Abstract
Background
Childhood overweight has increased dramatically, particularly among young girls. Genetic and environmental factors produce the overweight phenotype. Nonshared environments appear to account for a substantial proportion of the population variance in overweight but remain largely unspecified and unmeasured.
Objective
Our goal was to evaluate the influence of maternal control in feeding, an aspect of nonshared family environment, on daughters’ eating and relative weight.
Design
Structural equation modeling was used to test models that describe maternal influences on daughters’ eating and relative weight. The participants were 197 white, non-Hispanic families with 5-y-old daughters. The mothers’ own dietary restraint and their perceptions of their daughters’ risk of overweight were used to predict maternal control in feeding, which was used to predict the daughters’ eating and weight outcomes.
Results
Maternal body mass index was a modest predictor of daughters’ relative weight. The addition of the family-environment pathway provided a good fit and showed additional, independent prediction of daughters’ relative weight. Mothers’ dietary restraint and perceptions of their daughters’ risk of overweight predicted maternal child-feeding practices, which in turn predicted daughters’ eating and relative weight.
Conclusions
Child-specific aspects of the family environment, including mothers’ child-feeding practices and perceptions of their daughters’ risk of overweight, may represent important, nonshared, environmental influences on daughters’ eating and relative weight. The environmental effects noted were modest but comparable in magnitude to the direct association between maternal and child weight, which indicates that measuring family environmental factors can enhance our understanding of the etiology of childhood overweight.
INTRODUCTION
Familial patterns of adiposity are well established. The probability of being obese as an adult is ≥3 times higher for the young child with one parent who is obese, compared with a child who has no obese parents (1–3). In behavioral genetic approaches to the study of obesity, genetic and environmental contributions to the phenotype are estimated. Across studies, the estimated environmental effects on the variance in adiposity are substantial (1, 2, 4). Environmental effects can be categorized as either shared or nonshared. Shared environmental effects are perfectly correlated for family members and thus affect their phenotypes in the same way, but nonshared environmental effects are experienced differently and act to produce differences in phenotypes across family members (1, 2, 5). Research findings showed that nonshared environmental effects had a substantial influence on obesity, whereas the influence of shared environmental effects was negligible (1, 2, 4).
Traditionally, family environments were viewed as shared, whereas it was assumed that nonshared environmental influences were found outside the family (6). This view, in combination with the failure to find effects of shared environments, has led some to conclude that family environments do not matter (7). However, research on the effects of family environments on children’s development has shown that nonshared environmental influences are pervasive within families (6, 8, 9). Parents do not treat all their children alike; parenting practices are shaped by each child’s characteristics, including sex, age, birth order, physical appearance, and specific abilities (8, 10). Although siblings in the same household may eat from the same refrigerator and at the same table, children’s experiences with food and eating are generally of the nonshared variety, shaped in part by the child-feeding practices that they experience (11–13). For instance, Klesges et al (12) reported that parents used different kinds of prompts for eating with overweight and normal-weight children, and Waxman and Stunkard (13) reported that obese boys were given larger portions and treated differently at mealtimes than were their thinner siblings. Parental attempts to control and restrict children’s food intakes increase with increasing child overweight, especially if the child is a girl (11, 14, 15).
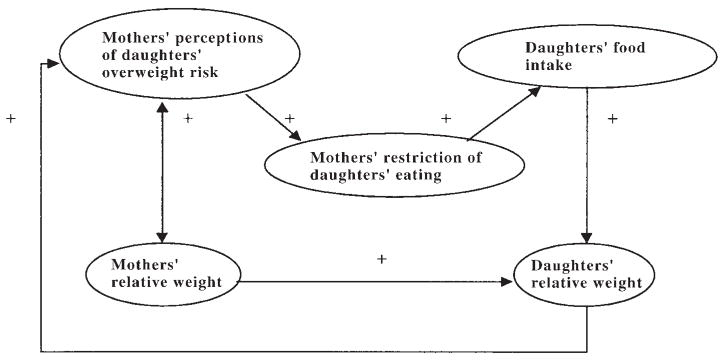
The purpose of the present study was to test a model that focuses on one aspect of the nonshared family environment, the effects of mothers’ child-feeding practices on their daughters’ eating and overweight (Figure 1). This model is based on our prior research (11, 14, 15) and was inspired by the obesity-proneness model presented by Costanzo and Woody (10). This theory says that parents will impose greater restrictive control over their daughters’ eating if 1) eating and appearance are particularly valued by, or problematic for, the parent, or 2) the child is perceived to be at risk of overweight. This research focused on girls and their mothers because problems of eating and energy balance, including weight concerns, chronic dieting, and eating disorders, differ by sex and are especially pervasive among females. Mothers may play an especially important role in their daughters’ developing controls of food intake, especially in the development of dieting and eating problems (16–20).
FIGURE 1.
Theoretical model depicting influences of the family environment on daughters’ relative weight.
SUBJECTS AND METHODS
Study design
In Figure 1 we show the hypothesized, full family-environment model, in which use of restrictive child-feeding practices predicted daughters’ self-control in eating, which in turn predicted daughters’ relative weight. Paths between weight-related risk characteristics of mothers and daughters and mothers’ child-feeding practices are shown on the left side of the figure. We measured risk factors including mothers’ perceptions of daughters’ risk of overweight, mothers’ concern regarding daughters’ overweight, and mothers’ use of dietary restraint to control their own weight. Paths between mothers’ control in child-feeding practices and girls’ eating and weight outcomes are shown on the right side of the figure. A direct path from mothers’ relative weight to daughters’ relative weight was included to reflect genetic and shared environmental effects on mothers’ and daughters’ relative weights. The full family-environment model was compared with a reduced model that evaluated only the direct relation between mothers’ and daughters’ weights.
Subjects
The participants were 197 girls aged ≈5 y (5.4 ± 0.02 y; 4.6–6.4 y) and their mothers. The girls lived with both biological parents, did not have severe food allergies or chronic medical problems affecting food intake, and were not consuming vegetarian diets during the period of data collection. Families were recruited for participation by using fliers and newspaper advertisements, and families with age-eligible daughters within a 5-county radius also received mailings and follow-up telephone calls (Metromail Corp, Lombard, IL). On average, mothers were in their mid-30s (35.4 ± 0.3 y). Almost two-thirds (63%) of the mothers were currently employed. Mothers in the sample worked an average of 18 h/wk. Reported family income was <$35000 for 29%, $35000–$50000 for 35%, and >$50000 for 36%. The parents were well-educated; the mean number of years of education was 15 ± 2 y (range: 12–20) for mothers and 15 ± 3 y (range: 12–20) for fathers.
Mothers’ measures
Mothers’ relative weight
We used body mass index (BMI; in kg/m2) as the measure of mothers’ relative weight. Subjects’ weight and height were measured by using procedures described by Lohman et al (21). All subjects were weighed and measured in light clothing without shoes. Height was measured in triplicate to the nearest 0.1 cm by using a stadiometer. Weight was measured in triplicate to the nearest 0.1 kg on an electronic scale (Seca Corp, Birmingham, United Kingdom).
Mothers’ restrained eating
The Eating Inventory (22) was used to measure mothers’ restrained eating. The Restraint Subscale (21 items) measures the cognitive intent to restrict food intake and consists of items such as, “When I have my quota of calories, I am usually good about not eating any more” and “I often stop eating when I am not really full as a conscious means of limiting the amount that I eat.” Restraint scores can range from 0 to 21, with high scores indicating high dietary restraint. The Restraint Subscale has been used widely for studying food-intake behavior and has a high internal consistency, with a Chronbach’s α ranging from 0.79 to 0.93 (23). The internal consistency for the Restraint Subscale in this sample was 0.87.
Mothers’ perceptions of daughters’ risk of overweight
Mothers’ perceptions of daughters’ risk of overweight were measured by using the Perceived Child Weight Subscale and the Concerns about Child Overweight Subscale from the Child-Feeding Questionnaire (CFQ; available from the authors on request). The CFQ is a self-report questionnaire that measures perceptions of daughters’ and parents’ overweight, concerns about overweight, and parents’ child-feeding attitudes and practices.
Perceived child weight
The CFQ Perceived Child Weight Subscale contains 6 items regarding perceptions of daughters’ weight status during several stages in childhood and has response options ranging from 1 (markedly underweight) to 5 (markedly overweight). Scores for the 6 items were averaged to obtain a total score for this subscale.
Concerns about child overweight
The CFQ Concerns about Child Overweight Subscale contains 3 items that assess whether mothers are concerned that their daughters will be overweight or will have to diet. Response options range from 1 (unconcerned) to 5 (concerned). Scores for the 3 items were averaged to obtain a total score for this scale. The internal consistency was 0.74 in this sample.
Mothers’ restriction of daughters’ eating
Mothers’ restriction of daughters’ eating was measured with the CFQ Restriction Subscale and CFQ Monitoring Subscale and the Restricted-Access Questionnaire (14).
CFQ Restriction Subscale
The CFQ Restriction Subscale contains 8 items that measure mothers’ attempts to control their daughters’ eating by restricting access to foods. It addresses restriction of both the types and amounts of foods and has response options of 1 (disagree) to 5 (agree). Examples of the items are, “I have to be sure that my child does not eat too many high-fat foods,” and “If I did not guide or regulate my child’s eating, she would eat too many junk foods.” Scores for the 8 items were averaged to obtain a total score for this scale. The internal consistency for the CFQ Restriction Subscale in this sample was 0.78.
CFQ Monitoring Subscale
The CFQ Monitoring Subscale contains 3 items that measure the extent to which mothers report monitoring their daughters’ consumption of energy-dense foods; it has response options of 1 (never) to 5 (always). Examples of the items are, “How much do you keep track of the snack food (potato chips, Doritos, cheese puffs) that your child eats?” and “How much do you keep track of the high-fat foods that your child eats?” Scores for the 3 items were averaged to create a total score for this subscale. The internal consistency for the CFQ Monitoring Subscale in this sample was 0.86.
Restricted-Access Questionnaire
The Restricted-Access Questionnaire (available from the authors on request) measures the extent to which mothers restrict their daughters’ access to 10 snack foods that were used in the free-access snack session described below. The instrument contains 10 items, each of which is asked about each snack food: 1) limiting the time of day that the food is allowed, 2) getting upset if the daughter obtained the food without asking, 3) monitoring the daughter’s consumption of the food, 4) generally limiting the amount consumed, 5) allowing second helpings, 6) generally limiting opportunities to consume the food, 7) providing the food relative to how often the daughter asks for it, 8) keeping the food out of reach, 9) limiting how often the food is in the home, and 10) limiting the type of eating occasions at which the food is provided. For each of the 10 items, responses were then summed across the 10 experimental foods. Scores for the 10 items were combined by using principal components analysis to create a total standardized score for this scale, because the items had different numbers of response options. The internal consistency for the Restricted-Access Questionnaire in this sample was 0.83 for mothers.
Daughters’ measures
Short-term control of energy intake: daughters’ intake in the absence of mothers’ supervision
Daughters’ short-term regulation of intake was measured by using 2 laboratory procedures: a short-term energy-compensation procedure (COMPX) and the free-access procedure.
COMPX procedure
The COMPX procedure measures the extent to which girls’ short-term energy intake is responsive to the energy density of foods (11). The COMPX procedure uses data from 2 separate eating occasions that differ in the energy (from carbohydrate) content of a fixed amount of a drink given as a preload (first course). On the first occasion, girls received a low-energy preload drink (25 kJ) and on the second occasion, they received a high-energy preload drink (649 kJ). On both occasions, the girls ate a self-selected lunch 20 min after the preload drink. The lunch offered to the girls was the same on both occasions and consisted of generous portions of bread (4 slices), sandwich meat (4 slices), carrots (20 g), applesauce (113 g), cheese (2 slices), cookies (2 medium), and milk (237 mL). At each compensation trial, 4–6 girls were seated together and several adults were present to ensure that foods were not shared among girls, dropped food was recorded and replaced, and food-related discussion was avoided. The COMPX score represents the adjustment in ad libitum lunch intake, expressed as a percentage of the energy difference between the 2 preload drinks (≈629 kJ). A compensation score of 100% would indicate that ad libitum lunch intake was precisely adjusted in response to the energy difference between the low- and high-energy preloads. In this case, ad libitum lunch intake would be 629 kJ greater after the low-energy preload than after the high-energy preload. The COMPX measure was reverse-scored so that composites with the free-access scores could be created for structural equation modeling analysis; therefore, higher COMPX scores indicated lower percentage compensation for energy.
Free-access procedure
The free-access procedure measured girls’ responsiveness to the presence of palatable foods in the absence of hunger. After a self-selected lunch (described above), each girl was interviewed one-on-one by a trained interviewer in a quiet room. The girls first indicated the extent to which they were hungry by using 3 cartoon figures that depicted an empty stomach, a half-empty stomach, and a full stomach. To minimize the influence of hunger on the assessment of snack food intake, cases in which girls indicated that they were still hungry after lunch were not included in these analyses. Next, a rank-order food preference assessment was performed to ensure that each girl had an opportunity to taste each of 10 sweet and savory snack foods, which differed in fat content (24). In this procedure, the child took small tastes of foods and placed them in front of cartoon faces that depicted “yummy,” “yucky,” and “just okay.” After the preference assessment, the girl was shown various toys that were available for a play session. Next, generous portions of the 10 snack foods were presented. The foods were popcorn (6 g), potato chips (58 g), pretzels (60 g), nuts (44 g), fig bars (51 g), chocolate chip cookies (66 g), fruit-chew candy (66 g), chocolate bars (66 g), ice cream (168 g), and frozen yogurt (168 g). The girl was told that she could play with the toys or eat any of the foods while the experimenter did some work in the adjacent room. The experimenter then left the room for 10 min. Manufacturers’ information was used to convert gram weight consumption of each food into energy intake. Total energy intake for the free-access procedure was calculated by summing the energy intakes for all the snack foods eaten during this period.
Daily energy intake: mothers’ reports of daughters’ 24-h energy intake
Daughters’ daily energy intakes were estimated by conducting three 24-h recalls. Recalls were conducted with the mother and the daughter by trained staff at The Pennsylvania State Nutrition Center. The staff used the computer-assisted NUTRITION DATA SYSTEM (Nutrient Database version 12A, Food Database version 27, release date 1996; Nutrition Coordinating Center, University of Minnesota). The database had no missing data for energy and 3% estimated data for energy. Multiple-pass 24-h recall methodology was used; with this approach, participants first provide a free-recall list of all foods consumed within a 24-h period. This is followed by structured prompts regarding food descriptions and amounts and a final review of the recall information to solicit any changes or additions from the participant (25). Two weekdays and 1 weekend day were randomly selected over a 2-wk period during the summer. Portion-size posters were used as a visual aid for estimating amounts of foods eaten. Average daily energy intake was estimated from the nutrient data collected on the 3 recall days.
Daughters’ relative weight
Daughters’ relative weight was measured as weight-for-height z score; as discussed above, mothers’ relative weight was measured as BMI. Daughters’ weight and height measurements were obtained to determine weight-for-height z scores by using National Center for Health Statistics data (26; EPI INFO version 6.04, EpiNut Module; Centers for Disease Control and Prevention, Atlanta). Height and weight measurements were obtained by a trained staff member who used procedures described by Lohman et al (21). Girls were dressed in light clothing and were measured without shoes. Height was measured in triplicate to the nearest 0.1 cm. Weight was measured in triplicate to the nearest 0.1 kg.
Statistical analysis
Of the 197 mother-daughter pairs that participated, 156 pairs were included in the data analysis. Forty-one pairs were excluded for the following reasons: 1) interviewer ratings indicated that the girl had general behavioral difficulties throughout the interview day; 2) interviewer ratings indicated that the girl did not seem comfortable or did not understand the instructions during the ad libitum lunch or the free-access period; 3) the girl ate a combined total of < 1257 kJ at breakfast, the snack, and the lunch; and 4) the girl consumed < 80% of the compulsory preload in the COMPX procedure. Descriptive statistics were generated for all variables of interest.
Model specification
Structural equation modeling was conducted with LISREL (version 8.30 for Windows; Scientific Software International Inc, Chicago) to test models that described maternal influences on daughters’ relative weight.
Full model
The full model (Figure 1) started with the direct relation between mothers’ and daughters’ relative weights. However, this model also included paths reflecting the influence of the family environment on daughters’ eating and weight outcomes. Mothers’ perceptions of daughters’ risk of overweight and mothers’ own restrained eating were evaluated as predictors of restrictive child-feeding practices. In turn, mothers’ restriction of daughters’ eating was evaluated as a predictor of daughters’ eating and weight outcomes.
The constructs that indicated mothers’ perceptions of daughters’ risk of overweight and daughters’ short-term regulation of food intake were created before model testing by adding z scores for all the variables representing the construct. For mothers’ perceptions of daughters’ overweight risk, we added the z scores for mothers’ perception of child weight and mothers’ concerns about child overweight. For girls’ short-term regulation of food intake, we added the z scores for the short-term compensation procedure and the free-access procedure. However, mothers’ restriction of daughters’ eating was treated as a latent construct within the model by separately measuring the loadings for each of 3 measures of mother’s restrictive feeding practices (CFQ Restriction Subscale, CFQ Monitoring Subscale, and Restricted-Access Questionnaire) as part of the model-fitting procedure.
Reduced model
The reduced model tested only the relation between mothers’ and daughters’ relative weights. For comparison purposes, this model also included estimates of construct variances, error terms, and item loadings that were included in the full model.
Indicators of model fit
We followed the advice of Byrne (27) and focused on 4 indexes that assess how well the model fits the data: the chi-square test, the Non-Normed Fit Index (NNFI), the Comparative Fit Index (CFI), and the Root Mean Square Error of Approximation (RMSEA). The chi-square test indicates how well the model fits the data: small, nonsignificant chi-square values indicate little discrepancy between the structure of the observed data and the hypothesized model. The NNFI and CFI indexes compare the hypothesized model with a null or worst-fitting model, taking into account model complexity, and indicate a well-fitting model with values > 0.90 (approaching an upper limit of 1). The RMSEA reflects how closely the model fit approximates a reasonably fitted model and indicates good model fit with values < 0.05. These 4 types of fit statistics were generated separately for the full and reduced models. The full model was also compared with the reduced model by evaluating the change in chi-square relative to the change in degrees of freedom between the 2 models.
RESULTS
In Table 1 and Table 2 we show the descriptive statistics for mothers’ and daughters’ variables. The mean BMI indicated that the sample of mothers was overweight. Mothers’ average dietary restraint scores were in the normal range (23). Daughters’ average weight-for-height z scores corresponded to 62 ± 2% for age and sex, indicating that the average sample value was somewhat above the median. With respect to the girls’ self-regulation of energy intake during the COMPX procedure, the average percentage compensation was 50%. This indicated that the girls compensated for about half of the energy difference between preloads in the subsequent ad libitum lunch, a finding comparable with previous research (11). During the free-access procedure, when we measured daughters’ response to the availability of snack foods in the absence of their mothers’ supervision, the girls consumed substantial amounts of energy, even though they had just consumed lunch and indicated that they were not hungry. Daughters’ mean energy intake in the free-access procedure was ≈503 kJ (range: 0–1567 kJ), representing ≈6% of the recommended dietary allowance (RDA) for girls of this age (1800 kcal or 7542 kJ) (28). The girls’ average energy intake as estimated from 24-h recalls, 6398 kJ, was lower than the RDA for girls of this age.
TABLE 1.
Descriptive statistics for mothers’ variables1
| Construct and related variables | Value |
|---|---|
| Mothers’ relative weight | |
| Body mass index (in kg/m2) | 26.0 ± 0.4 (17.7–44.1) |
| Mothers’ restrained eating | |
| Eating Inventory Restraint Subscale (0–21) | 8.4 ± 0.4 (0.0–20.0) |
| Mothers’ perceptions of daughters’ overweight risk | |
| CFQ Perceived Child Weight Subscale (1–5) | 2.8 ± 0.0 (1.3–4.0) |
| CFQ Concerns About Child Overweight Subscale (1–5) | 2.3 ± 0.1 (1.0–5.0) |
| Mothers’ restriction of daughters’ eating | |
| CFQ Restriction Subscale (1–5) | 3.0 ± 0.1 |
| CFQ Monitoring Subscale (1–5) | 3.7 ± 0.1 (1–5) |
| Restricted-Access Questionnaire (standardized) | 0.16 ± 0.16 (25.9 to 4.8) |
x̄ ± SEM with range in parentheses; n = 156. CFQ, Child-Feeding Questionnaire.
TABLE 2.
Descriptive statistics for daughters’ intake and weight variables1
| Construct and related measures | Value |
|---|---|
| Short-term control of energy intake | |
| COMPX (%) | 50 ± 5 (−121 to 218) |
| Free-access procedure (kJ) | 503 ± 29 (0–1567) |
| Daily energy intake | |
| 24-h dietary recall (kJ) | 6398 ± 109 (3482–11011) |
| Daughters’ relative weight | |
| Weight-for-height (%) | 62 ± 2 (6–99) |
| Percentage of sample overweight (≥85% of weight-for-height) | 22 |
x̄ ± SEM with range in parentheses; n = 162. COMPX, compensation procedure.
Fit statistics for the full family-environment model
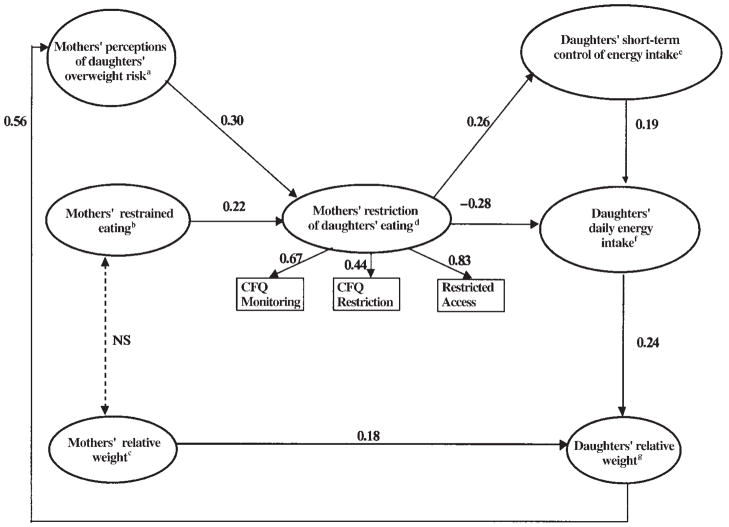
The nonsignificant chi-square value indicated that the specified full family-environment model was not different from the underlying data structure (P2 = 19.31, df = 24, P = 0.74). With use of generally accepted cutoffs (27), the NNFI, RMSEA, and CFI fit values obtained for the model were also consistent in indicating that the family-environment model provided an excellent fit to the data (NNFI = 1.04, CFI = 1.00, RMSEA = 0.00). Although of modest size, all path coefficients in the model were significant, which showed that each made a meaningful contribution to the model. The full family-environment model provided a much better fit than the reduced model (P2 = 110.16, df = 17, P = 0.00), which evaluated only the direct relation between mothers’ and daughters’ relative weights (Δχ2 = 90.85, Δdf = 7, P < 0.001).
Description of the full family-environment model
In Figure 2 we show the standardized parameter estimates for the full family-environment model. The path coefficients in the structural equation model were adjusted for all other relations in the model and can be interpreted in the same manner as β weights in regression analyses. Consistent with previous studies that included parents and young children, a modest relation was noted between mothers’ relative weights and daughters’ relative weights (β = 0.18). The family-environment model evaluated mothers’ and daughters’ eating and weight-related characteristics as predictors of maternal control in feeding. The model includes a feedback loop such that as daughters’ relative weight increased, mothers’ perception of daughters’ overweight and mothers’ concerns about daughters’ overweight risk increased (β = 0.56). The inclusion of the feedback loop was essential to the model’s excellent fit to the data, as indicated by the change in chi-square with its removal (Δχ2 = 48.5, Δdf = 1, P < 0.001). These maternal perceptions of daughters’ overweight risk predicted mothers’ reports of restricting daughters’ eating (β = 0.30). This model also evaluated the influence of mothers’ own dietary restraint on the amount of restriction that mothers reported using to influence their daughters’ eating. Higher degrees of maternal dietary restraint were related to higher degrees of mothers’ restriction of daughters’ food intake (β = 0.22).
FIGURE 2.
Structural equation full model that tested the influences of the family environment on girls’ eating and weight outcomes. aChild-Feeding Questionnaire (CFQ) Perceived Child Weight Subscale and Concerns About Child Overweight Subscale; bEating Inventory Restraint Subscale; cBody mass index (in kg/m2); dMothers’ restriction of daughters’ eating, with factor loadings for the CFQ Monitoring and CFQ Restriction Subscales and Restricted-Access Questionnaire; ecompensation procedure (COMPX, reverse scored) and free-access procedure; f24-h recalls; gNational Center for Health Statistics weight-for-height z scores.
The second aspect of the family-environment model evaluated mothers’ restrictive control in feeding as a determinant of their daughters’ short-term control of energy intake, daily energy intake, and relative weight. Greater maternal restriction predicted less adequate short-term regulation of energy intake by daughters (β = 0.26). That is, greater maternal restriction was associated with a combination of 1) less ability to compensate or adjust for preload energy during the subsequent ad libitum lunch (COMPX), and 2) higher free-access intakes of palatable snacks after lunch in the absence of hunger. This measure of daughters’ short-term energy regulation was positively related to daughters’ 24-h energy intakes (β = 0.19), and 24-h energy intakes were related to daughters’ relative weight (β = 0.24). Mothers’ restriction of daughters’ eating was also directly associated with mothers’ reports of daughters’ daily energy intake; mothers who reported greater restriction of daughters’ intake also reported lower 24-h energy intakes (β = −0.28) for daughters. The model provided an excellent fit to the data, yet residual variances for the dependent variables ranged from 0.70–0.93; this indicated that although the environmental model provided an excellent fit to the data, a great deal of variance remained unexplained.
DISCUSSION
In the present study, the relation between mothers’ and daughters’ relative weights was significant and of the same magnitude as that reported in family studies of obesity that included parents and young children (2, 29). This modest relation between the parent’s and child’s relative weights has been one of the critical pieces of evidence used in establishing familial patterns of adiposity (4). The model shown in Figure 2 illustrates 2 paths that influence daughters’ relative weight: 1) the direct path between mothers’ and daughters’ relative weights, which reflects both genetic and shared environmental effects; and 2) a second path that depicts an environmental influence, maternal control in feeding, on daughters’ eating and relative weight. The full family-environment model provided a substantially better fit to the data than did the simple path model that evaluated only mothers’ BMI as a predictor of daughters’ relative weight. Moreover, although the path coefficients that reflect environmental effects of maternal control in feeding on daughters’ eating and relative weight are moderate, they are of comparable magnitude with the path coefficient reflecting genetic factors, which directly links the mother’s and daughter’s relative weights. These results indicate that mothers’ child-feeding practices influence daughters’ risk of becoming overweight, and the results show one way that family environmental factors can work synergistically with genetic factors to produce intergenerational similarities in eating and overweight. Heavier mothers have heavier daughters, and these results indicate that these familial resemblances arise from genetic factors and the use of child-feeding practices that foster problems in eating and increase daughters’ relative weight.
The findings of this research support the bidirectionality of influence between parents and children within families; this bidirectional flow of influence is the basis for nonshared environments in families. In the present example, daughters’ weight status influenced mothers’ perceptions of daughters’ risk of overweight, which in turn influenced mothers’ child-feeding practices. The model tested here included only one of many possible nonshared family environmental effects that may influence daughters’ eating and relative weight, namely mothers’ child-feeding practices. Other possible aspects of the nonshared family environment, such as interactions with siblings, the influence of television and other media, and the effects of physical activity patterns, are not included in this model. The results of this research are promising because they show that specifying and measuring even a single, limited aspect of the nonshared family environment can substantially enhance our understanding of the ways in which family environments may foster the development of overweight phenotypes in children.
These findings also provide support for important aspects of Costanzo and Woody’s (10) obesity proneness model. This model explains how excessive parental control in feeding can result when 1) parents are particularly invested in their children’s eating; 2) children are perceived as being at risk of developing eating problems, weight problems, or both; and 3) parents have trouble controlling their own food intake and assume that their children cannot do so either. Consistent with these points, our results confirmed that a mother’s efforts to control her own weight, as measured by dietary restraint, in combination with her perceptions of her daughter’s risk of overweight, predicted the mother’s use of greater restrictive control in child feeding. These findings are consistent with those of other recent research on parenting practices and children’s development in suggesting that effects flow not only from parent to child, but also from child to parent in that parenting practices are shaped and influenced by the child (8). In particular, these findings strengthen previous findings indicating that child-feeding practices are influenced by the weight status of the child (11–13).
Costanzo and Woody (10) contend that excessive control in feeding diminishes children’s capacity for self-regulation. In the current study, this contention was supported by the finding that greater maternal restriction was associated with evidence of difficulties in self-control of food intake by daughters. Daughters’ self-control difficulties were measured by using a composite method that reflected 1) less evidence of adjustments in food intake in response to changes in the energy density of foods, and 2) greater intakes of palatable snack foods in the absence of hunger. These findings contribute to a growing body of evidence indicating that use of stringent controls and restrictive child-feeding practices does not produce the intended effect of helping daughters to establish adequate self-control of food intake. Rather, parents’ use of controlling feeding practices may actually promote patterns of intake that are readily influenced by the presence and availability of palatable foods (15). Additional research is warranted, given that our contemporary environment is characterized by the pervasive availability of large portions of palatable, inexpensive, energy-dense foods (30).
The possibility that parents facilitate or promote early dieting and weight concerns among older children and young adolescents was explored in previous research (16, 20, 31). Daughters who diet by adolescence tend to have mothers who diet, and mothers may encourage daughters to diet and provide coaching on how to do so (17). The findings of the current study suggest that the intergenerational transfer of eating and weight problems between mothers and daughters may begin during the preschool period, very early in girls’ development. In the present study, mothers with restrained eating styles imposed more control on their daughters’ eating and had daughters who showed evidence of reduced self-control of energy intake. Edmunds and Hill (20) recently found that 12-y-old children who reported the highest degree of dietary restraint also reported the highest degree of parental control over eating. These findings highlight a need for longitudinal data to examine the role of parental control, especially restriction and monitoring, as a developmental precursor of daughters’ subsequent use of self-imposed dietary restraint for weight control. In particular, more work is needed to evaluate whether control in feeding promotes problematic regulation of eating in young girls that persists into the period when dieting, weight concerns, and restrained eating begin to emerge.
The present study showed that specification and measurement of environmental factors can begin to delineate ways in which nonshared environmental factors operating within families can influence childhood overweight. However, this research involved a variety of limitations, including the fact that the sample consisted exclusively of white, 2-parent families. We chose this sample because the prevalence of dieting and eating problems in this group is particularly high. However, this restricted sample precludes generalization of the findings to other socioeconomic, ethnic, and racial groups. This is especially problematic because the prevalence of overweight is even higher among Hispanic and black children than among non-Hispanic white children (32). At the same time, the reported prevalences of dieting and weight concerns are lower in racial and ethnic groups other than non-Hispanic whites, which suggests that pathways of influence may differ between racial and ethnic groups. Research on family environmental factors that may promote overweight in these groups is urgently needed. Other limitations relate to our reliance on mothers as the sole source of information about child-feeding practices and daughters’ food intake and perceived risk of overweight. Mothers’ reports of their own dietary restraint and child-feeding practices include bias, but such self-reports are essential in measuring perceptions and attitudes, which play crucial roles in determining parental behavior (33).
In conclusion, child-specific aspects of the family environment, including mothers’ perceptions of children’s risk of overweight and mothers’ child-feeding practices, may represent important nonshared environmental influences on daughters’ eating and relative weight. The high prevalence of childhood overweight (32) and recent evidence linking childhood overweight to increased morbidity and mortality (34, 35) have led to a consensus that programs to prevent the development of childhood overweight should be of high priority (36). Given the multifactorial nature of the problem, such prevention efforts will have to include a variety of components that focus on influencing energy intake and expenditure. These results contribute to a growing body of literature (11, 15) suggesting that preventive interventions for childhood overweight should incorporate anticipatory guidance addressing child-feeding practices and their effects on children’s eating and relative weight.
Footnotes
Supported in part by NIH grant RO1 HD32973 and the National Dairy Council.
References
- 1.Grilo CM, Pogue-Geile MF. The nature of environmental influences on weight and obesity: a behavior genetics analysis. Psychol Bull. 1991;110:520–37. doi: 10.1037/0033-2909.110.3.520. [DOI] [PubMed] [Google Scholar]
- 2.Maes HHM, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–51. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- 3.Whitaker RC, Wright JA, Pepe MS, Seidd RD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–73. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard C, editor. The genetics of obesity. Boca Raton, FL: CRC Press; 1998. [Google Scholar]
- 5.Plomin R. Genetics and experience: the interplay between nature and nurture. Thousand Oaks, CA: Sage Publications; 1994. [Google Scholar]
- 6.Dunn J, Plomin R. Separate lives: why siblings are so different. New York: Harper Collins; 1990. [Google Scholar]
- 7.Harris JR. The nurture assumption: why children turn out the way they do. New York: Free Press; 1998. [Google Scholar]
- 8.Holden GW, Miller PC. Enduring and different: a meta-analysis of the similarity in parents’ child rearing. Psychol Bull. 1999;125:223–54. doi: 10.1037/0033-2909.125.2.223. [DOI] [PubMed] [Google Scholar]
- 9.McHale SM, Crouter A, McGuire SA, Updegraff KA. Congruence between mothers’ and fathers’ differential treatment of siblings: links with family relations and children’s well-being. Child Dev. 1995;66:116–28. doi: 10.1111/j.1467-8624.1995.tb00859.x. [DOI] [PubMed] [Google Scholar]
- 10.Costanzo PR, Woody EZ. Domain-specific parenting styles and their impact on the child’s development of particular deviance: the example of obesity proneness. J Soc Clin Psychol. 1985;3:425–45. [Google Scholar]
- 11.Johnson SL, Birch LL. Parents’ and children’s adiposity and eating style. Pediatrics. 1994;94:653–61. [PubMed] [Google Scholar]
- 12.Klesges RC, Malott JM, Boschee PF, Weber JM. The effects of parental influences on children’s food intake, physical activity and relative weight. Int J Eat Disord. 1986;5:335–46. [Google Scholar]
- 13.Waxman M, Stunkard AJ. Caloric intake and expenditure of obese boys. J Pediatr. 1980;98:187–93. doi: 10.1016/s0022-3476(80)80800-6. [DOI] [PubMed] [Google Scholar]
- 14.Fisher JO, Birch LL. Restricting access to foods and children’s eating. Appetite. doi: 10.1006/appe.1999.0231. in press. [DOI] [PubMed] [Google Scholar]
- 15.Fisher JO, Birch LL. Restricting access to palatable foods affects children’s behavioral response, food selection, and intake. Am J Clin Nutr. 1999;69:1264–72. doi: 10.1093/ajcn/69.6.1264. [DOI] [PubMed] [Google Scholar]
- 16.Hill AJ, Weaver C, Blundell JE. Dieting concerns of 10-year-old girls and their mothers. Br J Clin Psychol. 1990;29:346–8. doi: 10.1111/j.2044-8260.1990.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 17.Pike KM, Rodin J. Mothers, daughters, and disordered eating. J Abnorm Psychol. 1991;100:198–204. doi: 10.1037//0021-843x.100.2.198. [DOI] [PubMed] [Google Scholar]
- 18.Cutting TM, Fisher JO, Grimm-Thomas K, Birch LL. Like mother, like daughter: familial patterns of overweight are mediated by mothers’ dietary disinhibition. Am J Clin Nutr. 1999;69:608–13. doi: 10.1093/ajcn/69.4.608. [DOI] [PubMed] [Google Scholar]
- 19.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101:539–49. [PubMed] [Google Scholar]
- 20.Edmunds H, Hill A. Dieting and the family context of eating in young adolescent children. Int J Eat Disord. 1999;25:435–40. doi: 10.1002/(sici)1098-108x(199905)25:4<435::aid-eat8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Lohman TG, Roche AF, Martorell M. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 22.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition, and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 23.Gorman BS, Allison DB. Measures of restrained eating. In: Allison DA, editor. Handbook of assessment methods of eating behaviors and weight-related problems: measures, theory, and research. Thousand Oaks, CA: Sage Publications; 1995. pp. 149–84. [Google Scholar]
- 24.Birch LL. Preschool children’s food preferences and consumption patterns. J Nutr Educ. 1979;11:189–92. [Google Scholar]
- 25.Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc. 1996;96:1140–4. doi: 10.1016/S0002-8223(96)00293-3. [DOI] [PubMed] [Google Scholar]
- 26.Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF, Moore WM. Physical growth: National Center for Health Statistics percentiles. Am J Clin Nutr. 1979;32:607–29. doi: 10.1093/ajcn/32.3.607. [DOI] [PubMed] [Google Scholar]
- 27.Byrne BM. Structural equation modeling with LISREL, PRELIS, and SIMPLIS: basic concepts, applications, and programming. Mahwah, NJ: Lawrence Erlbaum Associates; 1998. [Google Scholar]
- 28.National Research Council. Recommended dietary allowances. 10. Washington, DC: National Academy Press; 1989. [Google Scholar]
- 29.Bouchard C, Perusse L. Genetics of obesity: family studies. In: Bouchard C, editor. The genetics of obesity. Boca Raton, FL: CRC Press; 1994. pp. 79–92. [Google Scholar]
- 30.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–4. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 31.Hill AJ, Franklin JA. Mothers, daughters, and dieting: investigating the transmission of weight control. Br J Clin Psychol. 1998;37:3–13. doi: 10.1111/j.2044-8260.1998.tb01275.x. [DOI] [PubMed] [Google Scholar]
- 32.Troiano RP, Flegal KM. Overweight children and adolescents: description, epidemiology, and demographics. Pediatrics. 1998;101:497–504. [PubMed] [Google Scholar]
- 33.Darling N, Steinberg L. Parenting style as context: an integrative model. Psychol Bull. 1993;113:487–96. [Google Scholar]
- 34.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998;101:518–25. [PubMed] [Google Scholar]
- 35.Goran MI. Measurement issues related to studies of childhood obesity: assessment of body composition, body fat distribution, physical activity, and food intake. Pediatrics. 1998;101:505–18. [PubMed] [Google Scholar]
- 36.Hill JO, Trowbridge FL. Childhood obesity: future directions and research priorities. Pediatrics. 1998;101:570–4. doi: 10.1542/peds.101.3.570. [DOI] [PubMed] [Google Scholar]