris, 2006), based on mammalian literature (Dasmahapatra et al., 2001), that binding of BaP to the AhR on CYP19A1 may interfere with normal transactivation and expression of the gene, but this remains to be tested.
In Fundulus CYP19A1 mRNA expression was not detectably decreased in adult oocytes by in situ hybridization following 15 day BaP exposures up to 100 µg/L, but aromatase activity was decreased in adult fish exposed similarly to only 10 µg/L BaP (Patel et al., 2006). Perhaps a compensatory mechanism is initiated during the 15 day continuous exposure in adults wherein oocytes can counteract aromatase inhibition so that the message levels did not appear to be decreased but the protein activity is still decreased. We have not been able to identify an effective antibody for Fundulus CYP19 to test whether actual protein concentrations are inhibited by BaP. Therefore, BaP also may be acting as an aromatase activity inhibitor as suggested by in vitro experiments with flounder ovarian tissue where 15 µM BaP inhibited steroidogenesis and estradiol production (Monteiro et al., 2000). However BaP at 10 µM did not decrease rainbow trout ovarian microsomal aromatase activity in vitro (Hinfray et al., 2006).
BaP decreased CYP19A2 expression in embryo and adult Fundulus brains
In contrast to CYP19A1, CYP19A2 was detectable in Fundulus embryo brains as early as 3 dpf. In this study we measured the effects of a 10 day exposure of BaP on CYP19A2 embryo expression at 14 dpf and found significant decreases in brain expression particularly in the hypothalamus and ventral telencephalon. The long-term developmental significance of decreased embryonic CYP19A2 expression is currently incompletely understood in teleosts and deserves more research. When protogynic fish like zebrafish were exposed to tributyltin, an aromatase inhibitor, from 0 to 70 days posthatch, a male biased population with a high incidence of fish with sperm lacking flagella resulted (McAllister & Kime, 2003). Similarly, dietary fadrozole exposure between 35 and 71 dpf can cause masculization of zebrafish (Fenske & Segner, 2004). However, these studies both represent larval exposures in contrast to the embryo exposures reported here in Fundulus.
CYP19A2 was also expressed in adrenal-like cells of the head kidney in embryos but interestingly this expression was not decreased by BaP exposure (data not shown, note kidney cells are not in same focal plane as brains in Figure 1). To date, the significance of CYP19A2 expression in the kidney is unknown, but adrenocortical tissue does possess enzymes involved in steroid synthesis (Bara, 1968; Vermeulen et al., 1995; Gonzáles & Piferrer, 2003).
Three previous studies have investigated BaP’s effects on CYP19A2 expression using qPCR. When zebrafish juveniles were exposed to 10 µM (2520 µg/L) BaP for 3 days and RNA was isolated from whole fish, CYP19A2 expression was significantly induced (Kazeto et al., 2004). CYP19A2 expression was also induced in zebrafish heads by a 56 day exposure to 3.35 µg/L (Hoffman & Oris, 2006). Work in our laboratory with Fundulus found no significant effect of BaP on whole brain CYP19A2 expression due to high interfish variability, but an increase in aromatase activity following winter but not summer BaP exposures was detected (Patel et al., 2006). Using in situ hybridization, BaP-mediated inhibition of CYP19A2 was statistically significant in the pituitary and hypothalamus regions. Discrepancies in BaP’s effects among all of these studies could be related to species, tissues from which RNA was isolated (whole fish vs. heads vs. brain), exposure (both concentrations and durations), and detection method sensitivities (PCR vs. in situ). In wild Fundulus collected from New Bedford Harbor, a superfund site, CYP19A2 mRNA and male vitellogenin expression was induced compared to fish collected from a cleaner site (Greytak et al., 2005). However, the New Bedford Harbor is most highly contaminated with PCBs and other estrogenic contaminants (i.e. not PAHs), hence induction was consistent with the established induction of CYP19A2 by estrogenic compounds. The idea that certain BaP metabolites may be slightly estrogenic has been suggested as a reason for BaP’s induction of CYP19A2 in previous studies (Kazeto et al., 2004; Hoffman & Oris, 2006; Patel et al., 2006). However, in this study CYP19A2 expression was decreased.
When considering the response elements in the Fundulus CYP19A2 promoters that might be contributing to transcriptional expression, we found putative ER, AR, PR, AhR and CAR/RXR binding sites. The ER binding site has been consistently reported in other fish CYP19A2 promoters (Tchoudakova et al., 2001; Kazeto et al., 2001) but the presence of a possible AhR has only been reported in zebrafish to date (Tong & Chung, 2003). However, transient transfection assays and DNA binding assays using the zebrafish CYP19A2 promoter suggested that while TCDD downregulated estrogen-induced expression, the dioxin response element sites were not functional (Cheshenko et al., 2008). Similar studies are ongoing in our laboratory with truncated Fundulus CYP19 promoters to attempt to further understand the transcriptional regulation of BaP on CYP19A2.
Both CYP19A2 expression and brain aromatase activity are much higher than gonadal expression in fish. The physiological significance of this high expression is not completely resolved but has been implicated in control of reproduction, sexual behaviors, and estrogen neurogenesis (recently reviewed in (Cheshenko et al., 2008)). BaP significantly decreased adult expression of CYP19A2 in both the pituitary and the hypothalamus but had no effect on expression in the ventral telencephalon or olfactory bulb. The inhibition was not sex dependent. As mentioned above, complicated interactive feedback loops control steroid synthesis in vertebrates and many compensatory systems may help counteract a stressors effect on one aspect of the system. While BaP significantly decreased CYP19A2 expression in two regions, whole brain aromatase activity was not decreased (Patel et al., 2006), and GSI was not affected in either males or females. The MacLatchy laboratory has recently optimized a short-term Fundulus reproduction assay (Peters et al., 2007) so in the future, we may be able to also test whether reproductive parameters are also adversely impacted by BaP exposure.
Fadrozole inhibited aromatase activity
An in vivo exposure to up to 100 µg/L fadrozole, a well established aromatase inhibitor, did not measurably decrease either CYP19A1 or CYP19A2 expression in these experiments. However, following exposure, whole brain or ovarian aromatase enzyme activity was inhibited as expected. Furthermore, fadrozole significantly increased male GSI but did not affect female GSI. Effects of fadrozole on GSI have also been reported in fathead minnows exposed to 100 µg/L for 21 days. Similar to our studies GSI increased in males, but a decrease in GSI was reported in females (Panter et al., 2004). In another fathead minnow study where doses ranged from 2 to 50 mg/L for 21 days, fadrozole decreased plasma estrogen and vitellogenin but had no effect on female GSI while it increased testosterone, 11-ketotestosterone and GSI in males (Ankley et al., 2002). At the messenger RNA level, the effects of fadrozole as measured by PCR are more complicated. Dietary exposure of zebrafish to fadrozole during gonadal differentiation resulted in 100% masculinization, decreased CYP19A1, but increased CYP19A2 expression (Fenske & Segner, 2004). Villeneuve and colleagues found when fathead minnows were exposed to fadrozole both brain activity and CYP19A2 expression were decreased, however CYP19A1 expression was increased and ovarian activity showed an inverted U-shaped dose response (Villeneuve et al., 2006). In a subsequent study the CYP19A2 inhibition was replicated, but the CYP19A1 expression also showed a U-shaped response (Villeneuve et al., 2007). These studies suggest, as concluded by Villeneuve and coworkers, that there are not particularly robust relationships between brain or ovary aromatase activity and isoforms specific mRNA expression (Villeneuve et al., 2006). Our work with BaP supports this conclusion and further suggests complex relationships between message expression and enzyme activity.
In conclusion, we found that 10 or 15 day exposures to BaP did decrease both CYP19A1 (in immature oocytes) and CYP19A2 (in embryo brains and adult hypothalamus and pituitary regions) mRNA expression in Fundulus. Importantly, these differences have not been detected previously using whole tissue homogenates. Furthermore, BaP and fadrozole worked through different mechanisms to alter aromatase. Additional studies of both promoter regulation and reproductive consequences of BaP exposure will hopefully add further insight into the physiological significance of the CYP19 inhibition that we have detected in embryonic, juvenile and adult Fundulus.
Supplementary Material
PCR primer locations, putative response elements and transcription initiation sites are indicated. GenBank Accession nos. EU045309 and EU045310, respectively.
Acknowledgements
The project described was supported by grant number R01 ES012710 from the National Institute of Environmental Health Sciences (NIEHS), NIH and the contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH. Parental killifish were collected and provided by Dr. Patricia McClellan-Green, Duke University. We appreciate the help of Dr. Asok Dasmahapatra for training with the aromatase assay and Novartis for providing the fadrozole used in these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonso LOB, Campbell PM, Iwama GK, Devlin RH, Donaldson EM. The effect of the aromatase inhibitor fadrozole and two polynuclear aromatic hydrocarbons on sex steroid secretion by ovarian follicles of coho salmon. Gen. Comp. Endocrinol. 1997;106:169–174. doi: 10.1006/gcen.1996.6855. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Kahl MD, Jensen KM, Hornung MW, Korte JJ, Makynen EA, Leino RL. Evaluation of the aromatase inhibitor fadrozole in a short-term reproduction assay with the fathead minnow (Pimephales promelas) Toxicol. Sci. 2002;67:121–130. doi: 10.1093/toxsci/67.1.121. [DOI] [PubMed] [Google Scholar]
- Armstrong PB, Child JS. Stages in the normal development of Fundulus heteroclitus. Biol. Bull. 1965;128:143–169. [Google Scholar]
- ATSDR. Toxicological profile for polycyclic aromatic hydrocarbons (PAHs) Atlanta, Georgia: Agency for Toxic Substances and Disease Registry; 1995. p. 1-458. Ref Type: Report. [PubMed] [Google Scholar]
- Bara G. Histochemical study of 3-beta-, 3-alpha-, 11-beta-, and 17-betahydroxysteroid dehydrogenases in the adrenocortical tissue and the corpuscles of Stannius of Fundulus heteroclitus. Gen. Comp Endocrinol. 1968;10:126–137. doi: 10.1016/0016-6480(68)90018-x. [DOI] [PubMed] [Google Scholar]
- Bradford CS, Taylor MH. Semilunar changes in estradiol and cortisol coincident with gonadal maturation and spawning in the killifish Fundulus heteroclitus. Gen. Comp. Endocrinol. 1987;66:71–78. doi: 10.1016/0016-6480(87)90351-0. [DOI] [PubMed] [Google Scholar]
- Burnett KG, Bain LJ, Baldwin WS, Callard GV, Cohen S, Di Giulio RT, Evans DH, Gomez-Chiarri M, Hahn ME, Hoover CA, Karchner SI, Katoh F, MacLatchy DL, Marshall WS, Meyer JN, Nacci DE, Oleksiak MF, Rees BB, Singer TD, Stegeman JJ, Towle DW, Van Veld PA, Vogelbein WK, Whitehead A, Winn RN, Crawford DL. Fundulus as the premier teleost model in environmental biology: Opportunities for new insights using genomics. Comp Biochem. Physiol Part D. Genomics Proteomics. 2007;2:257–286. doi: 10.1016/j.cbd.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda J, Subhedar N, Reich G, Wallace RA, Selman K. Oocyte sensitivity to serotonergic regulation during the follicular cycle of the teleost Fundulus heteroclitus. Biol. Reproduct. 1998;59:53–61. doi: 10.1095/biolreprod59.1.53. [DOI] [PubMed] [Google Scholar]
- Cheshenko K, Brion F, Le PY, Hinfray N, Pakdel F, Kah O, Segner H, Eggen RI. Expression of zebra fish aromatase cyp19a and cyp19b genes in response to the ligands of estrogen receptor and aryl hydrocarbon receptor. Toxicol. Sci. 2007;96:255–267. doi: 10.1093/toxsci/kfm003. [DOI] [PubMed] [Google Scholar]
- Cheshenko K, Pakdel F, Segner H, Kah O, Eggen RI. Interference of endocrine disrupting chemicals with aromatase CYP19 expression or activity, and consequences for reproduction of teleost fish. Gen. Comp Endocrinol. 2008;155:31–62. doi: 10.1016/j.ygcen.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Cochran RC, Zabludoff SD, Paynter KT, DiMichele L, Palmer RE. Serum hormone levels associated with spawning activity in the mummichog, Fundulus heteroclitus. Gen. Comp. Endocrinol. 1988;70:345–354. doi: 10.1016/0016-6480(88)90154-2. [DOI] [PubMed] [Google Scholar]
- Contractor RG, Foran CM, Li S, Willett KL. Evidence of gender- and tissue-specific promoter methylation and the potential for ethinylestradiol-induced changes in japanese medaka (Oryzias latipes) estrogen receptor and aromatase genes. J. Toxicol. Environ. Health. 2004;67:1–22. doi: 10.1080/15287390490253633. [DOI] [PubMed] [Google Scholar]
- Cooke GM. Effect of organotins on human aromatase activity in vitro. Toxicol. Lett. 2002;126:121–130. doi: 10.1016/s0378-4274(01)00451-9. [DOI] [PubMed] [Google Scholar]
- Dasmahapatra AK, Wimpee BAB, Trewin AL, Hutz RK. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases steady-state estrogen receptor-B mRNA levels after CYP1A1 and CYP1B1 induction in rat granulosa cells in vitro. Mol. Cell. Endocrin. 2001;182:39–48. doi: 10.1016/s0303-7207(01)00545-7. [DOI] [PubMed] [Google Scholar]
- Dong W, Willett KL. Local expression of CYP19A1 and CYP19A2 in developing and adult killifish (Fundulus heteroclitus) Gen. Comp Endocrinol. 2008;155:307–317. doi: 10.1016/j.ygcen.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube MG, MacLatchy DL. Identification and treatment of a waste stream at a bleached-kraft pulp mill that depresses a sex steroid in the mummichog (Fundulus heteroclitus) Environ. Toxicol. Chem. 2001;20:985–995. doi: 10.1897/1551-5028(2001)020<0985:iatoaw>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Fenske M, Segner H. Aromatase modulation alters gonadal differentiation in developing zebrafish (Danio rerio) Aquat. Toxicol. 2004;67:105–126. doi: 10.1016/j.aquatox.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Gonzáles A, Piferrer F. Aromatase activity in the European sea bass (Dicentrarchus labraz L.) brain. Distribution and changes in relation to age, sex, and the annual reproductive cycle. Gen. Comp. Endocrinol. 2003;132:223–230. doi: 10.1016/s0016-6480(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Greytak SR, Callard GV. Cloning of three estrogen receptors (ER) from killifish (Fundulus heteroclitus): Differences in populations from polluted and reference environments. Gen. Comp Endocrinol. 2007;150:174–188. doi: 10.1016/j.ygcen.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Greytak SR, Champlin D, Callard GV. Isolation and characterization of two cytochrome P450 aromatase forms in killifish (Fundulus heteroclitus): differential expression in fish from polluted and unpolluted environments. Aquatic. Toxicol. 2005;71:371–389. doi: 10.1016/j.aquatox.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Hallgren SL, Linderoth M, Olsen KH. Inhibition of cytochrome p450 brain aromatase reduces two male specific sexual behaviours in the male Endler guppy (Poecilia reticulata) Gen. Comp Endocrinol. 2006;147:323–328. doi: 10.1016/j.ygcen.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Hinfray N, Porcher JM, Brion F. Inhibition of rainbow trout (Oncorhynchus mykiss) P450 aromatase activities in brain and ovarian microsomes by various environmental substances. Comp Biochem. Physiol C. Toxicol. Pharmacol. 2006;144:252–262. doi: 10.1016/j.cbpc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Hoffman JL, Oris JT. Altered gene expression: A mechanism for reproductive toxicity in zebrafish exposed to benzo[a]pyrene. Aquat. Toxicol. 2006;78:332–340. doi: 10.1016/j.aquatox.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Hsiao S-M, Limesand SW, Wallace RA. Semilunar follicular cycle of an intertidal fish: the Fundulus model. Biol. Reproduct. 1996;54:809–818. doi: 10.1095/biolreprod54.4.809. [DOI] [PubMed] [Google Scholar]
- Kazeto Y, Ijiri S, Place AR, Zohar Y, Trant JM. The 5'-flanking regions of CYP19A1 and CYP19A2 in zebrafish. Biochem. Biophys. Res. Commun. 2001;288:503–508. doi: 10.1006/bbrc.2001.5796. [DOI] [PubMed] [Google Scholar]
- Kazeto Y, Place AR, Trant JM. Effects of endocrine disrupting chemicals on the expression of CYP19 genes in zebrafish (Danio rerio) juveniles. Aquat. Toxicol. 2004;69:25–34. doi: 10.1016/j.aquatox.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Kishida M, Callard GV. Distinct cytochrome P450 aromatase isoforms in zebrafish (Danio rerio) brain and ovary are differentially programmed and estrogen regulated during early development. Endocrinology. 2002;142:740–750. doi: 10.1210/endo.142.2.7928. [DOI] [PubMed] [Google Scholar]
- Lee PS, Pankhurst NW, King HR. Effects of aromatase inhibitors on in vitro steroidogenesis by Atlantic salmon (Salmo salar) gonadal and brain tissue. Comp Biochem. Physiol A Mol. Integr. Physiol. 2006;145:195–203. doi: 10.1016/j.cbpa.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Simpson ER. Assay of Aromatase Activity. Methods in Enzyme. 2002;206:477–483. doi: 10.1016/0076-6879(91)06116-k. [DOI] [PubMed] [Google Scholar]
- Lotrich VA. Summer home range and movements of Fundulus heteroclitus (Pisces: Cyprinodontidae) in a tidal creek. Ecology. 1975;56:191–198. [Google Scholar]
- McAllister BG, Kime DE. Early life exposure to environmental levels of the aromatase inhibitor tributyltin causes masculinisation and irreversible sperm damage in zebrafish (Danio rerio) Aquat. Toxicol. 2003;65:309–316. doi: 10.1016/s0166-445x(03)00154-1. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Wassenberg DM, Karchner SI, Hahn ME, Di Giulio RT. Expression and inducibility of aryl hydrocarbon receptor pathway genes in wild-caught killifish (Fundulus heteroclitus) with different contaminant-exposeure histories. Environ. Tox. Chem. 2003;22:2337–2343. doi: 10.1897/02-495. [DOI] [PubMed] [Google Scholar]
- Monteiro PR, Reis-Henriques MA, Coimbra J. Polycyclic aromatic hydrocarbons inhibit in vitro ovarian steroidogenesis in the flounder (Platichthys flesus L.) Aquat. Toxicol. 2000;48:549–559. doi: 10.1016/s0166-445x(99)00055-7. [DOI] [PubMed] [Google Scholar]
- Monteverdi GH, Di Giulio RT. Oocytic accumulation and tissue distribution of 2,3,7,8-tetrachlorodibenzo-p-dioxin and benzo(a)pyrene in gravid Fundulus heteroclitus. Environ, Toxicol. Chem. 2000;19:2512–2518. [Google Scholar]
- Nicolas J-M. Vitellogenesis in fish and the effects of polycyclic aromatic hydrocarbon contaminants. Aquat. Toxicol. 1999;45:77–90. [Google Scholar]
- Panter GH, Hutchinson TH, Hurd KS, Sherren A, Stanley RD, Tyler CR. Successful detection of (anti-)androgenic and aromatase inhibitors in pre-spawing adult fathead minnows (Pimephales promelas) using easily measured endpoints of sexual development. Aquat. Toxicol. 2004;70:11–21. doi: 10.1016/j.aquatox.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Patel MR, Scheffler BE, Wang L, Willett KL. Effects of benzo(a)pyrene exposure on killifish (Fundulus heteroclitus) aromatase activities and mRNA. Aquat. Toxicol. 2006;77:267–278. doi: 10.1016/j.aquatox.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters RE, Courtenay SC, Cagampan S, Hewitt ML, MacLatchy DL. Effects on reproductive potential and endocrine status in the mummichog (Fundulus heteroclitus) after exposure to 17alpha-ethynylestradiol in a short-term reproductive bioassay. Aquat. Toxicol. 2007;85:154–166. doi: 10.1016/j.aquatox.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Petrino TR, Greeley MS, Selman K, Lin Y-W, Wallace RA. Steroidogenesis in Fundulus heteroclitus II. Production of 17α-hydroxy-20β-dihydroprogesterone, testosterone, and 17β-estradiol by various components of the ovarian follicle. Gen. Comp. Endocrinol. 1989a;76:230–240. doi: 10.1016/0016-6480(89)90154-8. [DOI] [PubMed] [Google Scholar]
- Petrino TR, Hoch KL, Lin Y-W, Wallace RA. Steroidogenesis in Fundulus heteroclitus: III. Evidence for involvement of cAMP and protein synthesis in the gonadotropic modulation of ovarian steroid production and aromatase activity. J. Exper. Zool. 1990;253:177–185. [Google Scholar]
- Petrino TR, Lin Y-W, Wallace RA. Steroidogenesis in Fundulus heteroclitus I. Production of 17α-hydroxy, 20β-dihydroprogesterone, testosterone, and 17β-estradiol by prematurational follicles in vitro. Gen. Comp. Endocrinol. 1989b;73:147–156. doi: 10.1016/0016-6480(89)90065-8. [DOI] [PubMed] [Google Scholar]
- Piskorska-Pliszczynska J, Keys B, Safe S, Newman MS. The cytosolic receptor binding affinities and AHH induction potencies of 29 polynuclear aromatic hydrocarbons. Toxicol. Lett. 1986;34:67–74. doi: 10.1016/0378-4274(86)90146-3. [DOI] [PubMed] [Google Scholar]
- Powell WH, Bright R, Bello SM, Hahn ME. Developmental and tissue-specific expression of AHR1, AHR2, and ARNT2 in dioxin-sensitive and -resistant populations of the marine fish, Fundulus heteroclitus. Toxicol. Sci. 2000;57:229–239. doi: 10.1093/toxsci/57.2.229. [DOI] [PubMed] [Google Scholar]
- Prince R, Cooper K. Comparisons of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on chemically impacted and nonimpacted subpopulations of Fundulus heteroclitus: I. TCDD toxicity. Environ. Toxicol. Chem. 1995;14:579–587. [Google Scholar]
- Roling JA, Bain LJ, Gardea-Torresdey J, Key PB, Baldwin WS. Using mummichog (Fundulus heteroclitus) arrays to monitor the effectiveness of remediation at a superfund site in Charleston, South Carolina, USA. Environ. Tox. Chem. 2007;26:1205–1213. doi: 10.1897/06-421r.1. [DOI] [PubMed] [Google Scholar]
- Sanderson JT. The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol. Sci. 2006;94:3–21. doi: 10.1093/toxsci/kfl051. [DOI] [PubMed] [Google Scholar]
- Sawyer SJ, Gerstner KA, Callard GV. Real-time PCR analysis of cytochrome P450 aromatase expression in zebrafish: gene specific tissue distribution, sex differences, developmental programming, and estrogen regulation. Gen. Comp Endocrinol. 2006;147:108–117. doi: 10.1016/j.ygcen.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Selman K, Wallace RA. Oogenesis in Fundulus heteroclitus. III. Vitellogenesis. J. Exp. Zool. 1983;226:441–457. doi: 10.1002/jez.1402260315. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. Aromatase - A brief overview. Annu. Rev. Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- Stine CB, Smith DL, Vogelbein WK, Harshbarger JC, Gudla PR, Lipsky MM, Kane AS. Morphometry of hepatic neoplasms and altered foci in the mummichog, Fundulus heteroclitus. Toxicol. Pathol. 2004;32:375–383. doi: 10.1080/01926230490440899. [DOI] [PubMed] [Google Scholar]
- Taylor MH. A suite of adaptations for intertidal spawning. Am. Zool. 1999;39:313–320. [Google Scholar]
- Tchoudakova AV, Kishida M, Wood E, Callard GV. Promoter characteristics of two cyp19 genes differentially expressed in the brain and ovary of teleost fish. J. Steroid biochem. Molec. Biol. 2001;78:427–439. doi: 10.1016/s0960-0760(01)00120-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. Teleost model for studying the effects of chemicals on female reproductive endocrine function. J. Exper. Zool. Suppl. 1990;4:126–128. doi: 10.1002/jez.1402560421. [DOI] [PubMed] [Google Scholar]
- Tong S-K, Chung B-C. Analysis of zebrafish cyp19 promoters. J. Steroid Biochem. Mol. Biol. 2003;86:381–386. doi: 10.1016/s0960-0760(03)00347-9. [DOI] [PubMed] [Google Scholar]
- Uchida D, Yamashita M, Kitano T, Iguchi T. An aromatase inhibitor or high water temperature induce oocyte apoptosis and depletion of P450 aromatase activity in the gonads of genetic female zebrafish during sex-reversal. Comp. Biochem. Physiol. 2004;137A:11–20. doi: 10.1016/s1095-6433(03)00178-8. [DOI] [PubMed] [Google Scholar]
- van Metre PC, Mahler BJ. Trends in hydrophobic organic contaminants in urban and reference lake sediments across the United States, 1970–2001. Environ. Sci. Technol. 2005;39:5567–5574. doi: 10.1021/es0503175. [DOI] [PubMed] [Google Scholar]
- Vermeulen GJ, Lambert JGD, Teitsma CA, Zandbergen MA, Goos HJT. Adrenal tissue in the male African catfish, Clarias gariepinus: localization and steroid hormone secretion. Cell Tissue Res. 1995;280:653–657. [Google Scholar]
- Villeneuve DL, Knoebl I, Kahl MD, Jensen KM, Hammermeister DE, Greene KJ, Blake LS, Ankley GT. Relationship between brain and ovary aromatase activity and isoform-specific aromatase mRNA expression in the fathead minnow (Pimephales promelas) Aquat. Toxicol. 2006;76:353–368. doi: 10.1016/j.aquatox.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Larkin P, Knoebl I, Miracle AL, Kahl MD, Jensen KM, Makynen EA, Durhan EJ, Carter BJ, Denslow ND, Ankley GT. A graphical systems model to facilitate hypothesis-driven ecotoxicogenomics research on the teleost brain-pituitary-gonadal axis. Environ. Sci. Technol. 2007;41:321–330. doi: 10.1021/es061739x. [DOI] [PubMed] [Google Scholar]
- Wallace RA, Selman K. Oogenesis in Fundulus heteroclitus. II. The transition from vitellogenesis into maturation. Gen. Comp Endocrinol. 1980;42:345–354. doi: 10.1016/0016-6480(80)90165-3. [DOI] [PubMed] [Google Scholar]
- Wallace RA, Selman K. Oogenesis in Fundulus heteroclitus. I. Preliminary observations on oocyte maturation in vivo and in vitro. Dev. Biol. 1978;62:354–369. doi: 10.1016/0012-1606(78)90222-1. [DOI] [PubMed] [Google Scholar]
- Wang L, Scheffler BE, Willett KL. CYP1C1 messenger RNA expression is inducible by benzo[a]pyrene in Fundulus heteroclitus embryos and adults. Toxicol. Sci. 2006;93:331–340. doi: 10.1093/toxsci/kfl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ. Health Perspect. 2004;112:1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett K, Steinberg MA, Thomsen J, Narasimhan TK, Safe SH, McDonald SJ, Beatty KB, Kennicutt MC. Exposure of killifish to benzo(a)pyrene: Comparative metabolism, DNA adduct formation and aryl hydrocarbon (Ah) receptor agonist activities. Comp. Biochem. Physiol. 1995;112B:93–103. [Google Scholar]
- Yoshiura Y, Senthilkumaran B, Watanabe M, Oba Y, Kobayashi T, Nagahama Y. Synergistic expression of Ad4BP/SF-1 and cytochrome P-450 aromatase (ovarian type) in the ovary of Nile tilapia, Oreochromis niloticus, during vitellogenesis suggests transcriptional interaction. Biol. Reprod. 2003;68:1545–1553. doi: 10.1095/biolreprod.102.010843. [DOI] [PubMed] [Google Scholar]
- Zarn JA, Bruschweiler BJ, Schlatter JR. Azole fungicides affect mammalian steroidogenesis by inhibiting sterol 14 alpha-demethylase and aromatase. Environ. Health Perspect. 2003;111:255–261. doi: 10.1289/ehp.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, John-Alder HB, Weis JS, Weis P. Endocrine disruption: Thyroid dysfunction in mummichogs (Fundulus heteroclitus) from a polluted habitat. Mar. Environ. Res. 2000;50:393–397. doi: 10.1016/s0141-1136(00)00042-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR primer locations, putative response elements and transcription initiation sites are indicated. GenBank Accession nos. EU045309 and EU045310, respectively.


