Abstract
Background
Longitudinal data regarding the influence of beverage intakes on calcium adequacy are lacking.
Objective
This study evaluated calcium intake from ages 5 to 9 y as a function of mother-daughter beverage choices and as a predictor of bone mineral status.
Design
Intakes of energy, calcium, milk, sweetened beverages, fruit juices, and non-energy-containing beverages were measured with the use of three 24-h dietary recalls in 192 non-Hispanic white girls aged 5, 7, and 9 y and their mothers. Calcium intakes from ages 5 to 9 y were categorized as either meeting or falling below recommended adequate intakes (AIs). The girls’ bone mineral status was assessed with dual-energy X-ray absorptiometry at age 9 y.
Results
The mean 5-y calcium intake was related to bone mineral density at age 9 y (β = 0.27, P < 0.001). The girls who met the AI for calcium were not heavier (P = 0.83) but had higher energy intakes (P < 0.0001) than did the girls who consumed less than the AI. Compared with the girls who consumed less than the AI, the girls who met the AI consumed, on average, almost twice as much milk (P < 0.0001), had smaller decreases in milk intake (P < 0.01), and consumed 18% less sweetened beverages (P < 0.01) from ages 5 to 9 y; the 2 groups did not differ significantly in juice and non-energy-containing beverage intakes. The girls who met the AI were also served milk more frequently than were the girls who consumed less than the AI (P < 0.0001) and had mothers who drank milk more frequently (P < 0.01) than did the mothers of the girls who consumed less than the AI.
Conclusions
These findings provide new longitudinal evidence that calcium intake predicts bone mineral status during middle childhood and reflects mother-daughter beverage choice patterns that are established well before the rapid growth and bone mineralization observed in adolescence.
Keywords: Sweetened beverages, milk, juice, calcium intake, bone mineral content, bone mineral density, maternal influence
INTRODUCTION
Obtaining adequate calcium intakes from childhood through early adulthood is central to osteoporosis prevention efforts aimed at influencing peak bone mass in females (1–3). In girls, calcium intake approximates recommendations during childhood (4, 5). During the period of rapid bone mineral acquisition occurring in puberty, however, girls’ calcium intakes are 30–40% below the 1300-mg/d recommendations.
Milk provides the main source of calcium in many children’s diets, representing ≈50% of total intake (6–8). Milk intake in girls during childhood also appears to set the course of milk intake during adolescence and young adulthood (9–12). As such, children’s milk consumption patterns are an important behavioral phenomenon because factors dictating the consumption of this single food may have lasting implications for nutrient adequacy and bone health. Despite the apparent centrality of milk intake to calcium adequacy, determinants of girls’ beverage choices are not well characterized.
From early childhood to late adolescence, milk intake decreases > 25% (13–15). In contrast, soft drink intake more than triples during the same period, so that by the end of this period, intake is more than double that of milk. National cross-sectional data show a negative relation between milk and sweetened beverage intakes among children and adolescents (13, 16–19). The nature of this relation has been questioned, in part because of the inherent limitations of using cross-sectional data to draw inferences about causality (20). In a smaller study, maternal milk and soft drink consumption predicted the apparent tradeoff between milk and soft drinks in the diets of preschool-age girls (21). The extent to which mother-child resemblances in the amount and type of milk consumed (22, 23) reflects social modeling influences, shared patterns of the availability of beverages, or both is not well understood.
This investigation used a longitudinal cohort design to evaluate calcium intake across middle childhood as a function of mother-daughter beverage choices and as a predictor of bone mineral status. We hypothesized that the girls who met calcium recommendations from ages 5–9 y would consume and have mothers who consumed more milk and less sweetened beverages than did girls who did not meet the recommendations. We also hypothesized that the girls who met calcium recommendations would be served milk more frequently and would have a higher bone mineral status than would girls who failed to meet the recommendations. Predictors of girls’ milk intakes from 5 to 9 y of age were also evaluated. We hypothesized that milk intake from 5 to 9 y of age would be negatively related to the daughters’ sweetened beverage intakes and positively related to milk-serving practices and the mothers’ own milk intakes.
SUBJECTS AND METHODS
Subjects
Participants were from central Pennsylvania and were part of a longitudinal study of the health and development of young girls. At the beginning of the study, 197 five-year-old girls and their parents were enrolled. Of the original sample, 192 were reassessed 2 y later at age 7 y, and 182 were seen again at age 9 y. The eligibility criteria for the girls’ participation included living with both biological parents, the absence of severe food allergies or chronic medical problems that would affect food intake, and the absence of dietary restrictions involving animal products. Families were recruited for participation in the study through the use of flyers and newspaper advertisements. In addition, families with age-eligible female children within a 5-county radius received mailings and follow-up phone calls (Metromail Inc, Lombard, IL). The mean (± SD) age of the girls at entry was 5.4 ± 0.4 y, and 6.3% were overweight on the basis of having a body mass index [BMI; weight (kg)/height2 (m)] greater than the 95th percentile (24, 25). On average, the mothers were in their mid-30s at the time of recruitment [mean (± SD) age: 35 ± 5 y]. The mothers were well educated [mean (± SD) education level: 15 ± 2 y], and 63% were employed. Approximately equal numbers of families reported incomes in the following ranges when the girls were 5 y of age: $20 000 –$35 000, $35 000 –$50 000, and > $50 000. At enrollment to the study, the mean (± SD) BMI of the mothers was 25.6 ± 5.3, and 52.3% of the mothers were overweight (BMI > 25). The analyses were conducted with the use of data from 192 participants with complete dietary data at 2 of 3 time points. The parents provided written consent for their own participation and for their daughters’ participation; all procedures were reviewed and approved by the Pennsylvania State University Institutional Review Board.
Beverage and calcium intakes
The mothers’ and daughters’ energy, calcium, milk, fruit juice, sweetened beverage, and non-energy-containing beverage intakes were estimated by using multiple-pass 24-h dietary recalls. The daughters’ dietary intakes were measured at ages 5 (n = 192), 7 (n = 192), and 9 (n = 182) y. The mothers were the primary reporters of the girls’ intakes at each age; the girls were asked to be present during all interviews to facilitate the recall process. Maternal dietary intake was assessed when the girls were aged 7 y (n = 187 mothers assessed) and 9 y (n = 181 mothers assessed). Three recalls were obtained per respondent at each time of measurement; 2 weekdays and 1 weekend day during the summer were randomly selected over a 2-wk period. Interviews were conducted by trained staff at The Pennsylvania State University Diet Assessment Center by using the computer-assisted NUTRITION DATA SYSTEM (Nutrition Coordinating Center, University of Minnesota, Minneapolis). NDS Version 2.91, nutrient database version 26, food database 11a, release date 1996 was used when the girls were 5 y old. The NUTRITION DATA SYSTEM FOR RESEARCH (NDS-R) was used when the girls were 7 and 9 y old. At age 7 y, version 4.01_30, release date 2000 was used and at age 9, version 4.02_31 release date 2001 was used. Food portion posters (2D Food Portion Visual; Nutrition Consulting Enterprises, Framingham, MA) were used as a visual aid for estimating the amounts of foods eaten.
The mean of 3 dietary recalls was used to estimate nutrient intake for each respondent for each period of measurement (ie, 5, 7, and 9 y of age). The average total calcium intake at each age was calculated as the mean daily intake from all foods, beverages, and calcium-containing supplements. The girls’ calcium intakes were categorized as either meeting or falling below recommendations across the 5-y period. Specifically, the girls’ calcium intakes at each age (ie, 5, 7, and 9 y of age) were expressed as a percentage of the recommended adequate intake (AI) for that particular age (26): the AI at 4 –8 y = 800 mg/d, and the AI at 9 –13 y = 1300 mg/d [ie, 75% of the AI at age 5 y = (600 mg/d observed calcium intake ÷ 800 mg/d recommended intake) × 100]. Meeting the AI for calcium from ages 5 to 9 y was defined as having a mean percentage AI of ≥ 100% across ages 5, 7, and 9 y.
Beverage output files were used to calculate average 3-d beverage consumption into 4 intake categories: milk, fruit juice, sweetened beverages, and non-energy-containing beverages. Because the focus of this research was to evaluate the role of beverage intake on meeting calcium recommendations, milk intake was quantified as that consumed as a beverage; milk consumed with other foods (eg, cereal) or as part of a recipe was not included in this category. Fruit juice was defined as a beverage that contained ≥ 50% fruit juice. Sweetened beverages included both energy-containing carbonated (soda) and noncarbonated beverages (fruit drinks, sport drinks, and sweetened iced tea) that contained little if any fruit juice. Energy-containing carbonated beverages represented ≈33% of the intake in this category for girls and ≈45% for the mothers. Non-energy-containing beverages were carbonated and noncarbonated beverages such as unsweetened tea, coffee, club soda, diet soda, and artificially sweetened “diet” beverages. The mothers’ and girls’ intakes of bottled and tap water were not assessed. Consumption in each category was expressed in g and as a percentage of total daily energy intake.
Milk-serving practices at meals and snacks
The availability of milk to girls was assessed at 9 y (n = 181). Maternal reports were used to assess the frequency with which milk as a beverage was served to daughters at breakfast, lunch, dinner, and snacks. This question did not attempt to delineate who served milk to the daughters, but rather quantified how frequently milk was made available to the daughters at various eating occasions. A 5-point Likert-type response option was used to quantify maternal reports of the frequency with which milk was served to the daughters at each eating occasion, where 1 = never, 2 = rarely, 3 = sometimes, 4 = most always, and 5 = always. A total score was calculated by taking the mean of the mothers’ responses across all eating occasions assessed.
Weight status
The girls’ weights and heights were measured at ages 5 y (n = 192), 7 y (n = 192), and 9 y (n = 182) with the use of procedures described by Lohman et al (27). All subjects wore light clothing and were weighed and measured without shoes. Height and weight were measured in triplicate to the nearest 0.1 cm and 0.1 kg, respectively. Age- and sex-specific BMI z scores were calculated by using growth charts from the Centers for Disease Control and Prevention (24).
Pubertal status
A trained nurse assessed pubertal status by visually inspecting breast development when the girls were 9 y (n = 168). The Tanner Rating System (28) was used where a score of 1 reflected no development and a score of 5 reflected mature development. Two raters’ scores were averaged to calculate a total breast development score. If scores did not fall on whole numbers (ie, girls had different ratings for each breast), then the scores were rounded down. Parental consent was obtained before this procedure was performed, and the parents were able to request being present for the procedure.
Bone mineral status
The girls’ bone mineral density (BMD) and bone mineral content (BMC) were assessed at age 9 y (n = 173) with dual-energy X-ray absorptiometry. A trained technician obtained measurements while the children were in a supine position and wearing light clothing and no shoes. Whole-body scans were obtained with the use of a Hologic QDR 4500W (Hologic Inc, Waltham, MA) instrument in the array scan mode. Scans were analyzed by using whole-body software (QDR4500 Whole Body Analysis; Hologic Inc). BMC was expressed in g, and BMD was expressed in g/cm2.
Data analysis
Girls with complete data at 2 of the 3 time points (ages 5, 7, and 9 y) were included in the analyses. As a result, data from 192 of 197 girls are presented in this report. All analyses were performed by using SAS (version 8.2; SAS Institute, Cary, NC). Age-related trends in girls’ calcium and beverage intakes were evaluated by using 2 × 3 [(those who met the AI/those who consumed less than the AI) × (ages 5, 7, and 9 y)] repeated-measures analysis of variance; Greenhouse-Geisser corrections were applied in cases in which the assumption of sphericity was not met (29). Multiple linear regression was used to evaluate relations between calcium intake and BMD and BMC with potential covariates (ie, pubertal status and weight; 30, 31). Logistic regression was used to predict the odds of meeting the AI for calcium intake at age 9 y on the basis of meeting the AI at age 5 y. Spearman rank-order correlations were used to evaluate calcium tracking across time. Analysis of variance and analysis of covariance were used to assess differences in mother-daughter beverage intake patterns between girls who met the AI for calcium and those who consumed less than the AI. Multiple linear regression was used to evaluate predictors of girls’ milk intakes. Descriptive statistics were presented as means ±SEMs unless otherwise indicated.
RESULTS
Forty-one percent (n = 78) of the girls were categorized as meeting the AI from ages 5 to 9 y, with a mean calcium consumption of 124 ± 2% of the AI. Of the 59% of girls (n = 114) who consumed less than the AI from ages 5 to 9 y, the mean calcium intake was 78 ± 2% of the AI. One of 192 girls was reported by their mothers as being lactose intolerant but was retained in the analyses because regular and lactose-free milk intakes were reported.
Calcium intake and tracking
Calcium intake increased by ≈10% from ages 5 to 9 y (P < 0.001), with mean intakes of 852 ± 25, 876 ± 22, and 930 ± 23 mg at ages 5, 7, and 9 y, respectively. The effect of calcium intake classification (meeting or consuming less than the AI) on girls’ calcium intakes did not vary significantly by age (P = 0.06). When the calcium intake at age 5 y was controlled for, however, the girls who met the AI showed a 277-mg/d increase from ages 5 to 9 y, whereas those consuming less than the AI showed a 67-mg/d decrease (P < 0.0001) over the same period (Table 1). Calcium intake showed moderate tracking from 5 to 9 y. The correlations for calcium intake from ages 5 to 7 y, 7 to 9 y, and 5 to 9 y were r = 0.52 (P < 0.0001), r = 0.48 (P < 0.0001), and r = 0.39 (P < 0.0001), respectively. More than 50% of all girls met the 800-mg/d recommendations at ages 5 y (55%) and 7 y (57%). In contrast, only 10% of the total sample consumed the recommended 1300 mg/d at age 9 y. The girls who met the AI at age 5 y, however, were 4.8 times (95% CI: 1.3, 17.0; P < 0.05) as likely to meet the AI for calcium at age 9 y as were the girls who consumed less than the AI at age 5 y. The average calcium density of the diet per 1000 kcal was 29% lower in the girls who consumed less than the AI for calcium than in the girls who met the recommendations (P < 0.0001).
TABLE 1.
Nutrient intake, weight, and bone mineral status in the girls who met or consumed less than the current recommended adequate intake (AI) of dietary calcium1
| Met AI (n = 78) | Consumed less than the AI (n = 114) | |
|---|---|---|
| Mean calcium intake per 1000 kcal from ages 5 to 9 y (mg/d)2 | 627 ± 123 | 447 ± 10 |
| Change in calcium intake from ages 5 to 9 y (mg/d)4 | 277 ± 363 | −67 ± 28 |
| Mean energy intake from ages 5 to 9 y (kcal) | 1804 ± 283 | 1593 ± 24 |
| Mean BMI z score from ages 5 to 9 y | 0.41 ± 0.10 | 0.38 ± 0.08 |
| Bone mineral content at age 9 y (g)5 | 976 ± 136 | 944 ± 11 |
| Bone mineral density at age 9 y (g/cm2)7 | 0.85 ± 0.0058 | 0.83 ± 0.004 |
All values are x̄ ± SEM. See reference 26 for AI.
Excluding calcium intake from supplements.
Significantly different from those who consumed less than the AI: 3P < 0.0001, 6 P = 0.06, 8 P < 0.001.
The calcium intake at age 5 y was controlled for.
n = 160; pubertal development and height at age 9 y were controlled for.
n = 160; pubertal development at age 9 y was controlled for.
Energy intake, overweight, and bone mineral status
As shown in Table 1, the girls who met the AI had higher mean energy intakes from ages 5 to 9 y than did the girls who did not meet the AI (P < 0.0001); energy intakes in both groups were consistent with age- and sex-specific estimated energy requirements (32). Girls who met the AI, however, were not heavier from age 5 to 9 y than were the girls who consumed less than the AI for calcium (P = 0.83).
BMC and BMD were evaluated when the girls were age 9 y (n = 173); mean values for the sample at age 9 y were 956 ± 12 g and 0.839 ± 0.003 g/cm2, respectively. The mean calcium intake from ages 5 to 9 y was positively related to BMD at age 9 y after control for the stage of pubertal development at age 9 y (n = 160; β = 0.27, P < 0.001) and was weakly related to BMC after control for pubertal development and height at age 9 y (n = 160; β = 0.12, P < 0.05). As shown in Table 1, the girls who met calcium recommendations had BMD values that were slightly higher than those of the girls who did not meet these recommendations.
Daughters’ beverage choices
Age-related trends in girls’ beverage intakes are shown in Table 2. The girls’ milk intakes did not vary significantly with age (P = 0.42). Juice intake decreased from ages 5 to 9 y by 26% (P < 0.001), whereas sweetened beverages increased by 21% (P < 0.0001). The girls’ intakes of non-energy-containing beverages (eg, diet drinks, unsweetened tea, and coffee) showed an age-related increase of > 200% (P < 0.0001) but was low in absolute amounts relative to intakes of milk and sweetened beverages. A main effect of calcium intake classification on beverage intakes from 5 to 9 y was observed for milk (P < 0.0001) and sweetened beverages (P < 0.01) but not for juice (P = 0.70) or non-energy-containing beverages (P = 0.96). However, the effects of calcium intake classification on girls beverage intakes at ages 5, 7, and 9 y did not vary significantly by age for girls’ intakes of milk (P = 0.12), sweetened beverages (P = 0.75), juice (P = 0.11), and non-energy-containing beverages (P = 0.48). Therefore, a mean value was used to summarize beverage intakes from ages 5 to 9 y in the main analyses below, which compared the beverage choices of girls who met calcium recommendations with those of the girls who consumed less than the AI.
TABLE 2.
Time-related trends in the girls’ beverage intakes by age
| Beverage | Age 5 y (n = 192) | Age 7 y (n = 192) | Age 9 y (n = 182) | Change from ages 5 to 9 y |
|---|---|---|---|---|
| g/d | g/d | g/d | % | |
| Milk | 298 ± 151 | 294 ± 14 | 288 ± 15 | −3 |
| Sweetened beverage2 | 313 ± 16 | 375 ± 15 | 380 ± 17 | 21 |
| Fruit juice3 | 163 ± 11 | 150 ± 12 | 121 ± 10 | −26 |
| Non-energy-containing beverage2 | 23 ± 6 | 30 ± 7 | 81 ± 10 | 252 |
x̄ ± SEM (all such values).
Significant effect of age: 2P < 0.0001, 3 P < 0.001.
Girls who met calcium recommendations from 5 to 9 y of age differed from girls who failed to meet recommendations primarily in their milk and sweetened beverage intakes. Girls who met the AI consumed daily almost twice the amount of milk (407 compared with 215 g/d; P < 0.0001) as did girls who consumed less than the AI (Table 3). Accordingly, girls who met the AI consumed a greater percentage of total daily calcium from milk as a beverage (49 ± 2% compared with 38 ± 1%; P < 0.0001) than did girls who consumed less than the AI for calcium. When baseline milk intake at age 5 y was controlled for, girls who consumed less than the AI showed a 56-g/d decrease in milk intake from 5 to 9 y of age, whereas those who met the AI showed an increase of similar magnitude in milk intake (45 g/d) over the same period (P < 0.01). Alternatively, when baseline sweetened beverage intake at age 5 y was controlled for, girls who consumed less than the AI for calcium had larger mean changes in sweetened beverage intake from ages 5 to 9 y than did those girls who met the calcium recommendations, although the between-group differences were not statistically significant (x̄ ± SEM: 90 ± 21-g increase in sweetened beverage intake from ages 5 to 9 y compared with a 45 ± 25-g increase; P = 0.17). However, girls who met the AI for calcium consumed 18% fewer sweetened beverages from ages 5 to 9 y (P < 0.01) than did girls who consumed less than the AI for calcium.
TABLE 3.
Mother-daughter beverage intakes in the girls who met or consumed less than the current recommended adequate intake (AI) of dietary calcium1
| Met AI (n = 78) | Consumed less than the AI (n = 114) | |
|---|---|---|
| Daughter | ||
| Milk intake from ages 5 to 9 y (g/d) | 407 ± 152 | 215 ± 12 |
| Sweetened beverage intake from ages 5 to 9 y (g/d) | 315 ± 193 | 384 ± 16 |
| Fruit juice intake from ages 5 to 9 y (g/d) | 148 ± 12 | 143 ± 10 |
| Non-energy-containing beverage intake from ages 5 to 9 y (g/d) | 43 ± 8 | 43 ± 7 |
| ΔMilk intake from ages 5 to 9 y (g/d)4 | 45 ± 223 | −56 ± 18 |
| ΔSoft drink intake from ages 5 to 9 y (g/d)4 | 45 ± 25 | 90 ± 21 |
| ΔFruit juice intake from ages 5 to 9 y (g/d)4 | −57 ± 16 | −34 ± 13 |
| ΔNon-energy-containing beverage intake from 5 to 9 y (g/d)4 | 68 ± 16 | 49 ± 13 |
| Mother | ||
| Milk intake (g/d) | 191 ± 193 | 129 ± 16 |
| Sweetened beverage intake (g/d) | 394 ± 31 | 425 ± 25 |
| Fruit juice intake (g/d) | 112 ± 13 | 86 ± 11 |
| Non-energy-containing beverage intake (g/d) | 491 ± 44 | 496 ± 36 |
All values are x̄ ± SEM. See reference 26 for AI.Δ, change.
Significantly different from those who consumed less than the AI: 2P < 0.0001, 3 P < 0.01.
Baseline intake at age 5 y was controlled for.
The contribution of each beverage to the total beverage intake and to the total daily energy intake is shown in Figure 1. Both groups of girls consumed ≈20% of their total daily energy intake from beverages. Milk constituted close to 50% of all beverages consumed (excluding water) by the girls who met the AI, which represented 11% of their total daily energy intake. In contrast, sweetened beverages represented close to 50% of all beverages consumed by the girls who failed to meet the AI, which represented 9% of their total daily energy intake. Finally, intake of fruit juice and non-energy-containing beverages did not differ significantly between girls who met the recommendations and those who did not (Table 3 and Figure 1).
FIGURE 1.
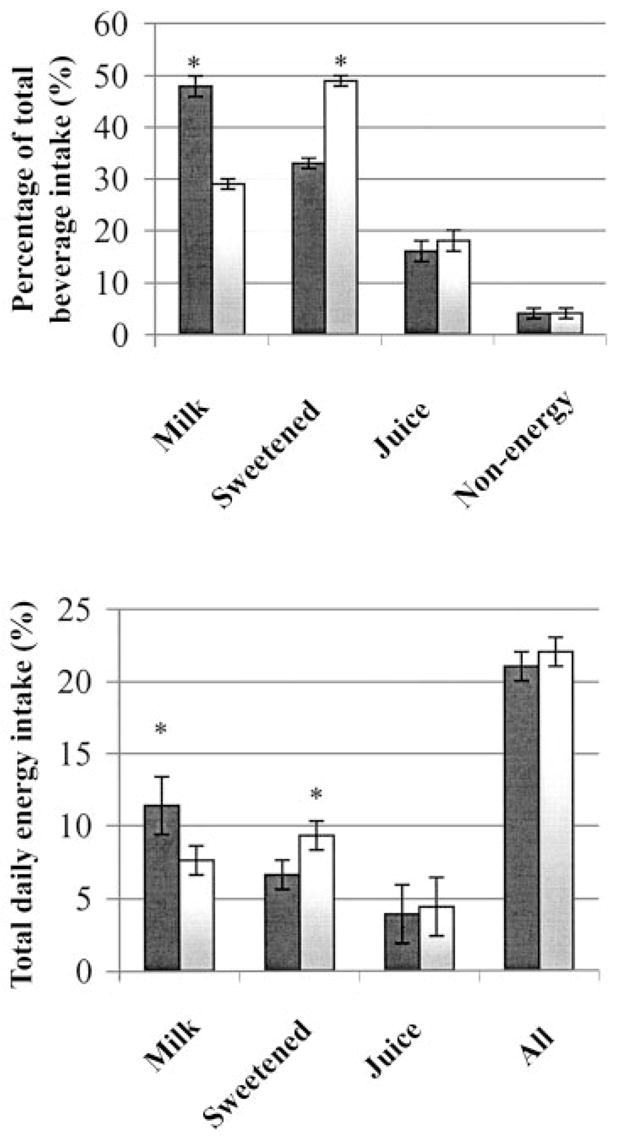
Mean (± SEM) percentage contribution of each beverage (milk, sweetened beverages, fruit juices, and non-energy-containing beverages) to the total beverage intake and to the total daily energy intake in the girls who met (■) or consumed less than (□) the current recommended adequate intake (AI; 26) of dietary calcium. n = 192. *Significantly different from the subjects who met the AI, P < 0.0001.
Association of sweetened beverage intake with changes in milk intake from ages 5 to 9 y
To evaluate whether increases in sweetened beverage intakes from ages 5 to 9 y were associated with decreases in girls’ milk intakes, mean sweetened beverage intakes and changes in sweetened beverage intakes from 5 to 9 y of age were used to predict changes in milk intake from ages 5 to 9 y. Greater decreases in milk intake from ages 5 to 9 y were associated with high initial milk intake at 5 y (β = −0.59, P < 0.0001), which indicated a possible regression toward the mean. When baseline milk intake at age 5 y was controlled for, greater decreases in milk intake from ages 5 to 9 y were associated with a greater mean sweetened beverage intake (β = −0.24, P < 0.0001) but were not associated with increases in sweetened beverage intake from ages 5 to 9 y (β = 0.02, NS). The upper and lower quartiles for mean sweetened beverage intake from ages 5 to 9 y were compared to further describe changes in beverage intakes over time among low and high consumers of sweetened drinks. When initial sweetened beverage intake at age 5 y was controlled for, girls who consumed an average of ≥ 444 mL/d (≈15 fluid oz/d) of sweetened beverages showed a 337-g/d increase in sweetened beverage intake and a 110-g/d decrease in milk intake from ages 5 to 9 y, whereas those girls who consumed ≤237 mL/d (≈8 fluid oz/d) of sweetened beverages showed a 200-g/d decrease in sweetened beverages and a 24-g/d increase in milk intake from ages 5 to 9 y (P < 0.01).
Maternal beverage intake and milk availability to daughters at meals and snacks
There was no association between the daughters’ and mothers’ intakes of fruit juice (r = 0.08, NS) and non-energy-containing beverages (r = 0.13, NS). The girls’ intakes of milk (r = 0.21, P < 0.01) and sweetened beverages (r = 0.37, P < 0.0001), however, were positively associated with their mothers’ intake of those beverages. Similarly, girls who met the AI for calcium had mothers who drank more (P < 0.05) milk. Girls who met the AI for calcium were also served milk more frequently at meals and snacks than were girls who consumed less than the AI (3.7 ± 0.1 compared with 3.2 ± 0.1, P < 0.0001, n = 181; where 1 = never, 3 = sometimes, and 5 = always serve at daughter’s meals and snacks). Maternal milk consumption and the frequency with which milk was made available to the daughters at meals and snacks were positively associated (β = 0.37, P < 0.0001). The finding that the girls’ milk consumption increased with the frequency of serving milk at meals and snacks (P < 0.0001), when the mothers’ milk intakes were controlled for, is illustrated in Figure 2. The girls who were served milk “almost always or always” at meals and snacks drank ≈56% more milk than did the girls who were “sometimes” served milk and almost double the amount consumed by the girls who were “never or rarely” served milk. The relative importance of maternal milk intake and the availability of milk to the daughters at meals and snacks were evaluated as predictors of the girls’ milk intakes from ages 5 to 9 y with the use of multiple regression. Only milk serving frequency predicted the girls’ intakes (β = 0.57, P < 0.0001), which indicated that the frequency with which milk was served at meals and snacks statistically mediated the relation between the mothers’ and daughters’ milk intakes.
FIGURE 2.
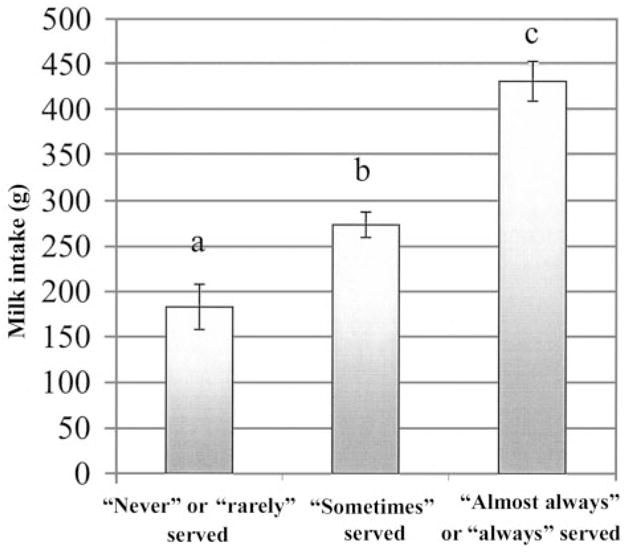
Mean (± SEM) milk intakes of the girls from ages 5 to 9 y and the frequency at which the girls were served milk at meals and snacks. n = 181. Means with different letters are significantly different (P < 0.05) after the mothers’ milk intakes were controlled for.
DISCUSSION
This prospective analysis was conducted to evaluate the role of mother-daughter beverage choices in meeting calcium recommendations during middle childhood in a sample of non-Hispanic white girls. This research showed that the girls’ calcium intakes from ages 5 to 9 y reflected the relative proportions of milk and sweetened beverages in their diets. Furthermore, the girls who met calcium recommendations were served milk more frequently than were the girls who failed to meet calcium recommendations and had mothers who drank more milk than did the mothers of girls who did not meet calcium recommendations. Milk availability to the daughters at meals and snacks appeared to explain the mother-daughter similarities in milk intake. Calcium intake from age 5 to 9 y predicted bone mineral status at age 9 y, which is evidence that maternal influences on daughters’ beverage choices are relevant to the girls’ bone health. Although cross-sectional data have shown parent-child similarities in intakes of calcium and calcium-containing foods (23, 33–35), the findings of the current longitudinal research showed that mother-daughter similarities in beverage choices were pervasive across middle childhood and appeared to influence the girls’ calcium intakes during that period in a manner that is associated with bone mineral status. These longitudinal influences are particularly noteworthy given that the girls’ milk intakes exerted considerable leverage on the ability to meet current recommendations for calcium intake.
In the current study, girls who met calcium recommendations from ages 5 to 9 y showed markedly different patterns of milk and sweetened beverage intakes than did those girls who consumed less calcium than recommended. Furthermore, the patterns of sweetened beverage and milk intakes that distinguished girls who met and did not meet calcium recommendations did not vary appreciably with time, which suggested that such patterns are maintained throughout middle childhood from the end of the preschool period. The girls who met calcium recommendations consumed, on average, 395 mL/d (13 fl oz/d) of milk, which represented 50% of all beverages consumed and roughly 50% of their total daily calcium intakes. This intake is ≈158 mL (5 oz or ⅔ cups) greater than the mean milk intake for 6 –11-y-old children reported in the Continuing Survey of Food Intakes by Individuals 1994 –1996 (15). The girls who met the recommendations also showed modest increases in milk intake from ages 5 to 9 y, whereas those girls who consumed less than the AI for calcium showed decreases in milk intake during the same period. Furthermore, this research found that the girls who met the AI had sweetened beverage intakes that were < 355 mL/d (12 oz/d) from ages 5 to 9 y and were 18% lower than those of girls who consumed less than the AI. Data from the third National Health and Nutrition Examination Survey (1988 –1994) indicate that children aged 6 –11 y consume 6.8% of total daily energy from soft drinks and fruit drinks (36). In the current study, girls who met the AI consumed a similar percentage of total daily energy from sweetened beverages (6.6% of total energy intake). In contrast, sweetened beverages represented 9% of the total daily energy intake and 50% of all beverages consumed by girls who failed to meet calcium recommendations from ages 5 to 9 y, with a mean intake > 355 mL/d (12 fl oz/d). Sweetened beverages may contribute energy (8, 36) to the diet but offer little other nutritional value relative to 100% fruit juice and milk (17, 37) and have been associated with an increased risk of obesity in childhood (38). Soft drink consumption, in particular, has been associated with low micronutrient intakes, including calcium, in children and adolescents (16, 18, 39). Harnack et al (18) observed that 6–12-y-old children who were nonconsumers of soft drinks had calcium intakes that were 26% higher than those of children who consumed > 266 mL/d (9 fl oz/d).
Our findings corroborate the well-documented negative relation between milk and sweetened beverages in the diets of children and adolescents (13, 16–19, 40, 41). Whether sweetened beverages displace milk from the children’s diets or reflect the choices of low-milk consumers has been debated. The prospective nature of this investigation offers the advantage over previous cross-sectional analyses on this topic of evaluating individual changes in beverage intakes over time. In the current study, girls who consumed less than the recommended amounts of calcium showed decreases in milk and increases in sweetened beverage intakes. Similarly, higher average intakes of sweetened beverages from 5 to 9 y were associated with greater decreases in milk intake from 5 to 9 y, although the size of this relation was modest. The strongest evidence of the displacement of milk by sweetened beverages in the young girls’ diets would be provided by showing that the increases in the girls’ soft drink consumption were closely associated with decreases in milk intake over the same period of time.
Changes in the girls’ milk intake from ages 5 to 9 y were not associated with changes in their consumption of sweetened beverages over the same period. Thus, these prospective data do not provide strong evidence that sweetened beverages displace milk in young girls’ diets. Rather, the findings indicate that milk intake during middle childhood decreases in avid consumers of sweetened beverages. Unfortunately, inferences regarding the causality of this relation are difficult to draw without data on the girls’ relative preferences for and exposure to both beverages. Such data are critical for understanding the extent to which reducing the availability of sweetened beverages to young girls might positively influence milk intake in those who tend to consume large amounts of sweetened beverages.
The findings of this research provide new evidence that the availability of milk at meals and snacks has a strong positive influence on the milk intake of young girls. The mothers and daughters had similar patterns of sweetened beverage and milk intakes. The mothers’ sweetened beverage intake, however, did not differentiate girls who met the recommendations from those who did not. Rather, the mothers’ milk intake was positively associated with the daughters’ milk intake and was higher in girls who met the AI than in girls who consumed less than the AI. Previous studies have reported mother-daughter similarities in the amount (21, 23) and type (22) of milk consumed during childhood and in lifetime milk intakes (35). In the current study, the mother-daughter similarity in milk intake was statistically mediated by the extent to which the mothers made milk available to their daughters at meals and snacks. This finding suggests that maternal milk consumption drives the extent to which milk is made available to daughters, and that milk availability at meals and snacks is the main influence on the milk intake of young girls. This interpretation is qualified by the fact that the questions asked did not assess who served the milk to the daughters but rather only how often milk was served. Because most of the mothers in this sample reported being responsible for the feeding of their children (91% reported being responsible for child feeding “most of the time” or “always”), maternal reports of milk-serving practices likely reflect maternal behavior. An influence of availability on intake is consistent with the findings of studies that showed a positive association of children’s fruit, juice, and vegetable intakes with the availability of those foods in the home and at neighborhood food establishments (42–44). Taken together, these findings suggest that making milk and other calcium-rich foods routinely available to young girls is necessary to ensure that such foods are consumed in the amounts needed to achieve adequate calcium intakes.
Diets low in milk are associated with low calcium intakes and low bone mineralization in children (45). In the current study, the mean calcium intake from ages 5 to 9 y was positively related to BMD at age 9 y and showed a weak positive association with BMC. This finding is consistent with many different lines of evidence that suggest a positive role of dietary calcium intake in bone health. These findings are consistent with those of many other studies that showed positive associations between spontaneous calcium intake and bone mineral status in girls (46–50). Additionally, whereas the positive effects of supplemental calcium on bone mineral status are not retained after supplementation ends (51–53), the benefits of milk-derived calcium supplements persist 3–5 y after discontinuation in prepubertal girls (54). Finally, milk intake during childhood and adolescence was associated with higher bone density and lower fracture risk in adulthood (10, 55), even when statistically adjusted for current milk and calcium intakes (56).
In addition to its role in bone health, there is growing interest in the potential role of calcium in weight maintenance (57–60). In the current study, the girls who met the calcium recommendations had higher energy intakes, but not higher BMI z scores, than did the girls who failed to meet the recommendations. The extent to which these findings might reflect a weight-sparing effect of calcium, however, is beyond the substantive focus of this investigation and is not adequately addressed by the data presented herein.
This investigation described beverage patterns associated with meeting calcium recommendations in a sample of prepubertal, non-lactose-intolerant, non-Hispanic white girls. This population is considered to be at risk of developing osteoporosis in adulthood on the basis of ethnicity and sex. The extent to which these findings generalize to other racial or ethnic groups or to boys is unclear; for instance, black children tend to consume less milk and less carbonated soda but more fruit drinks than do non-Hispanic white children (61). An important research need is to identify dietary patterns that contribute to adequate calcium intake in girls with low milk and dairy intakes, including those with perceived or actual intolerances to lactose and those who dislike milk and dairy products. The influence of season on dietary intake may also qualify the generalizability of the results reported herein. Dietary data were collected primarily in the summer and early fall. The data for the adolescents showed that milk intake is lower in summer, whereas that of fruit juice and soft drinks is higher than in other seasons (16). Despite this potential limitation, however, the mean milk intake in the current sample was similar to that reported for 6–11-y-old female participants in the Continuing Survey of Food Intakes by Individuals 1994–1996. Also, the contribution of all beverages to total energy in this sample is similar to the 20% of total daily energy intake for 6–11-y-old participants in the third National Health and Nutrition Examination Survey (36).
These longitudinal data provide new evidence that young girls’ calcium intakes and their consequent relation to bone mineral status reflect mother-daughter beverage intake patterns that are established well before the rapid growth and bone mineralization seen in adolescence. In the current study, greater sweetened beverage intakes, lower milk intakes, and greater decreases in milk intakes over the 5-y period clearly differentiated girls who did not meet calcium recommendations from those who did meet recommendations. Perhaps most noteworthy is that these distinguishing beverage intake profiles appeared to vary little from ages 5 to 9 y, which indicates that such patterns may be in place by the end of the preschool period. Furthermore, we found that the frequency with which milk was made available at meals and snacks was a strong predictor of the girls’ milk intakes. As a result, the single relatively simple routine of serving milk may have a central role in supporting habits that promote adequate calcium during periods of bone growth and bone mineral accrual. The current study and others (62, 63) show that calcium intake tracks moderately across childhood. For this reason, prevention efforts aimed at optimizing girls’ calcium intakes need to begin in the preschool years and focus on ways to increase routine exposure to calcium-rich foods. Future research should expand these findings to consider the role of beverage availability in settings outside the home (eg, daycare centers and school) and the social influences of peers and caregivers within these settings.
Acknowledgments
All authors contributed to the conceptual approach, statistical analyses, interpretation of the results, and manuscript preparation. MLM, DCM, and JOF participated in data collection. None of the authors had any financial or personal interest in organizations sponsoring this research.
Footnotes
Supported in part by NIH grant RO1 HD32973, The National Dairy Council, the General Clinical Research Center NIH grant M01 RR10732, and the Nutrition Coordinating Center of The Pennsylvania State University, University Park.
References
- 1.Heaney RP, Matkovic V. Inadequate peak bone mass. In: Riggs BL, Melton LJI, editors. Osteoporosis: etiology, diagnosis, and management. Philadelphia: Lippincott-Raven Publishers; 1995. pp. 115–31. [Google Scholar]
- 2.Matkovic V, Ilich JZ. Calcium requirements for growth: are current recommendations adequate? Nutr Rev. 1993;51:171– 80. doi: 10.1111/j.1753-4887.1993.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 3.Weaver CM. The growing years and prevention of osteoporosis in later life. Proc Nutr Soc. 2000;59:303– 6. doi: 10.1017/s0029665100000331. [DOI] [PubMed] [Google Scholar]
- 4.Alaimo K, McDowell MA, Briefel RR, et al. Dietary intake of vitamins, minerals, and fiber of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1988–91. Adv Data. 1994;258:1–28. [PubMed] [Google Scholar]
- 5.US Department of Agriculture, Agricultural Research Service. Food and nutrient intakes, 1994–96, 1998. [accessed 9 September 2003];1999 Internet: http://www.barc.usda.gov/bhnrc/foodsurvey/products.html.
- 6.Albertson A, Tobelmann R, Engstrom A. Nutrient intakes of 2- to 10-year-old American children: 10-year trends. J Am Diet Assoc. 1992;12:1492– 6. [PubMed] [Google Scholar]
- 7.Fleming KH, Heimbach JT. Consumption of calcium in the U. S.: food sources and intake levels. J Nutr. 1994;124:1426S–30S. doi: 10.1093/jn/124.suppl_8.1426S. [DOI] [PubMed] [Google Scholar]
- 8.Subar AF, Krebs-Smith SM, Cook A, Kahle LL. Dietary sources of nutrients among US children, 1989–1991. Pediatrics. 1998;102:913–23. doi: 10.1542/peds.102.4.913. [DOI] [PubMed] [Google Scholar]
- 9.Sandler RB, Slemenda CW, LaPorte RE, et al. Postmenopausal bone density and milk consumption in childhood and adolescence. Am J Clin Nutr. 1985;42:270– 4. doi: 10.1093/ajcn/42.2.270. [DOI] [PubMed] [Google Scholar]
- 10.Soroko S, Holbrook TL, Edelstein S, Barrett-Connor E. Lifetime milk consumption and bone mineral density in older women. Am J Public Health. 1994;84:1319–22. doi: 10.2105/ajph.84.8.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teegarden D, Lyle RM, Proulx WR, Johnston CC, Weaver CM. Previous milk consumption is associated with greater bone density in young women. Am J Clin Nutr. 1999;69:1014–7. doi: 10.1093/ajcn/69.5.1014. [DOI] [PubMed] [Google Scholar]
- 12.Welten DC, Kemper HC, Post GB, Van Staveren WA, Twisk JW. Longitudinal development and tracking of calcium and dairy intake from teenager to adult. Eur J Clin Nutr. 1997;51:612– 8. doi: 10.1038/sj.ejcn.1600454. [DOI] [PubMed] [Google Scholar]
- 13.Bowman SA. Beverage choices of young females: changes and impact on nutrient intakes. J Am Diet Assoc. 2002;102:1234–9. doi: 10.1016/s0002-8223(02)90273-7. [DOI] [PubMed] [Google Scholar]
- 14.Rampersaud GC, Bailey LB, Kauwell GP. National survey beverage consumption data for children and adolescents indicate the need to encourage a shift toward more nutritive beverages. J Am Diet Assoc. 2003;103:97–100. doi: 10.1053/jada.2003.50006. [DOI] [PubMed] [Google Scholar]
- 15.Smiciklas-Wright H, Mitchell D, Mickle S, Cook A, Goldman J. Foods commonly eaten in the United States: quantities consumed per eating occasion and in a day, 1994–96. Beltsville, MD: US Department of Agriculture, Agricultural Research Service; 2002. p. 252. [Google Scholar]
- 16.Guenther PM. Beverages in the diets of American teenagers. J Am Diet Assoc. 1986;86:493–9. [PubMed] [Google Scholar]
- 17.Guthrie JF, Morton JF. Food sources of added sweeteners in the diets of Americans. J Am Diet Assoc. 2000;100:43–51. doi: 10.1016/S0002-8223(00)00018-3. (quiz 49–50) [DOI] [PubMed] [Google Scholar]
- 18.Harnack L, Stang J, Story M. Soft drink consumption among US children and adolescents: nutritional consequences. J Am Diet Assoc. 1999;99:436– 41. doi: 10.1016/S0002-8223(99)00106-6. [DOI] [PubMed] [Google Scholar]
- 19.Yen ST, Lin B. Beverage consumption among US children and adolescents: full-information and quasi maximum-likelihood estimation of a censored system. Eur Rev Agric Econ. 2002;29:85–103. [Google Scholar]
- 20.Forshee RA, Storey ML. The role of added sugars in the diet quality of children and adolescents. J Am Coll Nutr. 2001;20:32– 43. doi: 10.1080/07315724.2001.10719012. [DOI] [PubMed] [Google Scholar]
- 21.Fisher J, Mitchell D, Smiciklas-Wright H, Birch L. Maternal milk consumption predicts the tradeoff between milk and soft drinks in young girls’ diets. J Nutr. 2001;131:246–50. doi: 10.1093/jn/131.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennison BA, Erb TA, Jenkins PL. Predictors of dietary milk fat intake by preschool children. Prev Med. 2001;33:536– 42. doi: 10.1006/pmed.2001.0939. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RK, Panely CV, Wang MQ. Associations between the milk mothers drink and the milk consumed by their school-aged children. Fam Econ Nutr Rev. 2001;13:27–36. [Google Scholar]
- 24.Kuczmarski RJ, Ogden C, Grummer-Strawn LM, et al. CDC growth charts: United States. Hyattsville, MD: National Center for Health Statistics; 2000. [Google Scholar]
- 25.Centers for Disease Control and Prevention. Use and interpretation of the CDC growth charts. [accessed 19 August 2003];2000 Internet: http://www.cdc.gov/nccdphp/dnpa/growthcharts/training.htm.
- 26.Institute of Medicine, National Academy of Sciences. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Washington, DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- 27.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 28.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Algina J, Keselman HJ. Detecting repeated measures effects with univariate and multivariate statistics. Psychol Methods. 1997;2:208–18. [Google Scholar]
- 30.Boot AM, de Ridder MA, Pols HA, Krenning EP, de Muinck Keizer-Schrama SM. Bone mineral density in children and adolescents: relation to puberty, calcium intake, and physical activity. J Clin Endocrinol Metab. 1997;82:57– 62. doi: 10.1210/jcem.82.1.3665. [DOI] [PubMed] [Google Scholar]
- 31.Abrams SA, Stuff JE. Calcium metabolism in girls: current dietary intakes lead to low rates of calcium absorption and retention during puberty. Am J Clin Nutr. 1994;60:739– 43. doi: 10.1093/ajcn/60.5.739. [DOI] [PubMed] [Google Scholar]
- 32.Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients) Washington, DC: Institute of Medicine; 2002. [DOI] [PubMed] [Google Scholar]
- 33.Oliveria SA, Ellison RC, Moore LL, Gillman MW, Garrahie EJ, Singer MR. Parent-child relationships in nutrient intake: the Framingham Children’s study. Am J Clin Nutr. 1992;56:593– 8. doi: 10.1093/ajcn/56.3.593. [DOI] [PubMed] [Google Scholar]
- 34.Picard D, Imbach A, Couturier M, Lepage R, Picard M. Familial resemblance of bone mineral density between females 18 years and older and their mothers. Can J Public Health. 2001;92:353– 8. doi: 10.1007/BF03404978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulrich CM, Georgiou CC, Snow-Harter CM, Gillis DE. Bone mineral density in mother-daughter pairs: relations to lifetime exercise, lifetime milk consumption, and calcium supplements. Am J Clin Nutr. 1996;63:72–9. doi: 10.1093/ajcn/63.1.72. [DOI] [PubMed] [Google Scholar]
- 36.Troiano RP, Briefel RR, Carroll MD, Bialostosky K. Energy and fat intakes of children and adolescents in the United States: data from the National Health and Nutrition Examination Surveys. Am J Clin Nutr. 2000;72(suppl):1343S–53S. doi: 10.1093/ajcn/72.5.1343s. [DOI] [PubMed] [Google Scholar]
- 37.Johnson RK, Frary C. Choose beverages and foods to moderate your intake of sugars: the 2000 dietary guidelines for Americans—what’s all the fuss about? J Nutr. 2001;131:2766S–71S. doi: 10.1093/jn/131.10.2766S. [DOI] [PubMed] [Google Scholar]
- 38.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505– 8. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 39.Ballew C, Kuester S, Gillespie K. Beverage choices affect adequacy of children’s nutrient intakes. Arch Pediatr Adolesc Med. 2000;154:1148–52. doi: 10.1001/archpedi.154.11.1148. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Artalejo F, Garcia EL, Gorgojo L, et al. Consumption of bakery products, sweetened soft drinks and yogurt among children aged 6–7 years: association with nutrient intake and overall diet quality. Br J Nutr. 2003;89:419–29. doi: 10.1079/BJN2002787. [DOI] [PubMed] [Google Scholar]
- 41.Whiting SJ, Healey A, Psiuk S, Mirwald R, Kowalski K, Bailey DA. Relationship between carbonated and other low nutrient dense beverages and bone mineral content of adolescents. Nutr Res. 2001;21:1107–15. [Google Scholar]
- 42.Baranowski T, Domel S, Gould R, et al. Increasing fruit and vegetable consumption among 4th and 5th grade students: results from focus groups using reciprocal determinism. J Nutr Educ. 1993;25:114–20. [Google Scholar]
- 43.Edmonds J, Baranowski T, Baranowski J, Cullen K, Myres D. Ecological and socioeconomic correlates of fruit, juice, and vegetable consumption among African-American boys. Prev Med. 2001;32:476– 81. doi: 10.1006/pmed.2001.0831. [DOI] [PubMed] [Google Scholar]
- 44.Hearn M, Baranowski T, Baranowski J, et al. Environmental influences on dietary behavior among children: availability and accessibility of fruits and vegetables enable consumption. J Health Educ. 1998;29:26–32. [Google Scholar]
- 45.Black RE, Williams SM, Jones IE, Goulding A. Children who avoid drinking cow milk have low dietary calcium intakes and poor bone health. Am J Clin Nutr. 2002;76:675– 80. doi: 10.1093/ajcn/76.3.675. [DOI] [PubMed] [Google Scholar]
- 46.Barr SI, Petit MA, Vigna YM, Prior JC. Eating attitudes and habitual calcium intake in peripubertal girls are associated with initial bone mineral content and its change over 2 years. J Bone Miner Res. 2001;16:940–7. doi: 10.1359/jbmr.2001.16.5.940. [DOI] [PubMed] [Google Scholar]
- 47.Chan GM. Dietary calcium and bone mineral status of children and adolescents. Am J Dis Child. 1991;145:631– 4. doi: 10.1001/archpedi.1991.02160060049019. [DOI] [PubMed] [Google Scholar]
- 48.Molgaard C, Thomsen BL, Michaelsen KF. The influence of calcium intake and physical activity on bone mineral content and bone size in healthy children and adolescents. Osteoporos Int. 2001;12:887–94. doi: 10.1007/s001980170042. [DOI] [PubMed] [Google Scholar]
- 49.Sentipal JM, Wardlaw GM, Mahan J, Matkovic V. Influence of calcium intake and growth indexes on vertebral bone mineral density in young females. Am J Clin Nutr. 1991;54:425– 8. doi: 10.1093/ajcn/54.2.425. [DOI] [PubMed] [Google Scholar]
- 50.Ruiz JC, Mandel C, Garabedian M. Influence of spontaneous calcium intake and physical exercise on the vertebral and femoral bone mineral density of children and adolescents. J Bone Miner Res. 1995;10:675– 82. doi: 10.1002/jbmr.5650100502. [DOI] [PubMed] [Google Scholar]
- 51.Fehily AM, Coles RJ, Evans WD, Elwood PC. Factors affecting bone density in young adults. Am J Clin Nutr. 1992;56:579– 86. doi: 10.1093/ajcn/56.3.579. [DOI] [PubMed] [Google Scholar]
- 52.Lee WT, Leung SS, Leung DM, Cheng JC. A follow-up study on the effects of calcium-supplement withdrawal and puberty on bone acquisition of children. Am J Clin Nutr. 1996;64:71–7. doi: 10.1093/ajcn/64.1.71. [DOI] [PubMed] [Google Scholar]
- 53.Slemenda CW, Peacock M, Hui S, Zhou L, Johnston CC. Reduced rates of skeletal remodeling are associated with increased bone mineral density during the development of peak skeletal mass. J Bone Miner Res. 1997;12:676– 82. doi: 10.1359/jbmr.1997.12.4.676. [DOI] [PubMed] [Google Scholar]
- 54.Bonjour JP, Chevalley T, Ammann P, Slosman D, Rizzoli R. Gain in bone mineral mass in prepubertal girls 3.5 years after discontinuation of calcium supplementation: a follow-up study. Lancet. 2001;358:1208–12. doi: 10.1016/S0140-6736(01)06342-5. [DOI] [PubMed] [Google Scholar]
- 55.Chumlea WC, Guo SS. CERIN Symposium Nutrition et Personnes Agees au-dela des apports recommandes. Paris: CERIN; 1997. Milk consumption in childhood and bone mineral in adulthood: the Fels Longitudinal Study Proceedings; pp. 125–34. [Google Scholar]
- 56.Kalkwarf HJ, Khoury JC, Lanphear BP. Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in US women. Am J Clin Nutr. 2003;77:257– 65. doi: 10.1093/ajcn/77.1.257. [DOI] [PubMed] [Google Scholar]
- 57.Heaney RP, Davies KM, Barger-Lux MJ. Calcium and weight: clinical studies. J Am Coll Nutr. 2002;21:152S–5S. doi: 10.1080/07315724.2002.10719213. [DOI] [PubMed] [Google Scholar]
- 58.Teegarden D. Calcium intake and reduction in weight or fat mass. J Nutr. 2003;133:249S–51S. doi: 10.1093/jn/133.1.249S. [DOI] [PubMed] [Google Scholar]
- 59.Parikh SJ, Yanovski JA. Calcium intake and adiposity. Am J Clin Nutr. 2003;77:281–7. doi: 10.1093/ajcn/77.2.281. [DOI] [PubMed] [Google Scholar]
- 60.Zemel MB. Regulation of adiposity and obesity risk by dietary calcium: mechanisms and implications. J Am Coll Nutr. 2002;21(suppl):146S–51S. doi: 10.1080/07315724.2002.10719212. [DOI] [PubMed] [Google Scholar]
- 61.Forshee RA, Storey ML. Total beverage consumption and beverage choices among young children and adolescents. Int J Food Sci Nutr. 2003;54:297–307. doi: 10.1080/09637480120092143. [DOI] [PubMed] [Google Scholar]
- 62.Lee WT, Leung SS, Lui SS, Lau J. Relationship between long-term calcium intake and bone mineral content of children aged from birth to 5 years. Br J Nutr. 1992;70:235– 48. doi: 10.1079/bjn19930120. [DOI] [PubMed] [Google Scholar]
- 63.Singer MR, Moore LL, Garahie EJ, Ellison RC. The tracking of nutrient intake in young children: The Framingham Children’s Study. Am J Public Health. 1995;85:1673–7. doi: 10.2105/ajph.85.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]


