Abstract
(23S,25S)-N-Benzyl-1α,25-dihydroxyvitamin D3-26,23-lactam ((23S,25S)-N-benzyl- 1α,25-(OH)2D3-26,23-lactam, (23S,25S)-DLAM-1P) antagonizes nuclear vitamin D receptor (VDR)-mediated differentiation of human promyelocytic leukemia (HL-60) cells [Bioorg. Med. Chem. Lett. 14, 2579–2583(2004)]. To enhance its VDR antagonistic actions, we synthesized multiple analogues of 1α,25-(OH)2D3-26,23-lactam. Among these analogues, (23S,25S)-N-phenetyl-1α,25-(OH)2D3-26,23-lactam, ((23S,25S)- DLAM-2P) had the strongest VDR binding affinity, which was 3 times higher than that of (23S,25S)-DLAM-1P. The 1α,25-(OH)2D3-26,23-lactam analogues never induced HL-60 cell differentiation even at 10−6M, but (23S,25S)-DLAM-1P and (23S,25S)-DLAM-2P significantly and dose-dependently inhibited HL-60 differentiation induced by 10−8M 1α,25-dihydroxyvitamin D3 (1α,25-(OH)2D3). These compounds also inhibited human and mouse cultures of osteoclast formation by marrow cells treated with 1α,25-(OH)2D3. Moreover, the 1α,25-(OH)2D3-26,23-lactam analogues minimally induced 25-hydroxy- vitamin D3-24-hydroxylase gene expression in HL-60 cells and human and mouse osteoblastic cells, but 10−6M (23S,25S)-DLAM-1P or (23S,25S)-DLAM-2P significantly blocked 24-hydroxylase gene expression induced by 10−8M 1α,25-(OH)2D3. (23S,25S)- DLAM-2P was 5 to 12 times more potent as a Vitamin D antagonist than (23S,25S)-DLAM-1P in HL-60 cells, human and mouse bone marrow cultures. These results demonstrate that (23S,25S)-DLAM-1P and (23S,25S)-DLAM-2P antagonize HL-60 cell differentiation and osteoclast formation by human and mouse osteoclast precursors induced by 1α,25-(OH)2D3 through blocking VDR-mediated gene transcription. In contrast, (23S)-25-deoxy-1α-hydroxyvitamin D3-26,23-lactone, which only blocks human VDR, these vitamin D antagonists can block VDR in human cells and rodent cells.
Keywords: Vitamin D receptor antagonist; 1α; 25-dihydroxyvitamin D3-26,23-lactam analogues; HL-60 cell differentiation; osteoclast formation; gene expression
Introduction
To date, a large number of vitamin D3 analogues have been synthesized; however, almost all of these compounds are vitamin D agonists [1]. 1β,25-Dihydroxyvitamin D3 (1β,25-(OH)2D3) is a well known vitamin D antagonist, which acts through rapid nongenomic actions via a putative membrane vitamin D receptor (VDR). It does not act as a vitamin D antagonist for nuclear VDR-mediated gene transcription induced by 1α,25-dihydroxyvitamin D3 (1β,25-(OH)2D3) [2,3]. Currently, only three types of 1α,25-(OH)2D3 analogues, which showed nuclear VDR antagonistic actions, have been described. These are the (23S)- and (23R)-25-dehydro-1α-hydroxyvitamin D3-26,23- lactones (TEI-9647 and TEI-9648, respectively) [4–6], the 25-carboxylic ester derivatives of 1α,25-(OH)2D3 (ZK159222 and ZK168281) [7,8], and the (23S,25S)-N-benzyl- 1α,25-dihydroxyvitamin D3-26,23-lactam ((23S,25S)-N-benzyl-1α,25-(OH)2D3-26,23- lactam, (23S,25S)-DLAM-1P) [9].
Previously, we reported that a 1α,25-dihydroxyvitamin D3-26,23-lactone (1α,25-(OH)2D3-26,23-lactone) analogue, TEI-9647, inhibits monocyte differentiation of human promyelocytic leukemia (HL-60) cells, and blocks osteoclast (OCL) formation by human bone marrow cells treated with 1α,25-(OH)2D3 [4–6,10,11]. TEI-9647 shows significant vitamin D antagonistic activity in terms of 25-hydroxyvitamin D3-24- hydroxylase (25-OH-D3-24-hydroxylase) and p21waf1/cip1 gene expression induced by 1α,25-(OH)2D3 in HL-60 cells and human osteosarcoma cells (SaOS-2 and MG-63 cells) [4,6,12]. Moreover, we demonstrated that TEI-9647 prevents heterodimer complex formation between the VDR and retinoid X receptor (RXR), and recruitment of steroid receptor coactivator-1 (SRC-1) to VDR [13].
Very recently, both Carlberg and our group reported that TEI-9647 displays vitamin D antagonistic actions in human cells but has vitamin D agonistic actions in rodent cells [14,15]. The vitamin D antagonistic actions of TEI-9647 depend on the primary structure of the carboxyl-terminal region of the VDR. The C-terminal regions (the last 67 residues) of the ligand binding domain of human and rodent VDR are highly conserved but differ from each other at the L378, C403 and C410 of human and the corresponding residues of rat VDR. We demonstrated that interaction between the exo-methylene structure and the cysteine residues at 403 and/or 410 in human VDR is critical for the antagonistic activity of TEI-9647 [14,15]. Unlike TEI-9647, ZK159222 and ZK168281, which are well-known VDR antagonists, exhibit their antagonistic effects regardless of the species of VDR [7,8,14–16].
Kato et al. recently reported that another type of vitamin D antagonist, (23S,25S)-DLAM-1P, which inhibits HL-60 cell differentiation induced by 1α,25-(OH)2D3, but its vitamin D antagonistic activity seems to be extremely weak compared to TEI-9647 [9]. Therefore, we synthesized multiple analogues of the 1α,25-(OH)2D3-26,23-lactam in order to enhance its vitamin D antagonistic activity, and evaluated these analogues in human and rodent cells. In this paper, we demonstrate that the (23S,25S)- N-phenetyl-1α,25-(OH)2D3-26,23-lactam ((23S,25S)-DLAM-2P) was a highly potent vitamin D antagonist in human and rodent cells.
Materials and Methods
Chemicals
1α,25-(OH)2D3, (23S,25R)-1α,25-(OH)2D3-26,23-lactone, TEI-9647 and 1α,25-(OH)2D3-26,23-lactam analogues were synthesized in our laboratory as described previously [9, 17–20]; their structures are shown in Fig. 1. Each compound was dissolved in absolute ethanol. [26,27-methyl-3H]1α,25-(OH)2D3 (specific activity, 6.623TBq/mmol) was purchased from GE Healthcare Bio-Sciences Corp. (Piscataway, NJ). Fetal bovine serum (FBS) was purchased from GIBCO-BRL Life Technologies, Inc. (Grand Island, NY). Nitro blue tetrazolium (NBT) was bought from Tokyo Kasei Kogyo Co. Ltd. (Tokyo, Japan). 12-O-Tetradecanoylphorbol-13-acetate (TPA) was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Kernechrot solution was obtained from Muto Pure Chemicals Co. Ltd. (Tokyo, Japan). All other chemicals and media were purchased from Sigma Chemical Corporation (St. Louis, MO), unless otherwise noted.
Figure 1.
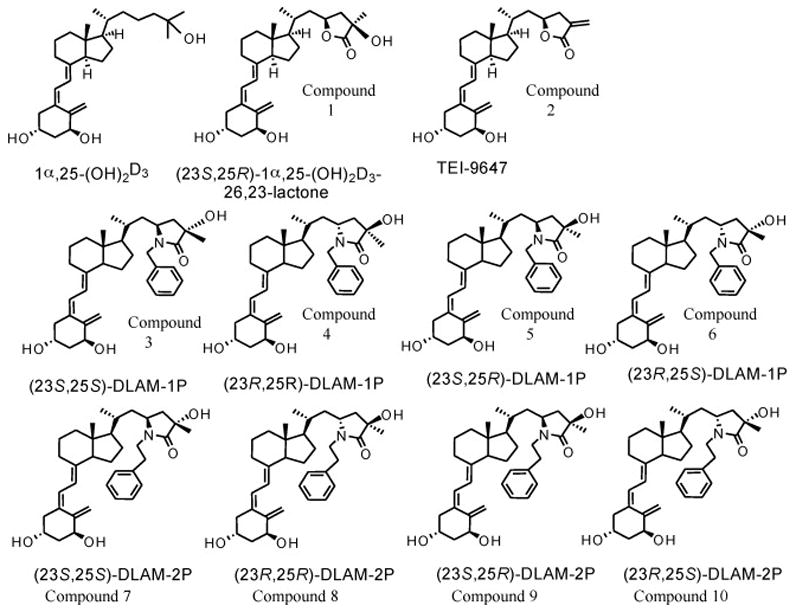
Structures of 1α,25-(OH)2D3-26,23-lactone analogues and 1α,25-(OH)2D3-26,23-lactam analogues. The (23S,25R)-1α,25-(OH)2D3-26,23-lactone is a naturally occurring metabolite derived from 1α,25-(OH)2D3.
Binding affinity for vitamin D receptor
Competitive receptor binding assays for 1α,25-(OH)2D3 and 1α,25-(OH)2D3-26,23-lactam analogues were performed using VDR from chick intestine as described previously [20]. In this assay, 0.1 mg protein/ml of chick VDR and 18,000 dpm of [26,27-methyl-3H]1α,25-(OH)2D3 (specific activity, 6.623TBq/mmol, 18.8pg) were used.
Cell and culture
HL-60 cells and a human osteoblastic cell line (HOS cells) were obtained from the Japanese Cancer Research Resources Bank. Cells were passaged twice a week to maintain them in an exponential proliferating phase. RPMI-1640 (GIBCO BRL, Life Technologies, Inc. Rockville, MO) containing 10% heat inactivated FBS (Bioserum, Lot No.: 01307-01) for the HL-60 cells, and Dulbecco’s modified Eagle’s medium (DMEM) (Nissui Pharmaceutical Co. Tokyo, Japan) supplemented with 10% dextran-coated charcoal-stripped FBS (JRH Bioscience, Dexton, KS), penicillin and streptomycin for the HOS cells, were used as culture media. Cell counts were determined with a hemocytometer, and the viability of cells was tested by trypan-blue dye exclusion.
Assay for HL-60 cell differentiation
NBT reducing activity was used as a marker of cell differentiation. HL-60 cells were cultured in RPMI-1640 medium supplemented with 10% heat inactivated FBS. Exponentially proliferating cells were collected, suspended in fresh media and seeded in culture vessels. 24-Well culture plates (Falcon, Becton Dickinson and Company, Franklin Lakes, NJ) were used. Cell concentration at seeding was adjusted to 2 × 104 cells/ml and seeding volume was 1 ml/well. 1α,25-(OH)2D3 and 1α,25-(OH)2D3-26,23-lactam analogues dissolved in ethanol were added to the culture media at 0.1% volume and the cells cultured for 96 hrs at 37°C in a humidified atmosphere of 5% CO2-air without media change. The same amount of vehicle was added to the control cultures. NBT reducing assays were performed as described previously [4] according to the method of Collins et al. [21].
Subjects and cell preparation
Bone marrow cells aspirated under 2% xylocaine anesthesia from the iliac crest of healthy normal volunteers into heparinized alpha-Minimal Essential Media (α-MEM) containing 5% FBS, as previously described [22]. Bone marrow mononuclear cells were then isolated by separation on Hypaque-Ficoll gradients (density 1.077g/ml), centrifuged at 400 g for 30 minutes and then washed three times with α-MEM, as described previously [22]. The Institutional Review Board of the University of Pittsburgh approved these studies.
Measles virus nucleocapsid protein (MVNP) gene transduction of human bone marrow cell
MVNP-transduced human marrow cells were used for these assays because they are hyper-responsive to 1α,25(OH)2D3 and form osteoclasts at concentrations of 1α,25(OH)2D3 that are 1–2 logs lower than normal cells. These cells were used as a more sensitive assay for the potential agonistic actions of the lactam analogues. Human bone marrow mononuclear cells were cultured for 2 days in α-MEM containing 10% FBS that contained 10ng/ml each of IL-3, IL-6 and stem cell factor (SCF) (Amgen Immunex Research and Development Corporation; Seattle, WA). The bone marrow cells were then cultured for an additional 48 hours at 37°C in a humidified atmosphere of 5% CO2-air at a density of 1 to 2 × 105 cells/ml with supernatant (10% v/v) containing the MVNP vector [23]. Cultures were supplemented with 4μg/ml of polybrene, 20ng/ml of IL-3, 50ng/ml of IL-6 and 100ng/ml of SCF as described previously [23]. MVNP-transduced cells were suspended at 106 cells/ml in α-MEM containing 1.2% methylcellulose, 30% FBS, 1% deionized bovine serum albumin (BSA) and 100pg/ml recombinant granulocyte-macrophage colony stimulating factor (GM-CSF) (Amgen Immunex Research and Development Corporation; Seattle, WA) with 250μg/ml of G418. Transduced cells were plated in a volume of 1 ml in 35-mm culture dishes (Corning; New York, NY) and incubated at 37°C in a humidified atmosphere of 5% CO2-air for 7 days, for isolation of G418 resistance colony-forming unit-granulocyte macrophage (CFU-GM) cells. The G418-resistant colonies were individually collected, using finally drawn pipettes, for use in OCL formation assays employing MVNP-transduced CFU-GM cells.
Osteoclast formation induced by 1α,25-(OH)2D3
OCL formation from MVNP- transduced CFU-GM cells induced by 1α,25-(OH)2D3 was performed as described previously [11,23]. Briefly, MVNP-transduced CFU-GM cells (106 cells/ml) were dispersed into α-MEM containing 20% horse serum, and were seeded in 96-well multi-plates (Becton Dickinson Labware, Frankin Lakes, NJ) at 100μl/well. 10−9M 1α,25-(OH)2D3, TEI-9647 (10−9M to 10−6M), 1α,25-(OH)2D3-26,23-lactam analogues (10−8M to 10−6M), alone or in combination with 1α,25-(OH)2D3 (10−9M) were added into a well. Half of the media was replaced two times a week, and the cultures were continued for 3 weeks at 37°C in an incubator of 5% CO2-air. The OCL that formed were then fixed with 2% formaldehyde and tested for cross-reactivity with the monoclonal antibody 23C6, which recognized the osteoclast vitronectin receptor (generously provided by Dr. Michael Horton, Rayne Institute, Bone and Mineral Center, London, United Kingdom), using a Vectastatin-ABC-AP kit (Vector Laboratories, Burlingame, CA), as described previously [22,23]. Cells that cross-reacted with the 23C6 antibody and had 3 or more nuclei were scored as OCL using an inverted microscope.
Osteoclast formation by mouse bone marrow cells treated with 1α,25-(OH)2D3
OCL formation by mouse bone marrow cells treated with 1α,25-(OH)2D3 was performed as described previously [24]. Briefly, six-week-old mice (ICR strain) were used for all experiments. The femora were disarticulated, soft tissues were dissected away, and then both ends of each bone were removed. The bone marrow cells were flushed out with a syringe containing α-MEM, and a single cell suspension was prepared by pipetting. One hundred microliter of unfractionated bone marrow cells (106 cells/ml) was plated in each well of 96-well multi-plates (Becton Dickinson Labware, Franklin Lakes, NJ) and cultured at 37°C in humidified 5% CO2-air incubator. The media consisted of αMEM containing 10% FBS that had been inactivated at 65°C for 30 min. After 1 day, the cells were transferred to media containing various concentrations of vitamin D analogues, and thereafter the cultures were fed every 3 days by replacing half the media. After 8 days of culture, the cells were fixed with 1% formaldehyde and stained with tartrate resistant acid phosphatase (TRAP) using a leukocyte acid phosphatase kit (Sigma Chemical Corporation, St. Louis, MO). The TRAP-positive multinucleated cells (> 3 nuclei/cell) were scored as OCL using an inverted microscope. The animal care and the experimental protocols were approved by the Animal Experimentation Ethics Committee of the University of Pittsburgh and the VA Pittsburgh Healthcare System.
25-Hydroxyvitamin D3-24-hydroxylase (25-OH-D3-24-hydroxylase) gene expression
To investigate the 25-OH-D3-24-hydroxylase gene expression induced by 1α,25-(OH)2D3 or 1α,25-(OH)2D3-26,23-lactam analogues, HL-60 cells (105 cells/ml medium in a 35mm-diameter dish, 3 ml) were incubated at 37°C in a RPMI-1640 media containing 10% heat inactivated FBS with 10−8M 1α,25-(OH)2D3, or 10−6M 1α,25-(OH)2D3- 26,23-lactam analogues alone, or in combination for 24 hrs. In the case of HOS cells and primary mouse osteoblastic cells, culture media were changed to DMEM and αMEM, respectively. After incubation, total RNA was extracted by the acid guanidium thiocyanate-phenol method (Isogen, Nippon Gene, Tokyo, Japan). cDNA was synthesized from 1μg of total RNA using random hexamers and reverse transcriptase (GeneAmp; RNA PCR Core kit, Applied Biosystems, Foster City, CA) according to their manual. For gene amplification of cDNAs for 25-OH-D3-24-hydroxylase and β-actin (RNA 50ng and 12.5ng, respectively), were added to 100 pmoles of each PCR primer and 2.5 units Taq polymerase (Applied Biosystems, Foster City, CA). Both samples (total 50μl reaction volume) were subjected to PCR amplification in a programmed thermal cycler. For human 25-OH-D3-24-hydroxylase amplification, the PCR primers were 5′ to 3′ GGGAAGTGATGAAGCTGGAC (898–917) and TCATAGATTCTTTCAGACAGG (1526–1546). For mouse 25-OH-D3-24-hydroxylase amplification, the PCR primer was 5′ to 3′ ATTACCTGAGAATCAGAGGCCACG (1049–1068) and GCCAAATGCAGTTT AAGCTCTGCT (1524–1543). PCR cycles were as follows; 60 sec at 94°C for denaturation, 60 sec at 60°C for annealing, 90 sec at 72°C for extension, 26 cycles. For β-actin gene amplification, the PCR primers were 5′ to 3′ ACCACAGTCCATGCCATCAC and TCCACCACCCTGTTGCTGTA. PCR cycles were as follows; 60 sec at 94°C for denaturation, 60 sec at 60°C for annealing, 90 sec at 72°C for extension, 25 cycles. PCR products were analyzed by 2% agarose gel electrophoresis (648 bp product and 495 bp product were obtained in human 25-OH-D3-24-hydroxylase gene and mouse 25-OH-D3-24-hydroxylase gene PCR, and 280bp product was obtained in β-actin gene PCR).
Reporter gene assay
The promoter region of the human 25-OH-D3-24-hydroxylase gene (−186 to −5), which contains two vitamin D responsive elements (VDRE; a gift from Dr. H. Eguchi, Teijin Institute for Bio-Medical Research, Tokyo, Japan), was cloned into a luciferase reporter vector pGL3-Basic Vector (Promega, Madison, WI) as described previously [26]. This plasmid construct was co-transfected with the β-galactosidase expression plasmid into NIH3T3 cells transduced with MVNP using the DMRIE-C Regent (Invitrogen Life Technology). Sixteen hours after transfection, vehicle (0.1% ethanol) or 10−9M 1α,25-(OH)2D3, or 10−6M (23S,25S)-DLAM-2P or combination of 10−9M 1α,25-(OH)2D3 and (23S,25S)-DLAM-2P (10−9M to 10−6M) were added. Twenty-four hours later, the cells were harvested and lysed in the cell lysate solution provided with the luciferase assay kit (Promega, Madison, WI). The luciferase activities of the cell lysates were measured with the luciferase assay kit according to the manufacturer’s instructions and were standardized by comparing the galactosidase activities of the same cell lysates as determined with a β-galactosidase enzyme assay system (Promega, Madison, WI).
Statistical analysis
Each data point is shown mean ± S.D. of triplicate or quadruplicate determinations. Results are representative of two or three independent experiments. The data were analyzed using a two-tailed Student’s t-test, with p< 0.05 considered significant.
Results
Figure 1 indicates the structures of 1α,25-(OH)2D3-26,23-lactam analogues, which are very similar with those of the 1α,25-(OH)2D3-26,23-lactone analogues. In the lactam structure, one nitrogen atom replaces an oxygen atom in the lactone ring. N-Benzyl- and N-phenetyl-1α,25-(OH)2D3-26,23-lactam have 2 asymmetric carbons at C23 and C25. Therefore, they have four diastereoisomers, respectively.
The binding affinities of the 1α,25-(OH)2D3-26,23-lactam analogues to chick VDR are shown in Table 1. The VDR binding affinities of (23S,25S)-DLAM-1P and (23S,25S)-DLAM-2P, which have 23S and 25S configurations, were 36.4 and 12.6 times weaker than that of 1α,25-(OH)2D3, respectively. The VDR binding affinity of (23S,25S)-DLAM-2P was about 3 times higher than that of (23S,25S)-DLAM-1P. The other stereoisomers of 1α,25-(OH)2D3-26,23-lactam had only a weak VDR binding affinity compared with (23S,25S)-DLAM-1P or (23S,25S)-DLAM-2P, respectively.
Table 1.
Binding affinities of 1α,25-(OH)2D3-26,23-lactam analogues to competitively inhibit [3H]1α,25-(OH)2D3 binding to chick intestinal VDR
| Compounds | 50% Displacement (pg/tube) | Molar Ratio |
|---|---|---|
| 1α,25-(OH)2D3 | 37.5 | 1 |
| (23S,25S)-DLAM-1P | 1,750 | 36.4 |
| (23R,25R)-DLAM-1P | 19,000 | 395.5 |
| (23S,25R)-DLAM-1P | 28,000 | 567.9 |
| (23R,25S)-DLAM-1P | 20,000 | 405.7 |
| (23S,25S)-DLAM-2P | 620 | 12.6 |
| (23R,25R)-DLAM-2P | 9,700 | 196.7 |
| (23S,25R)-DLAM-2P | 14,500 | 294.1 |
| (23R,25S)-DLAM-2P | 25,500 | 517.2 |
Molar ratio indicates the ratio of moles per liter of 1α,25-(OH)2D3 analogues over the moles per liter of 1α,25-(OH)2D3 required for 50% displacement of the [3H]1α,25-(OH)2D3 from the receptor. Each value of 50% displacement was calculated as the mean for duplicate determinations.
Previously, Kato et al. reported that (23S,25S)-DLAM-1P never induced HL-60 cell differentiation even after treatment at 10−6M, but that 10−6M (23S,25S)-DLAM-1P significantly inhibited HL-60 differentiation induced by 10−8M 1α,25-(OH)2D3 [9]. We examined the inhibitory actions of 1α,25-(OH)2D3-26,23-lactam analogues on HL-60 cell differentiation induced by 1α,25-(OH)2D3 in more detail, using NBT reducing activity as a cell differentiation marker. Concentrations of 10−9M to 10−7M 1α,25-(OH)2D3 dose-dependently induced differentiation of HL-60 cells during the 96 hrs culture period (data not shown). 10−8M 1α,25-(OH)2D3 differentiated >70% of the cells into NBT-reducing activity positive cells, however all diastereoisomers of N-benzyl-1α,25-(OH)2D3-26,23-lactam analogues and N-phenetyl-1α,25-(OH)2D3- 26,23-lactam analogues did not induce any cell differentiation even at concentrations of 10−6M or 2×10−6M (Figure 2A and 3A). However, (23S,25S)-DLAM-1P, (23S,25S)-DLAM-2P and (23S,25R)-DLAM-2P dose-dependently inhibited HL-60 cell differentiation induced by 10−8M 1α,25-(OH)2D3 (Figures 2B and 3B). The (23S,25S)-DLAM-1P and (23S,25R)-DLAM-2P showed a similar dose-response curve, but their suppressive effects were consistently weaker than that of (23S,25S)-DLAM-2P (Figures 2B and 3B). On the contrary, another type of vitamin D antagonist, TEI-9647 dose-dependently inhibited HL-60 cell differentiation induced by 10−8M 1α,25-(OH)2D3 (Figures 2B and 3B). Based on the HL-60 cell differentiation assays, the vitamin D antagonistic actions of (23S,25S)-DLAM-1P, (23S,25S)-DLAM-2P and (23S,25R)- DLAM-2P were estimated to be about 1.32, 8.33 and 0.75% of TEI-9647 activity, respectively.
Figure 2.
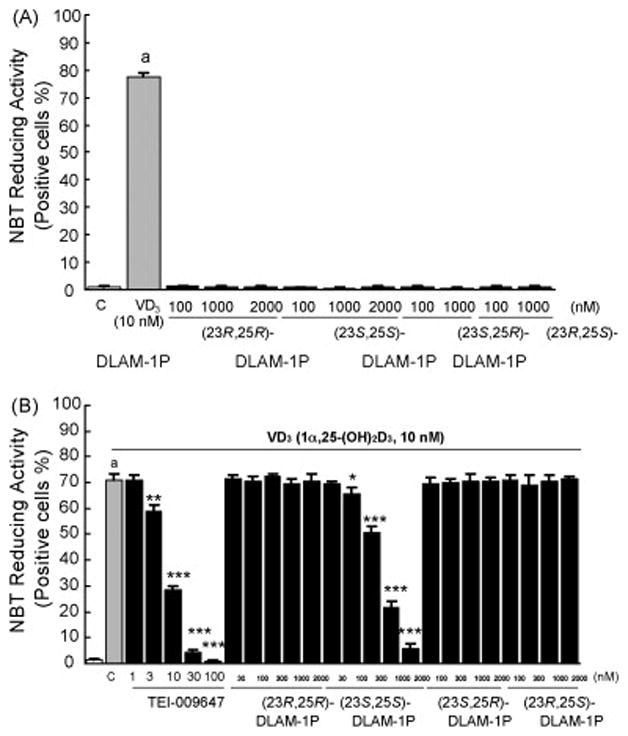
Effects of 4 diastereoisomers of N-benzyl-1α,25-(OH)2D3-26,23-lactam analogues on 1α,25-(OH)2D3-induced HL-60 cell differentiation as determined by NBT-reducing activity. (A) HL-60 cells were treated with 1α,25-(OH)2D3 alone or N-benzyl-1α,25-(OH)2D3-26,23-lactam alone for 96 hrs, and NBT-reducing activity was examined. (B) HL-60 cells were treated with N-benzyl-1α,25-(OH)2D3-26,23-lactam in the presence of 10−8M 1α,25-(OH)2D3 for 96 hrs, and NBT-reducing activity was examined. Rectangles and bars show mean ± S.D. of triplicate experiments, respectively. ap<0.001, compared with cells treated with media alone. *p<0.05, **p<0.01 and ***p<0.001, compared with cells treated with 10−8M 1α,25-(OH)2D3, respectively. Similar results were seen in two independent experiments.
Figure 3.
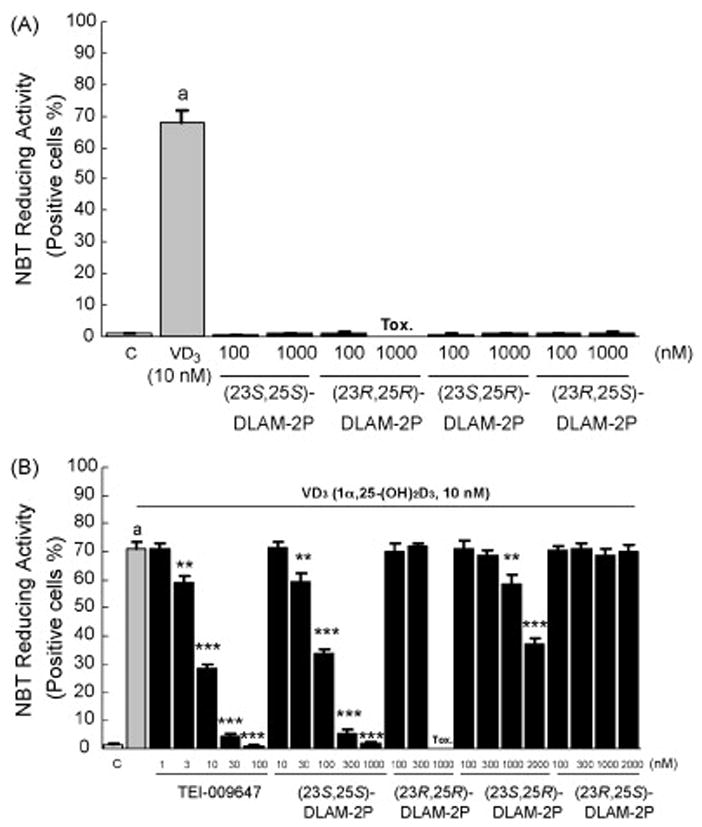
Effects of 4 diastereoisomers of N-phenetyl-1α,25-(OH)2D3-26,23-lactam analogues on 1α,25-(OH)2D3-induced HL-60 cell differentiation as determined by NBT-reducing activity. (A) HL-60 cells were treated with 1α,25-(OH)2D3 alone or N-phenetyl-1α,25-(OH)2D3-26,23-lactam alone for 96 hrs, and NBT-reducing activity was examined. (B) HL-60 cells were treated with N-phenetyl-1α,25-(OH)2D3-26,23-lactam in the presence of 10−8M 1α,25-(OH)2D3 for 96 hrs, and NBT-reducing activity was examined. Rectangles and bars show mean ± S.D. of triplicate experiments, respectively. ap<0.001, compared with cells treated with media alone. *p<0.05, **p<0.01 and ***p<0.001, compared with cells treated with 10−8M 1α,25-(OH)2D3, respectively. Similar results were seen in two independent experiments.
We previously demonstrated that TEI-9647 was a vitamin D antagonist in human cells but had weak vitamin D agonistic actions in rodent cells [14]. Therefore, we investigated whether the 1α,25-(OH)2D3-26,23-lactam analogues were vitamin D antagonists in both human cells and rodent cells, using OCL formation assays with human and mouse bone marrow cells. We confirmed our previous results that 1α,25-(OH)2D3 induced OCL formation through VDR-mediated transcription in marrow cultures from MVNP-transduced normal OCL precursors (MVNP-transduced CFU-GM cells) [11,25,26]. Using MVNP-transduced CFU-GM cells, 10−11M to 10−9M 1α,25-(OH)2D3 dose-dependently stimulated OCL formation. OCL formation in these cultures was significantly increased above control levels at 10−11M 1α,25-(OH)2D3 and reached maximum levels at 10−9M 1α,25-(OH)2D3 (data not shown). TEI-9647 did not stimulate OCL formation in any of the cultures, even at high concentrations (10−6M). In contrast, TEI-9647 dose-dependently blocked OCL formation induced by 10−9M 1α,25-(OH)2D3 (Figure 4A). All stereoisomers of N-benzyl-1α,25-(OH)2D3-26,23-lactam and N-phenetyl-1α,25-(OH)2 D3-26,23-lactam did not stimulate OCL formation in any of the cultures, even at 10−6M. (23S,25S)-DLAM-1P and (23S,25S)-DLAM-2P dose-dependently blocked OCL formation induced by 10−9M 1α,25-(OH)2D3. The OCL formation inhibitory action of (23S,25S)-DLAM-2P was about 5 times greater than that of (23S,25S)-DLAM-1P (Figure 4A). Similarly, the other stereoisomers of (23S,25S)-DLAM-1P and (23S,25S)-DLAM-2P, (23S,25R)-DLAM-1P and (23S,25R)- DLAM-2P dose-dependently blocked OCL formation induced by 10−9M 1α,25-(OH)2D3. However, these activities were significantly weaker compared to those of (23S,25S)-DLAM-1P and (23S,25S)-DLAM-2P (Figure 4A).
Figure 4.
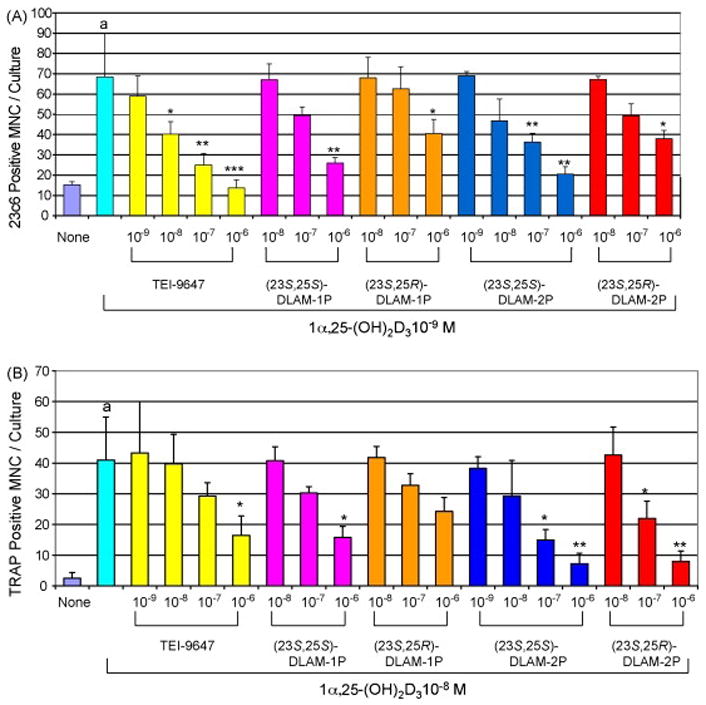
Effects of N-benzyl-1α,25-(OH)2D3-26,23-lactam analogues and N-phenetyl-1α,25-(OH)2D3-26,23-lactam analogues on OCL formation by human MVNP-transduced CFU-GM cells (A) and mouse bone marrow cells (B) treated with 1α,25-(OH)2D3. The experimental procedures for OCL formation from human MVNP-transduced CFU-GM cells and mouse bone marrow cells were carried out as described in Materials and Methods. Multinucleated cells that cross-reacted with the 23C6 antibody in human MVNP-transduced CFU-GM cells and TRAP-positive in mouse bone marrow cells and had three or more nuclei were scored an OCL. Data are expressed as the mean ± S.D. of quadruplicate determinations. ap<0.001, compared with cells treated with media alone. *p<0.05, **p<0.01 and ***p<0.001, compared with cells treated with 10−9M 1α,25-(OH)2D3 or 10−8M 1α,25-(OH)2D3, respectively. Similar results were seen in two independent experiments.
We previously demonstrated that 10−9M 1α,25-(OH)2D3 significantly stimulated OCL formation in mouse bone marrow cell cultures, and the number of OCL increased linearly up to 10−7M [24]. In mouse bone marrow cell cultures, all of the compounds tested showed similar inhibitory action on OCL formation induced by 10−8M 1α,25-(OH)2D3, except TEI-9647 (Figure 4B). The inhibitory action of TEI-9647 on OCL formation by normal mouse bone marrow cells treated with 10−8M 1α,25-(OH)2D3 was lower as compared to human bone marrow cells (Table 2).
Table 2.
Comparison of the OCL formation inhibitory actions of 1α,25-(OH)2D3-26,23-lactam analogues between human MVNP-transduced CFU-GM cultures and mouse bone marrow cultures.
| Compounds | OCL formation inhibitory action (IC50)
|
|
|---|---|---|
| 10−9M 1α,25-(OH)2D3 in MVNP-transduced CFU-GM cultures | 10−8M 1α,25-(OH)2D3 in mouse bone marrow cultures | |
| TEI-9647 | 7.5 × 10−9M | 1.5 × 10−7M |
| (23S,25S)-DLAM-1P | 2.1 × 10−7M | 1.8 × 10−7M |
| (23S,25R)-DLAM-1P | 8.6 × 10−7M | 5.0 × 10−7M |
| (23S,25S)-DLAM-2P | 4.0 × 10−8M | 1.5 × 10−8M |
| (23S,25R)-DLAM-2P | 3.9 × 10−7M | 4.4 × 10−8M |
The IC50 values for the OCL formation inhibitory action of 1α,25-(OH)2D3- 26,23-lactam were calculated from the results in Figure 4.
We then performed time-course and dose-response experiments to assess the antagonistic action of TEI-9647 and 1α,25-(OH)2D3-26,23-lactam analogues on VDR-mediated gene transcription using HL-60 cells, the human osteoblastic cell line, HOS cells, and primary mouse osteoblastic cells. We examined the time-course changes in 25-OH-D3-24-hydroxylase mRNA induced by 10−8M 1α,25-(OH)2D3 in HL-60 cells. The 25-OH-D3-24-hydroxylase mRNA was minimally detectable after 4–8 hrs treatment with 1α,25-(OH)2D3, but was expressed at significant levels after 24–48 hrs. 10−9M to 10−6M 1α,25-(OH)2D3 dose-dependently induced 25-OH-D3-24-hydroxylose mRNA, and maximum activity was observed at 10−7M (data not shown). Even at 10−6M concentration, neither TEI-9647 nor the 1α,25-(OH)2D3-26,23-lactam analogues by themselves induced 25-OH-D3-24-hydroxylase gene expression (lanes 3 to 7 in Figure 5A), although the structural analogue of 1α,25-(OH)2D3-26,23-lactam, 10−6M 1α,25- (OH)2D3-26,23-lactone, significantly induce it (lane 8 in Figure 5A). However, 10−6M TEI-9647 and 10−6M (23S,25S)-DLAM-2P almost completely suppressed the 25-OH-D3-24-hydroxylase gene expression induced by 10−8M 1α,25-(OH)2D3 in HL-60 cells (lanes 9 and 12 in Figure 5A). 10−6M (23S,25S)-DLAM-1P weakly but significantly suppressed 25-OH-D3-24-hydroxylase gene expression induced by 10−8M 1α,25-(OH)2D3 (lane 10 in Figure 5A). The inhibitory action of (23S,25S)-DLAM-1P was much weaker than that of (23S,25S)-DLAM-2P. On the contrary, stereoisomers of (23S,25S)-DLAM-1P and (23S,25S)-DLAM-2P, (23S,25R)-DLAM-1P and (23S,25R)- DLAM-2P, never and weakly inhibited 25-OH-D3-24-hydroxylase activity induced by 10−8M 1α,25-(OH)2D3 even at 10−6M, respectively (lanes 11 and 13 in Figure 5A). Moreover, 10−6M 1α,25-(OH)2D3-26,23-lactone enhanced the effects of 10−8M 1α,25-(OH)2D3 on 25-OH-D3-24-hydroxylase activity (lane 14 in Figure 5A). Similar results were seen with HOS cells and mouse osteoblastic cells. However, 10−6M (23S,25R)-DLAM-1P and (23S,25R)-DLAM-2P weakly but significantly blocked the 25-OH-D3-24-hydroxylase gene expression induced by 10−8M 1α,25-(OH)2D3 (lanes 11 and 13 in Figures 5B and 5C). In mouse osteoblastic cells, 10−6M TEI-9647 did not inhibit 25-OH-D3-24-hydroxylase gene expression induced by 10−8M 1α,25-(OH)2D3 a result different from that obtained using human cells(lane 9 in Figure 5C), but 10−6M (23S,25S)-DLAM-1P and (23S,25S)-DLAM-2P almost completely suppressed 25-OH-D3-24-hydroxylase gene expression induced by 10−8M 1α,25-(OH)2D3 (lanes 10 and 12 in Figure 5C). 1α,25-(OH)2D3-26,23-Lactone itself weakly but significantly induced 25-OH-D3-24-hydroxylase mRNA (lane 8 in Figure 5).
Figure 5.
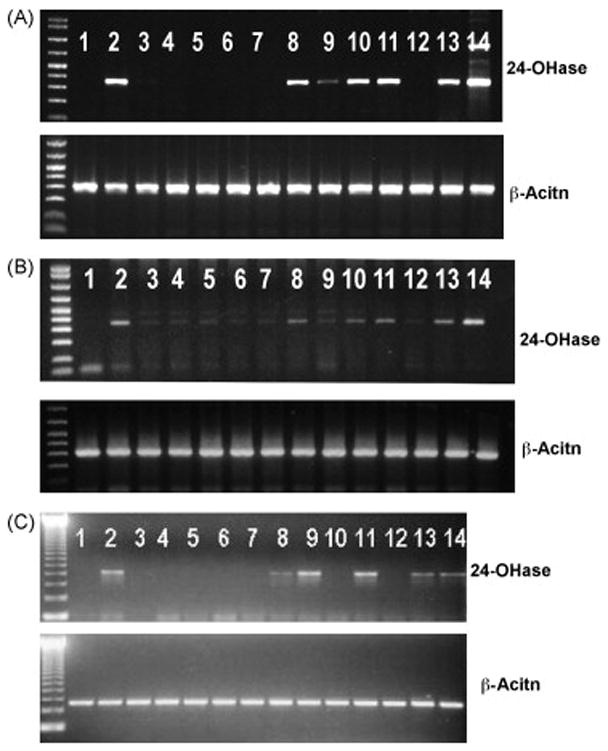
Effects of N-benzyl-1α,25-(OH)2D3-26,23-lactam analogues and N-phenetyl-1α,25-(OH)2D3-26,23-lactam analogues on 25-OH-D3-24-hydroxylase gene expression induced by 1α,25-(OH)2D3 in HL-60 cells (A), human osteoblastic cell line (HOS cells) (B) and primary mouse osteoblastic cells (C). The experimental procedures for gene expression induced by vitamin D analogues were carried out as described in Materials and Methods. 1, vehicle; 2, 10−8M 1α,25-(OH)2D3; 3, 10−6M TEI-9647; 4, 10−6M (23S,25S)-DLAM-1P; 5, 10−6M (23S,25R)-DLAM-1P; 6, 10−6M (23S,25S)-DLAM-2P; 7, 10−6M (23S,25R)-DLAM-2P; 8, 10−6M 1α,25-(OH)2D3-26,23-lactone; 9, 10−8M 1α,25-(OH)2D3 + 10−6M TEI-9647; 10, 10−8M 1α,25-(OH)2D3 + 10−6M (23S,25S)-DLAM-1P; 11, 10−8M 1α,25-(OH)2D3 + 10−6M (23S,25R)-DLAM-1P; 12, 10−8M 1α,25-(OH)2D3 + 10−6M (23S,25S)-DLAM-2P; 13, 10−8M 1α,25-(OH)2D3 + 10−6M (23S,25R)-DLAM-2P; 14, 10−8M 1α,25-(OH)2D3 + 10−6M 1α,25-(OH)2D3-26,23-lactone. Similar results were seen in two independent experiments.
Among 1α,25-(OH)2D3-26,23-lactam analogues, (23S,25S)-DLAM-2P showed the strongest inhibitory action of gene expression induced by 1α,25-(OH)2D3. Therefore, the VDR antagonistic potency of (23S,25S)-DLAM-2P was investigated using reporter gene assay in MVNP-transduced NIH3T3 cells. 10−9M 1α,25-(OH)2D3 significantly increased reporter gene activity as reported previously [26]. On the contrary, (23S,25S)-DLAM-2P never increased it even at 10−6M. However, (23S,25S)-DLAM-2P dose-dependently (10−9M to 10−6M) inhibited the reporter gene activity induced by 10−9M 1α,25-(OH)2D3. (23S,25S)-DLAM-2P, which about 10 times high concentration of 1α,25-(OH)2D3 showed 50% inhibition of 1α,25-(OH)2D3 action (Figure 6).
Figure 6.
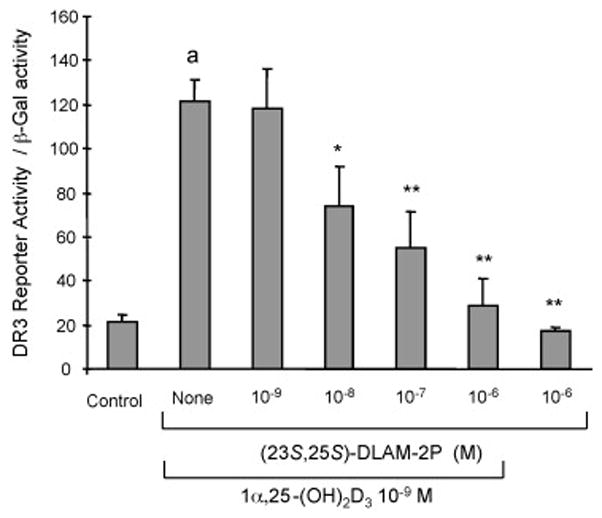
Effect of (23S,25S)-DLAM-2P on reporter gene activity induced by 1α,25-(OH)2D3 in MVNP-transduced NIH3T3 cells. The reporter plasmid containing 25-OH-D3-24-hydroxylase promoter and β-galactosidase vector were transfected into MVNP-transduced NIH3T3 cells. 10−9M 1α,25-(OH)2D3 and various concentration of (23S,25S)-DLAM-2P were added for 24 hours after the transfection of MVNP-transduced NIH3T3 cells. Results are expressed as the mean ± SEM. ap<0.001, compared with cells treated with media alone. *p< 0.01 and **p<0.001 compared with cells treated with 10−9M 1α,25-(OH)2D3. Similar results were seen in three independent experiments.
Discussion
TEI-9647, which is 1α,25-(OH)2D3-26,23-lactone analogue, functions as a vitamin D antagonist through VDR-VDRE mediated genomic actions of 1α,25-(OH)2D3 in human cells, but shows vitamin D agonistic actions in rodent cells [14]. Recently, it was reported that (23S,25S)-DLAM-1P, a 1α,25-(OH)2D3-26,23-lactone analogue, showed vitamin D antagonistic action on HL-60 cell differentiation induced by 1α,25-(OH)2D3 [9]. In the present study, we examined whether 1α,25-(OH)2D3-26,23-lactam analogues show vitamin D antagonistic action by human cells and rodent cells through a VDR-mediated genomic action of 1α,25-(OH)2D3. TEI-9647 inhibited 25-OH-D3-24- hydroxylase gene expression induced by 1α,25-(OH)2D3 in human cells but not rodent cells. On the other hand, (23S,25R)-diastereoisomers of 1α,25-(OH)2D3-26,23-lactam analogues blocked the vitamin D-dependent gene expression by not only human cells but also rodent cells treated with 1α,25-(OH)2D3. These results indicate that at least (23S,25S)-diastereoisomers of 1α,25-(OH)2D3-26,23-lactam analogues function as VDR antagonists that block the VDR-mediated genomic action of 1α,25-(OH)2D3 in multiple species.
As for OCL formation, we previously reported that the (23S,25R)- 1α,25-(OH)2D3-26,23-lactone, a major metabolite of 1α,25-(OH)2D3 in vitro and in vivo, blocks OCL formation by human and mouse bone marrow cells treated with 1α,25-(OH)2D3 through a VDR independent mechanism [24,28,29, Figure 5]. (23S,25R)-DLAM-1P and (23S,25R)-DLAM-2P, which are analogues of 1α,25-(OH)2D3-26,23-lactam, even at 10−6M, never or slightly inhibited VDR-mediated gene expression induced by 10−8M 1α,25-(OH)2D3. However, 10−8 M to 10−6M of these compounds dose-dependently blocked OCL formation by human and mouse bone marrow cultures treated with 10−8M 1α,25-(OH)2D3 (Figures 4 and 5). These results suggest that 1α,25-(OH)2D3-26,23-lactam analogues have two mechanism of inhibitory actions on OCL formation; one is a VDR-mediated antagonistic action and the other is VDR-independent 1α,25-(OH)2D3-26,23-lactone-like action. The mechanism of VDR-independent 1α,25-(OH)2D3-26,23-lactone-like action is still unknown.
The mechanism of VDR antagonistic action of TEI-9647 and 1α,25-(OH)2D3-26,23-lactam analogues is quite different. Recently, we demonstrated that the C-terminal region of the ligand binding domain of VDR plays an important role in determining the antagonistic/agonistic profile of TEI-9647 and suggested that one or both of the two cysteine residues at 403 and 410 in human VDR are key residues for the antagonistic activity of TEI-9647 [14,15]. Unlike TEI-9647, ZK159222 and ZK168281, which are also VDR antagonists, exhibited their antagonistic effect regardless of the species of VDR. This suggests the molecular mechanisms responsible for the antagonistic effects of TEI-9647, and ZK159222 or ZK168281 are different, although both types of compounds have relatively bulky ring structures in their side chains compared with 1α,25-(OH)2D3. However, the TEI-9647 side chain is not as bulky as that of ZK159222 and ZK168281. The extended side chains of ZK159222 and ZK168281 prevent the interaction between residues H397 and F422 [16,30,31]. Disturbance of the interaction with these residues makes helix 12 deviate from an optimized position for coactivator recruitment. In the case of 1α,25-(OH)2D3-26,23-lactam analogues, 1α,25-(OH)2D3-26,23-lactam, N-methyl- and N-isopropyl-1α,25-(OH)2D3-26,23-lactam analogues never act as vitamin D antagonists (unpublished data). On the contrary, N-benzyl-, N-phenetyl- and N-phenyl-butyl-1α,25-(OH)2D3-26,23-lactam analogues are vitamin D antagonists in human and rodent cells [19]. These results demonstrate that compounds which have a bulky group with a nitrogen atom in the lactam ring of 1α,25-(OH)2D3-26,23-lactam analogues showed VDR antagonistic action. The molecular mechanism responsible for the vitamin D antagonistic actions of N-benzyl- and N-phenetyl-1α,25-(OH)2D3-26,23-lactam analogues may be that they block the conformational change of helix 12 in VDR, and prevent coactivator recruitment, but the exact mechanism of action of these compounds is still unclear.
Acknowledgments
This work was supported by research funds from the NIH, Grant #PO1-AR049363 and research funds from Teijin Institute for Bio-Medical Research. The materials are the result of work supported with resources and the use of facilities at the VA Pittsburgh Healthcare System, Research and Development. We acknowledge the MUH-CTRC at the University of Pittsburgh for assistance with obtaining the marrow samples.
Abbreviations
- 1α
25-(OH)2D3, 1α,25-dihydroxyvitamin D3
- 1α
25-(OH)2D3-26,23- lactone, (23S,25R)-1 α,25-dihydroxyvitamin D3-26,23-lactone
- TEI-9647
(23S)-25-dehydro-1α-hydroxyvitamin D3-26,23-lactone
- TEI-9648
(23R)-25-dehydro-1α-hydroxyvitamin D3- 26,23-lactone
- 1α
25-(OH)2D3-26,23-lactam, 1α,25-dihydroxy-vitamin D3-26,23-lactam
- DLAM-1P
N-Benzyl-1α,25-dihydroxyvitamin D3-26,23-lactam
- DLAM-2P
N-Phenetyl- 1α,25-dihydroxyvitamin D3-26,23-lactam
- 25-OH-D3-24-hydroxylase
25-hydroxyvitamin D3- 24-hydroxylase
- VDR
vitamin D receptor
- VDRE
vitamin D responsive element
- MVNP
measles virus nucleocapsid protein
- CFU-GM
colony forming unit-granulocyte macrophage
- M-CSF
macrophage-colony stimulating factor
- GM-CSF
granulocyte- macrophage colony stimulating factor
- SCF
stem cell factor
- IL-3
interleukin-3
- IL-6
interleukin-6
- OCL
osteoclast
- NBT
nitro blue tetrazolium
- TRAP
tartrate-resistant acid phosphatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bouillon R, Okamura WH, Norman AW. Structure-function relationships in the vitamin D endocrine system. Endocr Rev. 1995;16:200–257. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- 2.Norman AW, Bouillon R, Farach-Carson MC, Bishop JE, Zhou LX, Nemere I, Zhao J, Muralidharan KR, Okamura WH. Demonstration that 1β,25-dihydroxyvitamin D3 is an antagonist of the nongenomic but not genomic biological responses and biological profile of the three A-ring diastereomers of 1α,25-dihydroxyvitamin D3. J Biol Chem. 1993;268:20022–20030. [PubMed] [Google Scholar]
- 3.Bhatia M, Kirkland JB, Meckling-Gill KA. Monocytic differentiation of acute promyelocytic leukemia cells in response to 1,25-dihydroxyvitamin D3 is independent of nuclear receptor binding. J Biol Chem. 1995;270:15962–15965. doi: 10.1074/jbc.270.27.15962. [DOI] [PubMed] [Google Scholar]
- 4.Miura D, Manabe K, Ozono K, Saito M, Gao Q, Norman AW, Ishizuka S. Antagonistic action of novel 1α,25-dihydroxyvitamin D3-26,23-lactone analogs on differentiation of human leukemia cells (HL-60) induced by 1α,25-dihydroxyvitamin D3. J Biol Chem. 1999;274:16392–16399. doi: 10.1074/jbc.274.23.16392. [DOI] [PubMed] [Google Scholar]
- 5.Miura D, Manabe K, Gao Q, Norman AW, Ishizuka S. 1α,25-Dihydroxy-vitamin D3-26,23-lactone analogs antagonize differentiation of human leukemia cells (HL-60 cells) but not of human acute promyelocytic leukemia cells (NB4 cells) FEBS Letters. 1999;460:297–302. doi: 10.1016/s0014-5793(99)01347-2. [DOI] [PubMed] [Google Scholar]
- 6.Ishizuka S, Miura D, Ozono K, Saito M, Eguchi H, Chokki M, Norman AW. (23S)- and (23R)-25-dehydro-1α-hydroxyvitamin D3-26,23-lactone function as antagonists of vitamin D receptor-mediated genomic actions of 1α,25-dihydroxyvitamin D3. Steroids. 2001;66:227–237. doi: 10.1016/s0039-128x(00)00146-x. [DOI] [PubMed] [Google Scholar]
- 7.Herdick M, Steinmeyer A, Carlberg C. Antagonistic actions of a 25-carboxylic ester analogue of 1α,25-dihydroxyvitamin D3 is mediated by a lack of ligand-induced vitamin D receptor interaction with coactivators. J Biol Chem. 2000;275:16506–16512. doi: 10.1074/jbc.M910000199. [DOI] [PubMed] [Google Scholar]
- 8.Toell A, Gonzalez MM, Ruf D, Steinmeyer A, Ishizuka S, Carlberg C. Different molecular mechanisms of vitamin D receptor antagonists. Mol Pharmacol. 2001;59:1478–1485. doi: 10.1124/mol.59.6.1478. [DOI] [PubMed] [Google Scholar]
- 9.Kato Y, Nakano Y, Sano H, Tanatani A, Kobayashi H, Shimazawa R, Koshino H, Hashimoto Y, Nagasawa K. Synthesis of 1α,25-dihydroxy vitamin D3-26,23- lactams (DLAMs), a novel series of 1α,25-dihydroxy vitamin D3 antagonist. Bioorg Med Chem Lett. 2004;14:2579–2583. doi: 10.1016/j.bmcl.2004.02.076. [DOI] [PubMed] [Google Scholar]
- 10.Ishizuka S, Kurihara N, Miura D, Takenouchi K, Cornish J, Cundy T, Reddy SV, Roodman GD. Vitamin D antagonist, TEI-9647, inhibits osteoclast formation induced by 1α,25-dihydroxyvitamin D3 from pagetic bone marrow cells. J Steroid Biochem Mol Biol. 2004;89–90:331–334. doi: 10.1016/j.jsbmb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Ishizuka S, Kurihara N, Cornish DJ, Cundy T, Reddy SV, Roodman GD. (23S)-25-Dehydro-1α-hydroxyvitamin D3-26,23-lactone, a vitamin D receptor antagonist that inhibits osteoclast formation and bone resorption in bone marrow cultures from patients with Paget’s disease. Endocrinology. 2005;146:2023–2030. doi: 10.1210/en.2004-1140. [DOI] [PubMed] [Google Scholar]
- 12.Ishizuka S, Miura D, Eguchi Ozono KH, Chokki M, Kamimura T, Norman AW. Antagonistic action of novel 1α,25-dihydroxyvitamin D3-26,23-lactone analogs on 25-hydroxyvitamin-D3-24-hydroxylase gene expression induced by 1α,25- dihydroxyvitamin D3 in human promyelocytic leukemia (HL-60) cells. Arch Biochem Biophys. 2000;380:92–102. doi: 10.1006/abbi.2000.1902. [DOI] [PubMed] [Google Scholar]
- 13.Ozono K, Saito M, Miura D, Michigami T, Nakajima S, Ishizuka S. Analysis of the molecular mechanism for the antagonistic action of a novel 1α,25- dihydroxyvitamin D3 analogue toward vitamin D receptor function. J Biol Chem. 1999;274:32376–32381. doi: 10.1074/jbc.274.45.32376. [DOI] [PubMed] [Google Scholar]
- 14.Ochiai E, Miura D, Eguchi H, Ohara S, Takenouchi K, Azuma Y, Kamimura T, Norman AW, Ishizuka S. Molecular mechanism of the vitamin D antagonistic actions of (23S)-25-dehydro-1α-hydroxyvitamin D3-26,23-lactone depends on the primary structure of the carboxyl-terminal region of the vitamin D receptor. Mol Endicrinol. 2005;19:1147–1157. doi: 10.1210/me.2004-0234. [DOI] [PubMed] [Google Scholar]
- 15.Perakyla M, Molnar F, Carlberg C. A structural basis for the species-specific antagonism of 26,23-lactones on vitamin D signaling. Chemistry Biology. 2004;11:1147–1156. doi: 10.1016/j.chembiol.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Bury Y, Steinmeyer A, Carlberg C. Structure activity relationship of carboxylic ester antagonists of the vitamin D3 receptor. Mol Pharmacol. 2000;58:1067–1074. doi: 10.1124/mol.58.5.1067. [DOI] [PubMed] [Google Scholar]
- 17.Ishizuka S, Oshida J, Tsuruta H, Norman AW. The stereochemical configuration of the natural 1α,25-dihydroxyvitamin D3-26,23-lactone. Arch Biochem Biophys. 1985;242:82–89. doi: 10.1016/0003-9861(85)90482-5. [DOI] [PubMed] [Google Scholar]
- 18.Manabe K, Ishizuka S, Tabe M, Tanaka H, Gao Q, Furuya M, Tomimori K, Sakuma Y, Hazato A. Synthesis of a novel vitamin D3-lactone analog. In: Norman AW, Bouillon R, Thomasset M, editors. Vitamin D: Chemistry, Biology and Clinical Applications of Steroid Hormone. 1997. pp. 79–80. [Google Scholar]
- 19.Nakano Y, Kato Y, Imai K, Ochiai E, Namekawa J, Miura D, Ishizuka S, Takenouchi K, Tanatani A, Hashimoto Y, Nagasawa K. A practical synthesis and evaluation of biological activities of VDR antagonists, 1α,25-dihydroxyvitamin D3-26,23-lactams (DLAMs), designed based on the helix 12-folding inhibition hypothesis. J Med Chem. 2006;49:2398–2406. doi: 10.1021/jm050738x. [DOI] [PubMed] [Google Scholar]
- 20.Ishizuka S, Bannai K, Naruchi T, Hashimoto Y. Studies on the mechanism of action of 1α,24-dihydroxyvitamin D3 II: Specific binding of 1α,24- dihydroxyvitamin D3 to chick intestinal receptor. Steroids. 1981;37:33–43. doi: 10.1016/0039-128x(81)90005-2. [DOI] [PubMed] [Google Scholar]
- 21.Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J Exp Med. 1979;149:969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kukita A, Chenu C, McManus LM, Mundy GR, Roodman GD. A typical multinucleated cells form in long-term marrow cultures from patients with Paget’s disease. J Clin Invest. 1990;85:1280–1286. doi: 10.1172/JCI114565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurihara N, Reddy SV, Menaa C, Anderson D, Roodman GD. Osteoclasts expressing the measles virus nucleocapsid gene display a pagetic phenotype. J Clin Invest. 2000;105:607–614. doi: 10.1172/JCI8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishizuka S, Kurihara N, Hakeda S, Maeda N, Ikeda K, Kumegawa M, Norman AW. 1α,25-Dihydroxyvitamin D3 [1α,25-(OH)2D3]-26,23-lactone inhibits 1,25-(OH)2D3-mediated fusion of mouse bone marrow mononuclear cells. Endocrinology. 1988;123:781–186. doi: 10.1210/endo-123-2-781. [DOI] [PubMed] [Google Scholar]
- 25.Menaa C, Barsony J, Reddy SV, Cornish J, Cundy T, Roodman GD. 1α,25-Dihydroxyvitamin D3 hypersensitivity of osteoclast precursors from patients with Paget’s disease. J Bone Miner Res. 2000;15:228–236. doi: 10.1359/jbmr.2000.15.2.228. [DOI] [PubMed] [Google Scholar]
- 26.Kurihara N, Reddy SV, Arai N, Ishizuka S, Ozono K, Cornish J, Cundy T, Singer FR, Roodman GD. TAFII-17 and the 1,25-(OH)2D3 hyper-responsivity of osteoclast precursors from patients with Paget’s disease. J Bone Miner Res. 2004;19:1154–1164. doi: 10.1359/JBMR.040312. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y, Inaba M, DeLuca HF, Mellon WS. Immunological identification of 1,25-dihydroxyvitamin D3 receptors in human promyelocytic leukemic cells (HL-60) during homologous regulation. J Biol Chem. 1989;264:13701–13705. [PubMed] [Google Scholar]
- 28.Ishizuka S, Sumitani K, Hiura K, Kawata T, Okawa M, Hakeda Y, Kumegawa M. Biological activity assessment of 1α,25-dihydroxyvitamin D3-26,23-lactone and its intermediate metabolites in vivo and in vitro. Endocrinology. 1990;127:695–701. doi: 10.1210/endo-127-2-695. [DOI] [PubMed] [Google Scholar]
- 29.Kurihara N, Ishizuka S, Tatsumi J, Arai F, Ikeda K, Roodman GD. 23(S)25(R)-1,25-(OH)2D3-26,23-Lactone, a naturally occurring metabolite of 1,25-(OH)2 vitamin D3, inhibits osteoclast-like cell formation in human bone marrow cultures. J Bone Miner Metab. 1998;16:5–10. [Google Scholar]
- 30.Vaisanen S, Perakyla M, Karkkainen JI, Steinmeyer A, Carlberg C. Critical role of helix 12 of the vitamin D3 receptor for the partial agonism of carboxylic ester antagonists. J Mol Biol. 2002;315:229–238. doi: 10.1006/jmbi.2001.5225. [DOI] [PubMed] [Google Scholar]
- 31.Carlberg C. Ligand-mediated conformational changes of the VDR are required for gene transactivation. J Steroid Biochem Mol Biol. 2004;89–90:227–232. doi: 10.1016/j.jsbmb.2004.03.112. [DOI] [PubMed] [Google Scholar]


