Abstract
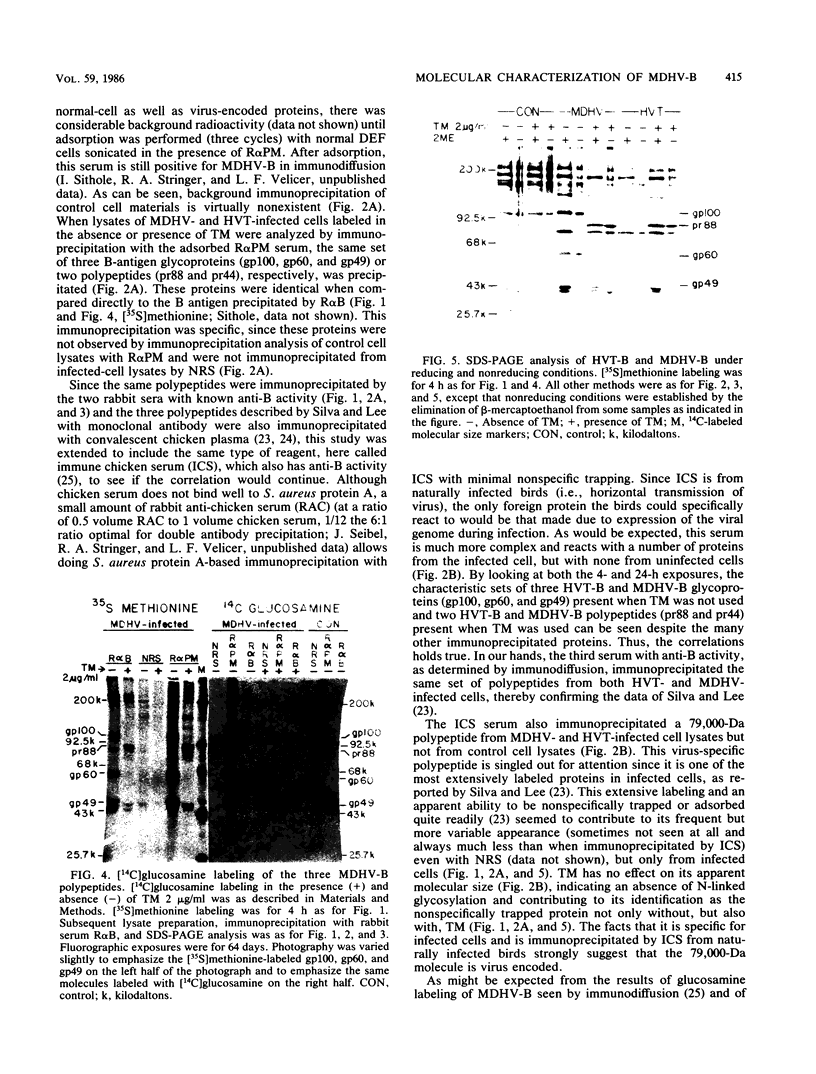
The Marek's disease herpesvirus (MDHV) B antigen (MDHV-B) was identified and molecularly characterized as a set of three glycoproteins of 100,000, 60,000, and 49,000 apparent molecular weight (gp100, gp60, and gp49, respectively) by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) after immunoprecipitation from [35S]methionine-labeled infected cells by specific rabbit antiserum directed against MDHV-B (R alpha B), as previously determined by immunodiffusion. Further identification was accomplished by blocking this immunoprecipitation with highly purified MDHV-B. The same set of three polypeptides was also immunoprecipitated from [35S]methionine- and 14C-labeled infected cells with two other sera shown to have anti-B activity, i.e., rabbit anti-MDHV-infected-cell plasma membrane (R alpha PM) and immune chicken serum from birds naturally infected with MDHV. The three herpesvirus of turkeys (HVT) B-antigen (HVT-B) glycoproteins immunoprecipitated with all three sera containing anti-B activity were also shown to be identical in size to those of MDHV-B by immunoprecipitation and SDS-PAGE. These data serve to clarify the molecular identification of the polypeptides found in common between MDHV and HVT by linking them to MDHV-B, previously identified only by immunodiffusion, and to a similarly sized set of immunologically related common glycoproteins called gp100, gp60, and gp49, detected with monoclonal antibody by other workers. Tunicamycin inhibition of N-linked glycosylation resulted in either nonglycosylated or O-linked glycosylated putative precursors of MDHV-B and HVT-B with apparent molecular weights of 88,000, called pr88, and 44,000, tentatively called pr44, both immunoprecipitable with all three sera. However, the relationships of these two polypeptides to each other and to the overall precursor-processing relationship of the MDHV-B complex remains to be elucidated. The three fully glycosylated B-antigen polypeptides were not connected by disulfide linkage. Collectively, the data presented here and by others support the conclusion that all three glycoproteins now identified as gp100, gp60, and gp49 have MDHV-B determinants. Finally, detection of the same three polypeptides with well-absorbed R alpha PM, which was directed against purified infected-cell plasma membranes, suggests that at least one component of the B-antigen complex has a plasma membrane location in the infected cell.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Churchill A. E., Chubb R. C., Baxendale W. The attenuation, with loss of oncogenicity, of the herpes-type virus of Marek's disease (strain HPRS-16) on passage in cell culture. J Gen Virol. 1969 Jun;4(4):557–564. doi: 10.1099/0022-1317-4-4-557. [DOI] [PubMed] [Google Scholar]
- Glaubiger C., Nazerian K., Velicer L. F. Marek's disease herpesviruses. IV. Molecular characterization of Marek's disease herpesvirus A antigen. J Virol. 1983 Mar;45(3):1228–1234. doi: 10.1128/jvi.45.3.1228-1234.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta K., Ueda S., Kato S., Hirai K. Identification with monoclonal antibodies of glycoproteins of Marek's disease virus and herpesvirus of turkeys related to virus neutralization. J Virol. 1984 Mar;49(3):1014–1017. doi: 10.1128/jvi.49.3.1014-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta K., Ueda S., Kato S., Hirai K. Processing of glycoprotein gB related to neutralization of Marek's disease virus and herpesvirus of turkeys. Microbiol Immunol. 1984;28(8):923–933. doi: 10.1111/j.1348-0421.1984.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Isfort R. J., Stringer R. A., Kung H. J., Velicer L. F. Synthesis, processing, and secretion of the Marek's disease herpesvirus A antigen glycoprotein. J Virol. 1986 Feb;57(2):464–474. doi: 10.1128/jvi.57.2.464-474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaden O. R., Dietzschold B. Alterations of the immunological specificity of plasma membranes from cells infected with Marek's disease and turkey herpes viruses. J Gen Virol. 1974 Oct;25(1):1–10. doi: 10.1099/0022-1317-25-1-1. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Long P. A., Clark J. L., Velicer L. F. Marek's Disease Herpesviruses II. Purification and Further Characterization of Marek's Disease Herpesvirus A Antigen. J Virol. 1975 May;15(5):1192–1201. doi: 10.1128/jvi.15.5.1192-1201.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long P. A., Kaveh-Yamini P., Velicer L. F. Marek's Disease Herpesviruses I. Production and Preliminary Characterization of Marek's Disease Herpesvirus A Antigen. J Virol. 1975 May;15(5):1182–1191. doi: 10.1128/jvi.15.5.1182-1191.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukàcs N., Thiel H. J., Mettenleiter T. C., Rziha H. J. Demonstration of three major species of pseudorabies virus glycoproteins and identification of a disulfide-linked glycoprotein complex. J Virol. 1985 Jan;53(1):166–173. doi: 10.1128/jvi.53.1.166-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki W., Purchase H. G., Burmester B. R. Protection against Marek's disease by vaccination with a herpesvirus of turkeys. Avian Dis. 1970 May;14(2):413–429. [PubMed] [Google Scholar]
- Ono K., Takashima M., Ishikawa T., Hayashi M., Yoshida I., Konobe T., Ikuta K., Nakajima K., Ueda S., Kato S. Partial protection against Marek's disease in chickens immunized with glycoproteins gB purified from turkey-herpesvirus-infected cells by affinity chromatography coupled with monoclonal antibodies. Avian Dis. 1985 Apr-Jun;29(2):533–539. [PubMed] [Google Scholar]
- Schwarz R. T., Rohrschneider J. M., Schmidt M. F. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol. 1976 Sep;19(3):782–791. doi: 10.1128/jvi.19.3.782-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R. F., Lee L. F. Monoclonal antibody-mediated immunoprecipitation of proteins from cells infected with Marek's disease virus or turkey herpesvirus. Virology. 1984 Jul 30;136(2):307–320. doi: 10.1016/0042-6822(84)90167-3. [DOI] [PubMed] [Google Scholar]
- Velicer L. F., Yager D. R., Clark J. L. Marke's disease herpesviruses. III. Purification and characterization of Marek's disease herpesvirus B antigen. J Virol. 1978 Jul;27(1):205–217. doi: 10.1128/jvi.27.1.205-217.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Witte O. N., Wirth D. F. Structure of the murine leukemia virus envelope glycoprotein precursor. J Virol. 1979 Feb;29(2):735–743. doi: 10.1128/jvi.29.2.735-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyn-Jones A. P., Kaaden O. R. Induction of virus-neutralizing antibody by glycoproteins isolated from chicken cells infected with a herpesvirus of turkeys. Infect Immun. 1979 Jul;25(1):54–59. doi: 10.1128/iai.25.1.54-59.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]








