Abstract
OBJECTIVE
To determine whether obesigenic families can be identified based on mothers’ and fathers’ dietary and activity patterns.
METHODS
A total of 197 girls and their parents were assessed when girls were 5 y old; 192 families were reassessed when girls were 7 y old. Measures of parents’ physical activity and dietary intake were obtained and entered into a cluster analysis to assess whether distinct family clusters could be identified. Girls’ skinfold thickness and body mass index (BMI) were also assessed and were used to examine the predictive validity of the clusters.
RESULTS
Obesigenic and a non-obesigenic family clusters were identified. Mothers and fathers in the obesigenic cluster reported high levels of dietary intake and low levels of physical activity, while mothers and fathers in the non-obesigenic cluster reported low levels of dietary intake and high levels of activity. Girls from families in the obesigenic cluster had significantly higher BMI and skinfold thickness values at age 7 and showed significantly greater increases in BMI and skinfold thickness from ages 5 to 7 y than girls from non-obesigenic families; differences were reduced but not eliminated after controlling for parents’ BMI.
CONCLUSIONS
Obesigenic families, defined in terms of parents’ activity and dietary patterns, can be used predict children’s risk of obesity.
Keywords: children, overweight, parents, family, obesigenic, environment
Introduction
Familial patterns in weight status are well established;1–4 overweight parents tend to have overweight children. This pattern is not surprising when you consider that, in addition to genetic resemblances, family members show similarities in behavioral risk factors associated with overweight including energy and percent fat intake,5–8 food preferences9 and physical activity.10–16 Furthermore, recent research has identified correlations within families across these factors. We recently found that relative to mothers with lower percentage fat intake, mothers with higher fat intake had higher body mass index (BMI) values, were less active, enjoyed activity less, had daughters with higher BMI values and higher percentage fat intake, and had husbands who were also less active and reported higher fat intake.17 A similar pattern of findings was reported in a recent study using an Australian cohort.18 While research indicates that risk factors for overweight tend to co-occur within the family, this familial pattern has been identified and presented in a very piecemeal fashion, generally in the form of numerous correlation or regression analyses. It appears, however, that there is one clear pattern: certain families display a configuration of dietary and activity patterns that are likely to promote the development of obesity among family members, particularly children, beyond that explained by genetic susceptibility. The goal of this study is to confirm this idea using a single summary analysis, namely cluster analysis.
Genetic contribution to overweight is substantial. Behavioral genetic studies illustrate that genetic factors explain at least 50% of the population variance in obesity among adults;19 a similar figure has been noted among pediatric populations.20–22 Research also points to the importance of environmental or behavioral factors, including percentage fat intake and physical activity, in explaining variability in adiposity or weight status among children.23 While these behavioral characteristics may, to some extent, have genetic origins, they have generally been interpreted as evidence of environmental effects. In the search for causes of overweight and obesity, less attention has been directed toward the possibility that genes and environment are likely to be highly correlated. That is, individuals who are overweight, or individuals with a propensity for accelerated weight gain, may select or create environments that promote the development of overweight (by for example overeating and exercising infrequently), thereby making genetic and environmental characteristics largely inseparable.24 For children growing up in the context of the family, the presence of such a gene – environment correlation markedly increases their risk of overweight because they may inherit a genetic susceptibility for weight gain from their parents and they are raised in an environment that promotes the development of overweight. A gene – environment interaction is also likely to operate in such a situation, as children of overweight parents are likely to be genetically more susceptible to the adverse effects of this environment than children of non-overweight parents.24
With this in mind, this study was designed to test the following ideas: (1) parents may create an environment that places their children at risk of overweight; (2) parents who create such an environment are likely to be overweight; and (3) children in these families are likely to show higher levels of adiposity or accelerated trajectories of weight gain across time. These ideas will be assessed using cluster analysis with a longitudinal sample of mothers and fathers and their young daughters. Specifically, cluster analysis will be used to determine whether distinct obesigenic and non-obesigenic family clusters can be identified based on measures of mothers’ and fathers’ dietary and activity practices. The predictive validity of the resulting clusters will be determined by assessing differences in parents’ BMI and girls’ BMI and skinfold thickness, and change in girls’ BMI and skinfold thickness between ages 5 and 7 y.
Methods
Participants
Data were collected from 197 girls and their parents when girls were 5 y old; 192 families were reassessed 2 y later when girls were 7 y old. Families were recruited for participation in a longitudinal study of the ‘health and development of young girls from age 5 to 9 y’ using flyers and newspaper advertisements. In addition, families with age-eligible female children within a five-county radius received letters inviting them to participate in the study. Eligibility criteria for girls’ (and parents’) enrollment in the project included living with both biological parents, the absence of severe food allergies or chronic medical problems affecting food intake, and the absence of dietary restrictions involving animal products; families were not recruited with regard to weight status. All families participating were non-Hispanic white and lived in Pennsylvania. The longitudinal project has been reviewed and approved by the Institutional Review Board of the associated university.
Procedures
Parents visited the laboratory and completed a series of questionnaires assessing their physical activity and dietary patterns. Activity and dietary variables will be referred to as the clustering variables because they were used as the basis of the cluster analysis. The clustering variables were assessed when girls were 5 y old with the exception of parents’ sport participation, which was assessed when girls were 7. In addition, at each time of assessment, trained staff measured girls’, mothers’ and fathers’ height and weight and girls’ tricep and subscapular skinfold thickness. These variables will be referred to as criterion variables because they were used to validate the results of the cluster analysis.
Clustering variables
Parents’ activity patterns
Research shows that physical activity is multi-dimensional, with any one measure providing only a limited indication of an individual’s activity patterns. In addition, it is acknowledged that it is difficult to obtain a reliable and valid measure of physical activity.25,26 Therefore, three measures of parents’ physical activity were collected including (1) sport participation, (2) weekly exercise frequency, and (3) enjoyment of sport and activity. Parents’ sport participation was assessed using an activity checklist. Parents were provided with a list of 22 sports (eg aerobics, golf, hiking, swimming and tennis) and asked to indicate how frequently they participated in each sport from 1 = never to 5 = always. A total frequency score (ranging from 22 to 110) was obtained by summing across each of the sports. Parents’ exercise frequency was assessed using the question ‘how many days a week do you exercise?’ Due to a substantial positive skew in the data, with most parents reporting low levels of exercise, responses were categorized into three groups: low activity = never exercise or participate in sports; medium activity = participate in sports or exercise 1 – 3 days a week; high activity = participate in sports or exercise more than 3 days a week. Finally, parents indicated how well the statement ‘I exercise for fun’ described them using a three-point response option (1 = does not describe me; 2 = sort of describes me; 3 = really describes me). Enjoyment of activity was included because it seems reasonable that parents who enjoy exercising are more likely to be active when faced with everyday obstacles to activity (including a lack of time) and are more likely to include their children in physical activities.
Parents’ dietary patterns
Similarly, three measures of parents’ dietary intake patterns were obtained including (1) total energy intake adjusted for body weight, (2) percentage of energy as fat, (3) and dietary disinhibition, or eating in an out-of-control manner in the absence of hunger. Total energy intake and percentage fat intake were assessed using a food frequency questionnaire (FFQ) developed by Kristal et al.27 Parents were asked to indicate the frequency with which they had consumed each food in the past 3 months and the approximate serving size. Previous research supports the reliability and validity of the FFQ used in this study.28 Mothers’ and fathers’ energy intakes were adjusted for body weight by regressing weight in kilograms onto energy intake and saving the residuals; the residuals were used in all analyses. The residuals were normally distributed with a mean of 0, thus satisfying criteria outlined by Allison et al.29 Parents’ dietary disinhibition was assessed using the mean of the emotional and external disinhibition subscales of the Dutch Eating Behavior Questionnaire.30 Previous research illustrates the reliability and validity of this scale.31,32 In this study the internal consistency co-efficient was 0.95 for mothers and 0.93 for fathers for total disinhibition.
Criterion variables
Girls’ and parents’ BMI
Girls’ and parents’ BMI (weight (kg)/height (m)2) was calculated using the average of three height and weight measurements obtained at each time of assessment. Girls were classified as overweight (BMI ≥ 17.2 at age 5 and BMI ≥ 18.0 at age 7) or obese (BMI ≥ 19.3 at age 5 and BMI ≥ 21.0 at age 7) based on recent age- and sex-specific definitions of overweight.33 In addition, girls BMI values were converted to BMI percentiles using the CDC 2000 growth charts.34 Parents were classified as overweight and obese based on BMI cut-offs of 25 and 30 respectively.35
Girls’ skinfold thickness
Girls’ subcutaneous body fat was assessed using the sum of tricep and subscapular skinfold measurements. All skinfold measurements were taken by a trained staff member according to procedures described by Harrison et al.36 Skinfold measurements were taken in duplicate on the right side of the body to the nearest tenth of a millimeter using Harpenden skinfold calipers (Crymych, UK). The intraclass correlation co-efficients at ages 5 and 7, respectively were 0.90 and 0.99 for subscapular skinfold and 0.97 and 0.97 for tricep skinfold. The inter-rater correlation was 0.99 for subscapular skinfold and 0.99 for triceps skin-fold when girls were 7 y old. No inter-rater data were available at age 5 as only one person collected skinfold measurements.
Statistical analyses
The three measures of activity were combined to form a composite activity score for each parent using principal components analysis (mean = 0, s.d. = 1). The three measures of dietary patterns were combined to create an intake composite score for each parent in a similar manner. Cluster analysis was then used to identify blocks or clusters of families who showed similar activity and dietary patterns among parents. Cluster analysis is an exploratory multivariate procedure that assesses whether cases (or in this instance families) can be grouped together based on similarity in a number of pertinent characteristics. The basic premise of cluster analysis is that differences among individuals can be used as a basis for understanding process, that is, ‘pattern represents process’.37 Using cluster analysis, we sought to identify possible processes by which children in particular families are at heightened risk of the development of overweight.
Using the PROC FASTCLUS procedure in SAS version 6.12 (Cary, NC, USA), a K-means cluster analysis was performed specifying a maximum of two clusters. Clustering occurred at the level of the family (ie not separately for mothers and fathers) and was based on mothers’ and fathers’ activity and dietary principal component scores (ie four variables per each family). The calculation and reiteration process of this program is summarized as follows;38 first, the data were arbitrarily divided into two clusters; second, the means or centroids of the clusters were calculated based on the referent variables of mothers’ and fathers’ dietary and activity practices; third, for a particular family, the distance from each centroid was calculated and if the family was closer to the mean of the other cluster, the family was reclassified; fourth, the centroids were recalculated; and fifth, the next family was then classified. This process continued until all families were placed in the cluster whose centroid they were closest.
Clusters should show distinct and interpretable differences on grouping variables. Therefore, in order to verify the internal consistency of the clustering procedure, the two clusters were compared based on the characteristics by which they were originally classified including the mean component intake and activity scores and the individual activity and dietary variables. We predicted that parents in one cluster (an obesigenic cluster) would have higher intake and lower activity scores than parents in the second cluster (a non-obesigenic cluster).
Finally, the predictive validity of the clusters was assessed by using cluster membership to predict differences on a criterion variable of interest that was not used in the clustering procedure. The criterions of interest in this study were girls’ BMI and skinfold thickness at ages 5 and 7 as well as change in BMI and skinfold thickness. Cluster differences in parents’ BMI when girls were 5 and 7 y of age were also assessed. We predicted that girls from families in the obesigenic cluster would show significantly higher BMI and skin-fold thickness at ages 5 and 7 and significantly greater increases between ages 5 and 7.
Results
Sample characteristics
At time of entry into the study, two thirds of parents reported a level of education higher than a high school diploma, all fathers and two-thirds of mothers were employed, and approximately equal proportions of families reported incomes below $35 000, between $35 000 and $50 000, and above $50 000. Girls’ mean age at each time of assessment was 5.4 (±0.3) and 7.3 (±0.3) y. Girls’ mean height in centimeters was 111.2 (±4.7) and 123.8 (±5.5) and mean weight in kilograms was 19.8 (±3.1) and 25.7 (±5.3) at ages 5 and 7, respectively. Sample means, standard deviations, and ranges for the clustering and criterion variables are reported in Table 1. Girls’ mean BMI values at ages 5 and 7 are similar to population-level patterns for white 5-and 7-year-old girls.39 Based on recent international definitions of overweight and obesity, 16% (age 5) and 19% (age 7) of girls were overweight and 3% (age 5) and 4% (age 7) were obese.33 A strong degree of tracking was noted in girls’ BMI (r = 0.87, P < 0.001) and skinfold thickness (r = 0.85, P < 0.0001) across ages 5 – 7 y. When girls were 5 y old, 54% of mothers and 76% of fathers were overweight. At age 7 these figures were 57% for mothers and 79% for fathers. Parents’ reports of their activity indicated that mothers and fathers exercised on average 3 days per week (see Table 1). Both mothers and fathers reported an average liking for physical activity. Mean energy intake, controlling for body weight (ie intake residualized for body weight), was slightly below zero for mothers and fathers, indicating that parents with higher BMI reported lower intake relative to their body weight. Both parents consumed a mean of 35% of their energy intake as fat. Finally, mothers and fathers reported an average degree of disinhibited eating, although mothers generally reported higher levels of disinhibition than fathers.
Table 1.
Sample characteristics
| Sample range | Mean | s.d. | |
|---|---|---|---|
| Clustering variables | |||
| Activity patterns | |||
| Mothers’ sport participationa | 22 – 62 | 38.8 | 8.2 |
| Fathers’ sport participationa | 24 – 69 | 43.2 | 9.3 |
| Mothers’ weekly frequency of exercise | 0 – 7 | 2.9 | 1.9 |
| Fathers’ weekly frequency of exercise | 0 – 7 | 2.8 | 2.1 |
| Mothers’ enjoyment of activityb | 1 – 3 | 1.7 | 0.7 |
| Fathers’ enjoyment of activityb | 1 – 3 | 1.9 | 0.8 |
| Dietary patterns | |||
| Mothers’ residualized energy intakec | −1433 – (2257) | −5.1 | 668.4 |
| Fathers’ residualized energy intakec | −1349 – (2972) | −12.1 | 710.6 |
| Mothers’ percent intake as fat | 12.6 – 50.6 | 35.5 | 7.3 |
| Fathers’ percent intake as fat | 14.4 – 53.9 | 35.7 | 7.2 |
| Mothers’ disinhibitiond | 1.2 – 4.6 | 2.8 | 0.7 |
| Fathers’ disinhibitiond | 1.3 – 4.1 | 2.3 | 0.6 |
| Criterion variables | |||
| Girls age 5 | |||
| Girls’ BMIe | 13.0 – 25.6 | 15.8 | 1.4 |
| Mothers’ BMI | 17.7 – 56.1 | 26.3 | 5.6 |
| Fathers’ BMI | 18.7 – 42.0 | 28.0 | 4.2 |
| Girls’ skinfold thickness | 10.0 – 45.9 | 17.3 | 5.1 |
| Girls age 7 | |||
| Girls’ BMIe | 12.5 – 32.5 | 16.7 | 2.5 |
| Mothers’ BMI | 18.0 – 53.5 | 26.8 | 6.1 |
| Fathers’ BMI | 19.9 – 41.9 | 28.4 | 4.3 |
| Girls’ skinfold thickness | 10.2 – 49.1 | 18.7 | 7.2 |
Sport participation = sum of (no. of activities×frequency of participation from 1 = never to 5 = always).
Enjoyment = ‘I exercise for fun’ (1 = does not describe me to 3 = really describes me).
Energy intake scores were residualized for weight in kilograms (0 = energy intake as expected for body weight; positive scores = greater than expected energy intake; negative scores = less than expected energy intake). Mean daily energy intake (kcal) unadjusted for body weight was 1802 (±679) for mothers and 2137 (±753) for fathers.
Disinhibition = score on disinhibition subscale of the Dutch Eating Behaviors Questionnaire (range = 1 – 5 with 5 indicating high levels of disinhibition).
Girls’ mean BMI percentile based on the CDC 2000 growth charts was 59.8 (±26.3) and 59.1 (±27.4) at age 7.
Formation of the clusters
The cluster analysis, based on the component activity and intake scores for mothers and fathers, resulted in the formation of two distinct clusters: one cluster showed clear behavioral patterns that promote the development of overweight while the other showed a clear absence of such patterns. Hence, the resulting clusters will be referred to respectively as the obesigenic and the non-obesigenic clusters. Differences in income for the two family clusters were assessed using chi-square analysis and differences in parents’ education and age were assessed using ANOVA. Results showed that families in the obesigenic cluster were significantly less likely to report a family income >$50 000 (28%) in comparison to families in the non-obesigence cluster (43%; χ2 (3192) = 8.33, P <0.05). No differences in parent education or age were noted after taking income into consideration. Therefore, family income was entered as a covariate in all analyses that follow.
As shown in Figure 1, mothers and fathers from the obesigenic cluster reported below-average (ie negative) activity component scores and above-average (ie positive) intake scores, relative to the sample. The opposite pattern was noted for parents in the non-obesigienic cluster; mothers and fathers in this cluster reported above-average activity scores and below-average intake scores. Results from a series of ANCOVAs, controlling for differences in family income, indicated that this pattern was also evident for the individual activity and intake variables (see Table 2). That is, mothers and fathers in the obesigenic cluster reported significantly lower activity scores and significantly higher intake scores across all individual variables than parents in the non-obesigenic cluster. Dietary disinhibition was the only exception. No differences were noted across the clusters in fathers’ disinhibition. A trend, however, was noted among mothers, with mothers from the obesigenic cluster reporting higher levels of disinhibition than mothers from the non-obesigenic cluster. All analyses were rerun, removing disinhibition from the creation of the intake principal component scores. The resulting clusters were not as distinct as those from the original analysis, thus we decided to keep disinhibition in the clustering procedure.
Figure 1.
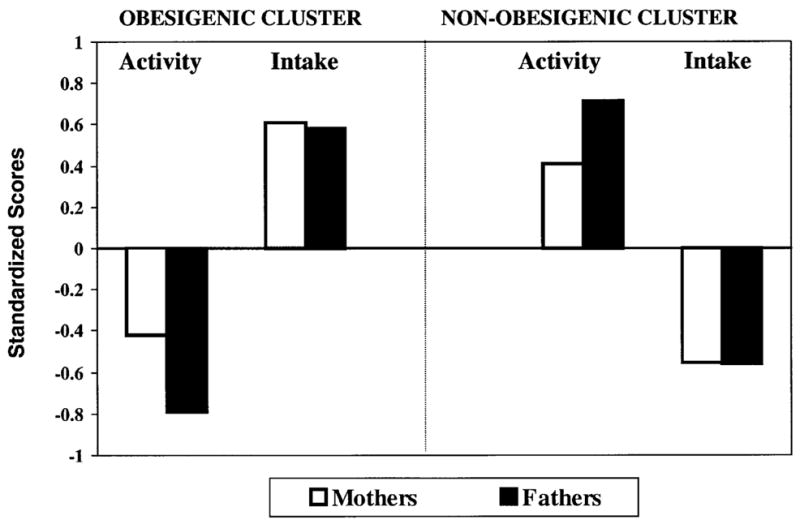
Mean activity and intake component scores for mothers and fathers in the obesigenic and non-obesigenic clusters.
Table 2.
Differences in individual activity and intake variables for mothers and fathers in the obesigenic and non-obesigenic clusters
| Non-obesigenic cluster n = 98 | Obesigenic cluster n = 94 | P-value | |
|---|---|---|---|
| Activity patterns | |||
| Mothers’ sport participationa | 41.1 (8.2) | 36.5 (7.6) | < 0.0001 |
| Fathers’ sport participationa | 46.6 (8.1) | 39.4 (9.0) | < 0.0001 |
| Mothers’ weekly frequency of exercise | 3.5 (1.7) | 2.4 (1.9) | < 0.0001 |
| Fathers’ weekly frequency of exercise | 3.7 (1.8) | 1.9 (2.0) | < 0.0001 |
| Mothers’ enjoyment of activityb | 1.9 (0.8) | 1.6 (0.6) | < 0.01 |
| Fathers’ enjoyment of activityb | 2.3 (0.7) | 1.6 (0.7) | < 0.0001 |
| Dietary patterns | |||
| Mothers’ total energy intakec | −147.4 (566) | 149.6 (736) | < 0.01 |
| Fathers’ total energy intakec | −290.0 (554) | 281.5 (741) | < 0.0001 |
| Mothers’ percent intake as fat | 31.7 (6.7) | 39.6 (5.6) | < 0.0001 |
| Fathers’ percent intake as fat | 32.9 (7.3) | 38.7 (5.9) | < 0.0001 |
| Mothers’ dietary disinhibitiond | 2.7 (0.7) | 2.9 (0.8) | < 0.10 |
| Fathers’ dietary disinhibitiond | 2.4 (0.6) | 2.3 (0.6) | NS |
Sport participation = Sum of (no. of activities×frequency of participation from 1 = never to 5 = always).
Enjoyment = ‘I exercise for fun’ (1 = does not describe me to 3 = really describes me).
Energy intake scores were residualized for weight in kilograms (0 = energy intake as expected for body weight; positive scores = greater than expected energy intake; negative scores = less than expected energy intake). Mean daily energy intake (kcal) unadjusted for body weight was 1633 (±553) and 1971 (±747) for mothers in the non-obesigenic and obesigenic clusters respectively. The corresponding values for fathers were 1860 (±624) and 2411 (±745).
Disinhibition = score on disinhibition subscale of the Dutch Eating Behaviors Questionnaire (range = 1 – 5 with 5 indicating high levels of disinhibition).
Validation of the clusters
The predictive validity of the obesigenic and non-obesigenic family clusters was determined by assessing differences in girls’ and parents’ body composition using ANCOVA controlling for differences in family income (see Table 3). Mothers in the obesigenic cluster had significantly higher BMI scores at both times of assessment and fathers had significantly higher BMI scores when girls were 7 (note that parents’ BMI was not used in the clustering procedure). More importantly, consistent differences were noted between the two clusters in girls’ body composition and change in body composition. Girls from families in the obesigenic cluster showed significantly greater increases in BMI between ages 5 and 7 (after controlling for BMI at age 5) and had significantly higher BMI scores at age 7. A similar pattern was noted for skinfold thickness; girls in the obesigenic cluster showed significantly higher skinfold thickness at ages 5 and 7 and greater increases in skinfold thickness between ages 5 and 7. When comparing the stability of girls’ weight status (ie overweight vs non-overweight) for the two clusters, girls from the obesigenic were more likely to become overweight between ages 5 and 7. That is, of the 14 girls who became overweight between ages 5 and 7, 11 of these girls (or 78%) were from families in the obesigenic cluster (not shown in Table 1).
Table 3.
Cluster validation: assessing cluster differences on mean BMI and skinfold thickness
| Non-obesigenic cluster n = 98 | Obesigenic cluster n = 94 | P-value not controlling for parent BMI | P-value controlling for mothers’ BMI | P-value controlling for fathers’ BMI | |
|---|---|---|---|---|---|
| Parents’ BMI | |||||
| Mothers’ BMI when girls age 5 | 25.1 (4.6) | 27.7 (6.5) | <0.05 | — | — |
| Fathers’ BMI when girls age 5 | 27.5 (3.6) | 28.6 (4.8) | NS | — | — |
| Mothers’ BMI when girls age 7 | 25.5 (4.9) | 28.1 (6.9) | <0.01 | — | — |
| Fathers’ BMI when girls age 7 | 27.7 (3.4) | 29.1 (4.9) | <0.05 | — | — |
| Girls’ BMI and skinfold thickness | |||||
| BMI at age 5a | 15.7 (1.3) | 15.9 (1.4) | NS | NS | NS |
| Skinfold thickness at age 5 | 16.4 (3.9) | 17.7 (5.1) | <0.05 | NS | < 0.05 |
| BMI at age 7b | 16.2 (2.0) | 16.9 (2.3) | <0.05 | <0.10 | < 0.10 |
| Skinfold thickness at age 7 | 17.4 (6.2) | 19.6 (7.3) | <0.05 | <0.05 | < 0.10 |
| Change in BMI from age 5 to 7 | 0.5 (1.0) | 1.0 (1.4) | <0.01 | <0.05 | < 0.05 |
| Change in skinfolds from age 5 to 7 | 0.90 (3.0) | 2.0 (3.7) | <0.10 | <0.10 | < 0.10 |
For all analyses assessing differences in changes scores, the variable at time 1 was entered as a control variable.
The corresponding mean BMI percentiles based on the CDC 2000 growth charts34 are 58.7 and 60.7 for the non-obesigenic and obesigenic clusters, respectively.
The corresponding mean BMI percentiles based on the CDC 2000 growth charts34 are 55.3 and 63.4 for the non-obesigenic and obesigenic clusters, respectively.
As noted above, parents from the obesigenic cluster had significantly higher BMI scores at both points in time. As a result, differences in girls’ body composition may simply reflect a genetic predisposition for weight gain passed on by parents, rather than the obesigenic environment that overweight parents create for their children. The obvious solution of controlling for parents’ BMI is not effective in this situation, however, because it removes both genetic and environmental associations between parents’ and girls’ BMI and controlling for both parents simultaneously is likely to exacerbate this problem. Therefore, as a compromise, differences in girls’ body composition were reassessed first controlling for mothers’ BMI and then controlling for fathers’ BMI (also shown in Table 3). As expected, the results were not as clear when we controlled for parent BMI; however, the general pattern of differences in girls’ BMI and skinfold thickness was still evident. That is, with few exceptions, differences were still significant or were reduced to a trend after controlling for parents’ BMI.
Discussion
The primary goals of this study were to determine whether distinct obesigenic and non-obesigenic family clusters could be identified based on mothers’ and fathers’ activity and dietary patterns, and to assess whether cluster membership could be used to predict differences in their daughters’ body composition. Cluster analysis clearly identified an obesigenic family cluster and a non-obesigenic cluster. Mothers and fathers in the obesigenic cluster reported higher intake scores and lower physical activity scores than mothers and fathers in the non-obesigenic cluster. More importantly, this obesigenic pattern could be used to predict differences in girls’ BMI and skinfold thickness and, to a greater extent, change in BMI and skinfold thickness across from age 5 to 7 y, with girls from families in the obesigenic cluster illustrating an increased risk of the development of overweight. Differences in girls’ body composition were apparent even after controlling for parents’ BMI. Results from this study illustrate the utility of identifying children at risk of overweight by examining parents’ activity and intake patterns and highlight the importance of focusing on the family unit rather than individual family members.
Mothers and fathers showed marked similarity in their intake and activity practices within each cluster and marked dissimilarity across clusters with one cluster showing patterns that promote the development of overweight and the other showing patterns that prevent the development of overweight. Actual differences in activity and intake patterns for families with obesigenic vs non-obesigenic tendencies may be even greater than those identified in this study as research shows that people tend to under-report dietary intake40 and over-report physical activity25 and suggests that this pattern may be more pronounced among overweight and obese persons.41–43 This bias was also present in this study as subjects who were heavier reported lower intakes than would be expected based on their weight (as shown by a negative sample mean value for energy intake residualized for body weight in Table 1). In addition to being less active and having higher dietary intake scores, mothers and fathers in the obesigenic cluster had higher BMI scores at both times of measurement. Thus, couples in each cluster showed remarkable similarities in their weight status, and activity and intake practices. Children from such families are likely to be at heightened risk of overweight due the combination of both a genetic susceptibility for overweight, passed on by parents, and living in an obesity-promoting environment. Indeed, in this study, clear differences in girls’ weight status were noted for girls in each of the family clusters and these differences were consistent with the type of environment that parents created.
In validating the formation of the clusters, the most consistent cluster differences were noted in girls’ change in body composition. That is, girls from families in the obesigenic cluster showed greater increases in BMI and skinfold thickness and were more likely to become overweight between ages 5 and 7 than girls from families in the non-obesigenic cluster. These results suggest that the risk of overweight for children in ‘obesigenic families’ may be increasingly evident with time and support previous research illustrating that children of obese parents, who are likely to create an obesity promoting family environment, tend to be more overweight than children of lean parents and that these differences increase with age.44 Differences identified in girls’ weight status may simply reflect genetic contribution to familial patterns in weight status, as parents in the obesigenic cluster were more overweight than parents in the non-obesigenic cluster. The confounding effect of parents’ weight status in this analysis is difficult to address because controlling for parents’ BMI effectively removes more variance than necessary; that is, it removes genetic and shared environmental contributions to similarity in girls’ and parents’ weight status. Despite this caveat, controlling for parent BMI did not result in the elimination of the cluster differences in girls’ weight status. Differences were, however, attenuated, thereby indicating the importance of both genetic and environmental factors in predicting children’s weight status and change in weight status.
There are a number of limitations to this study. The external validity of the results is limited by the sample characteristics. That is, results may differ for families from different ethnic and educational backgrounds (all subjects in this study were white and were generally well-educated) and for sons in such families. A greater degree of familial clustering in weight status, physical activity and dietary intake may be evidenced among African-American and Hispanic populations, who show elevated levels of overweight and its health co-morbities.45–47 In addition, it is also possible that boys living in families displaying obesigenic tendencies are less susceptible to the negative impact of such an environment because boys are more likely to have greater contact with systems outside the family, such as organized sports, that inhibit excessive weight gain.48 These ideas require additional research. This study is also limited by the use of self-report measures of activity and intake, which are problematic in terms of their reliability and validity.25,26,49 We attempted to overcome this problem by incorporating multiple measures that tap differing dimensions of each construct into a single score. This practice not only simplifies the interpretation of the results, it also prevents any one measure from unduly affecting the results. Finally, this study was not designed to separate the contribution of mothers and fathers to the problem of childhood overweight or to look at the relative contribution of activity and dietary practices; we have presented this information elsewhere.17
Despite these limitations, this study confirms the idea that a distinct set of families showing obesity-promoting characteristics can be identified based on parents’ dietary and activity patterns. The importance of including parents in childhood overweight prevention and treatment program has been previously discussed.50–52 Results from this study further confirm the need to focus intervention efforts at the level of the family as it is unlikely that a child’s elevated weight status, poor eating habits, and low levels of activity are isolated events in the family context, rather they are likely to reflect a general pattern of characteristics noted among parents and possibly siblings. Findings from this study also indicate that characteristics of the family environment explain differences in children’s weight status over and above that explained by genetic susceptibility. Overweight parents may adopt a fatalistic outlook, believing that their children are genetically predisposed to be overweight. Making parents aware of the additional impact of their own activity and intake patterns may promote parental participation in intervention and prevention programs for childhood overweight, thereby increasing their efficacy.
Acknowledgments
This research was supported by the National Institute of Health grant no. RO1 HD 32973.
References
- 1.Garn SM, Sullivan TV, Hawthorne VM. Fatness and obesity of the parents of obese individuals. Am J Clin Nutr. 1989;50:1308–1313. doi: 10.1093/ajcn/50.6.1308. [DOI] [PubMed] [Google Scholar]
- 2.Grilo CM, Pogue-Geile MF. The nature of environmental influences on weight and obesity: a behavior genetics analysis. Psychol Bull. 1991;110:520–537. doi: 10.1037/0033-2909.110.3.520. [DOI] [PubMed] [Google Scholar]
- 3.Maffeis C, Talamini G, Tato L. Influences of diet, physical activity and parents’ obesity on children’s adiposity: a four-year longitudinal study. Int J Obes Relat Metab Disord. 1998;22:758–764. doi: 10.1038/sj.ijo.0800655. [DOI] [PubMed] [Google Scholar]
- 4.Morrison JA, Payne G, Barton BA, Khoury PR, Crawford P. Mother – daughter correlations of obesity and cardiovascular disease risk factors in black and white households: the NHLBI Growth and Health Study. Am J Publ Health. 1994;84:1761–1767. doi: 10.2105/ajph.84.11.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérusse L, Tremblay A, Leblanc C, Cloninger CR, Reich T, Rice J, et al. Familial resemblance in energy intake: Contribution of genetic and environmental factors. Am J Clin Nutr. 1988;47:629–635. doi: 10.1093/ajcn/47.4.629. [DOI] [PubMed] [Google Scholar]
- 6.Laskarzewski P, Morrison JA, Khoury P, Kelly K, Glatfelter L, Larsen R, et al. Parent – child nutrient intake relationships in school children ages 6 to 19: the Princeton School District Study. Am J Clin Nutr. 1980;33:2350–2355. doi: 10.1093/ajcn/33.11.2350. [DOI] [PubMed] [Google Scholar]
- 7.Vauthier J, Lluch A, Lecomte E, Artur Y, Herberth B. Family resemblance in energy and macronutrient intakes: the Stanislas Family Study. Int J Epidemiol. 1996;25:1030–1037. doi: 10.1093/ije/25.5.1030. [DOI] [PubMed] [Google Scholar]
- 8.Oliveria SA, Ellison RC, Moore LL, Gillman MW, Garrahie EJ, Singer MR. Parent – child relationships in nutrient intake: the Framingham children’s study. Am J Clin Nutr. 1992;56:593–598. doi: 10.1093/ajcn/56.3.593. [DOI] [PubMed] [Google Scholar]
- 9.Borah-Giddens J, Falciglia GA. A meta-analysis of the relationship in food preferences between parents and children. J Nutr Educ. 1993;25:102–107. [Google Scholar]
- 10.Anderssen N, Wold B. Parental and peer influences on leisure-time physical activity in young adolescents. Res Q Exerc Sport. 1992;63:341–348. doi: 10.1080/02701367.1992.10608754. [DOI] [PubMed] [Google Scholar]
- 11.Sallis JF, Patterson TL, McKenzie TL, Nader PR. Family variables and physical activity in preschool children. J Devl Behav Pediat. 1988;9:57–61. [PubMed] [Google Scholar]
- 12.Vilhjalmsson R, Thorlindsson T. Factors related to physical activity: a study of adolescents. Soc Sci Med. 1988;47:665–675. doi: 10.1016/s0277-9536(98)00143-9. [DOI] [PubMed] [Google Scholar]
- 13.Gottlieb NH, Chen M. Sociocultural correlates of childhood sporting activities: their implications for heart health. Soc Sci Med. 1985;21:533–539. doi: 10.1016/0277-9536(85)90037-1. [DOI] [PubMed] [Google Scholar]
- 14.Wold B, Anderssen N. Health promotion aspects of family and peer influences on sport participation. Int J Sport Psychol. 1992;23:343–359. [Google Scholar]
- 15.Freedson PS, Evenson S. Familial aggregation in physical activity. Res Q Exerc Sport. 1991;62:384–389. doi: 10.1080/02701367.1991.10607538. [DOI] [PubMed] [Google Scholar]
- 16.Moore LL, Lombardi DA, White MJ, Campbell JL, Oliveria SA, Ellison C. Influences of parents’ physical activity levels on activity levels of young children. J Pediat. 1991;118:215–219. doi: 10.1016/s0022-3476(05)80485-8. [DOI] [PubMed] [Google Scholar]
- 17.Davison K, Birch L. Child and parent characteristics as predictors of change in girls’ body mass index. Int J Obes Relat Metab Disord. 2001;25:1834–1842. doi: 10.1038/sj.ijo.0801835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burke V, Beilin LJ, Dunbar D. Family lifestyle and parental body mass index as predictors of body mass index in Australian children: a longitudinal study. Int J Obes Relat Metab Disord. 2001;25:147–157. doi: 10.1038/sj.ijo.0801538. [DOI] [PubMed] [Google Scholar]
- 19.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–351. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- 20.Beunen GP, Maes HH, Vlietinck R, Malina RM, Thomas M, Feys E, et al. Univariate and multivariate genetic analysis of subcutaneous fatness distribution in early adolescence. Behav Genet. 1998;28:279–288. doi: 10.1023/a:1021671313974. [DOI] [PubMed] [Google Scholar]
- 21.Bodurtha JN, Mosteller M, Hewitt JK, Nance WE, Eaves LJ, Moskowitz WB, et al. Genetic analysis of anthropometric measures in 11-year-old twins: the Medical College of Virginia Twin Study. Pediatr Res. 1990;28:1–4. doi: 10.1203/00006450-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Faith MS, Pietrobelli A, Nunez C, Heo M, Heymsfield SB, Allison DB. Evidence for independent genetic influences on fat mass and body mass index in a pediatric twin sample. Pediatrics. 1999;104:61–67. doi: 10.1542/peds.104.1.61. [DOI] [PubMed] [Google Scholar]
- 23.Davison K, Birch L. Childhood overweight: a contextual model and recommendations for future research. Obes Rev. 2001;2:159–171. doi: 10.1046/j.1467-789x.2001.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wachs TD. The use and abuse of environment in behavior-genetic research. Child Devl. 1983;54:396–407. [PubMed] [Google Scholar]
- 25.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71:1–14. doi: 10.1080/02701367.2000.11082780. [DOI] [PubMed] [Google Scholar]
- 26.Goran M. Measurement Issues related to studies of childhood obesity: assessment of body composition, body fat distribution, physical activity, and food intake. Pediatrics. 1998;101:505–518. [PubMed] [Google Scholar]
- 27.Kristal AR, Shattuck AL, Williams AE. Food frequency questionnaires for diet intervention research. Paper presented at: 17th National Nutrient Database Conference; 7 – 10 June, 1992; Baltimore MD. [Google Scholar]
- 28.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 29.Allison D, Goran M, Poehlman E, Heymsfield S. Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord. 1995;19:644–652. [PubMed] [Google Scholar]
- 30.Van Strien T, Fritjers JER, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional and external eating behavior. Int J Eating Disord. 1986;5:295–315. [Google Scholar]
- 31.Van Strien T, Fritjers JER, van Staveren WA, Defares PB, Deurenberg P. The predictive validity of the Dutch Restrained Eating Scale. Int J Eating Disord. 1986;5:747–755. [Google Scholar]
- 32.Allison D. Handbook of assessment methods for eating behaviors and weight related problems. Sage; Thousand Oaks, CA: 1995. [Google Scholar]
- 33.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. Br Med J. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuczmarski R, Ogden C, Grummer-Strawn L, Flegal K, Guo S, Wei R, et al. CDC growth charts: United States. Advance data from vital and health statistics. National Center for Health Statistics; Hyattsville, MD: 2000. [PubMed] [Google Scholar]
- 35.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation on obesity; Geneva, 3 – 5 June, 1997; Geneva: World Health Organization; 1998. [PubMed] [Google Scholar]
- 36.Harrison GG, Buskirk ER, Carter L, Johnston FE, Lohman TG, Pollock ML, et al. Skinfold thickness and measurement technique. Anthropometric standardization reference manual. Human Kinetics; Champaigne, IL: 1991. [Google Scholar]
- 37.Sokal R, Sneath P. Principals of numerical taxonomy. WH Freeman; San Francisco, CA: 1963. [Google Scholar]
- 38.Affifi AA, Clark V. Computer-aided multivariate analysis. 4. Chapman & Hall/CRC; Washington, DC: 1996. [Google Scholar]
- 39.Rosner B, Prineas R, Loggie J, Daniels SR. Percentiles for body mass index in US children 5 to 17 y. J Pediatr. 1998;132:211–221. doi: 10.1016/s0022-3476(98)70434-2. [DOI] [PubMed] [Google Scholar]
- 40.Samaras K, Kelly PJ, Campbell LV. Dietary underreporting is prevalent in middle-aged British women and is not related to adiposity (percent body fat) Int J Obes Relat Metab Disord. 1999;23:881–888. doi: 10.1038/sj.ijo.0800967. [DOI] [PubMed] [Google Scholar]
- 41.Fisher JA, Johnson RK, Lindquist C, Birch LL, Goran MI. Influence of body composition on the accuracy of reported energy intake in children. Obes Res. 2000;8:597–603. doi: 10.1038/oby.2000.77. [DOI] [PubMed] [Google Scholar]
- 42.Bingham SA. The use of 24-h urine samples and energy expenditure to validate dietary assessments. Am J Clin Nutr. 1994;59(Suppl):227S–231S. doi: 10.1093/ajcn/59.1.227S. [DOI] [PubMed] [Google Scholar]
- 43.Livingstone M, Prentice A, Coward W, Strain J, Black A, Davies P, et al. Validation of estimates of energy intake by weighed dietary record and diet history in children and adolescents. Am J Clin Nutr. 1992;56:29–35. doi: 10.1093/ajcn/56.1.29. [DOI] [PubMed] [Google Scholar]
- 44.Garn SM, LaVelle M, Pilkington JJ. Obesity and living together. Marriage Family Rev. 1984;7:33–47. [Google Scholar]
- 45.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960 – 1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 46.Brown CD, Higgins M, Donato KA, Rohde F, Garrison R, Obarzanek E, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8:605–619. doi: 10.1038/oby.2000.79. [DOI] [PubMed] [Google Scholar]
- 47.Owens S, Gutin B, Barbeau P, Litaker M, Allison J, Humphries M, et al. Visceral adipose tissue and markers of the insulin resistance syndrome in obese black and white teenagers. Obes Res. 2000;8:287–293. doi: 10.1038/oby.2000.34. [DOI] [PubMed] [Google Scholar]
- 48.Sallis J, Prochaska J, Taylor W. A review of correlates of physical activity of children and adolescents. Med Sci Sports & Exerc. 2000;32:963–975. doi: 10.1097/00005768-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 49.McPherson RS, Hoelscher DM, Alexander M, Scanlon KS, Serdula MK. Dietary assessment methods among school-aged children: validity and reliability. Prev Med. 2000;31(Suppl):S11–S33. [Google Scholar]
- 50.Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year follow-up of behavioral, family-based treatment for obese children. JAMA. 1990;264:2519–2523. [PubMed] [Google Scholar]
- 51.Golan M, Weizman A, Apter A, Fainaru M. Parents as the exclusive agents of change in the treatment of childhood obesity. Am J Clin Nutr. 1998;67:1130–1135. doi: 10.1093/ajcn/67.6.1130. [DOI] [PubMed] [Google Scholar]
- 52.Glenny AM, O’Meara S, Melville A, Sheldon TA, Wilson C. The treatment and prevention of obesity: a systematic review of the literature. Int J Obes Relat Metab Disord. 1997;21:715–737. doi: 10.1038/sj.ijo.0800495. [DOI] [PubMed] [Google Scholar]


