Abstract
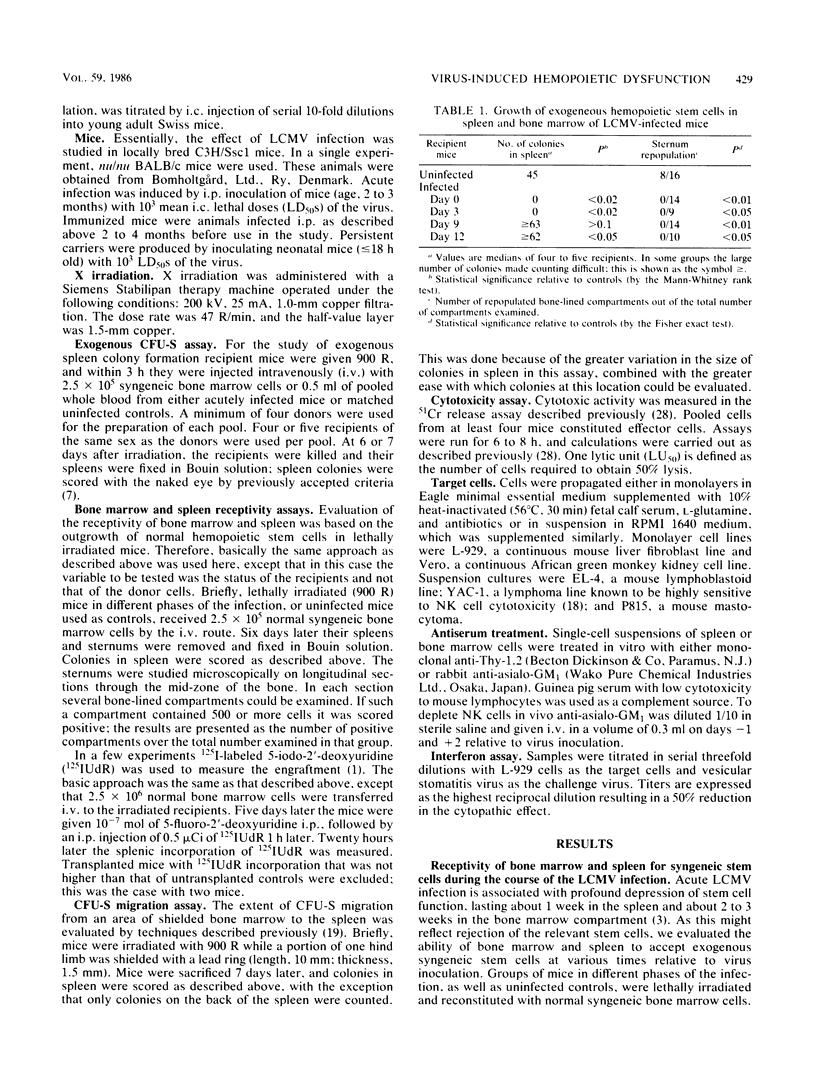
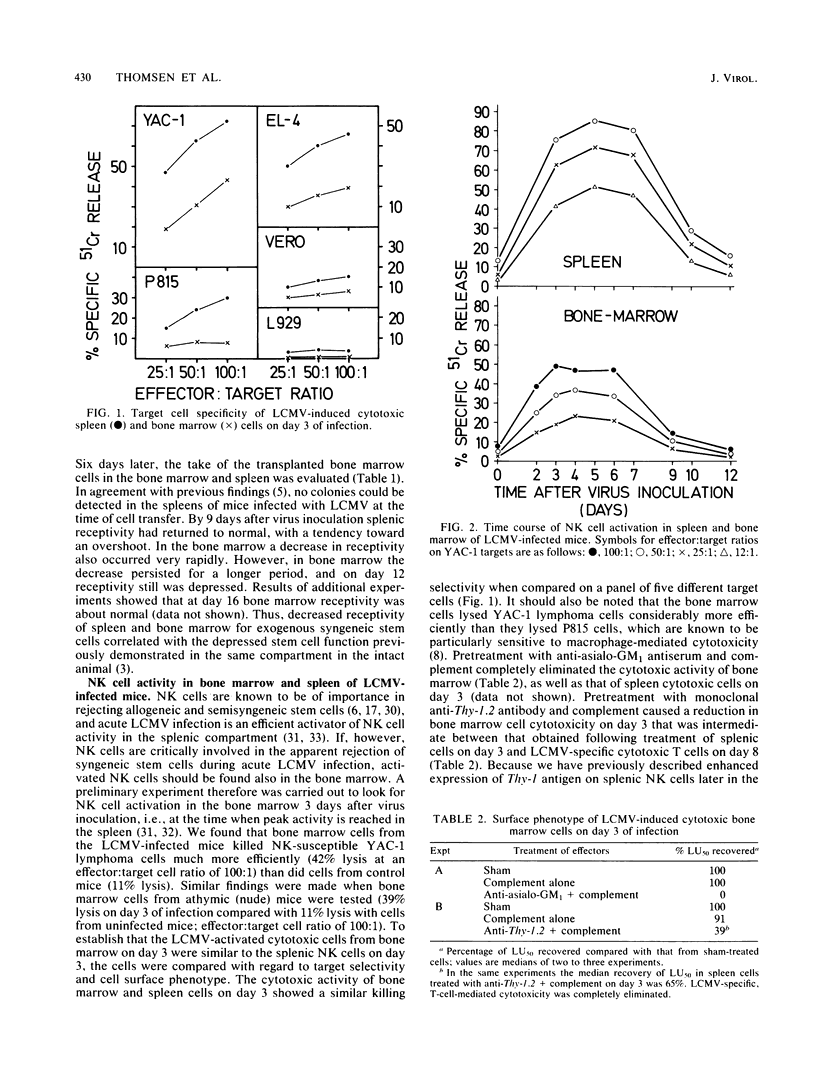
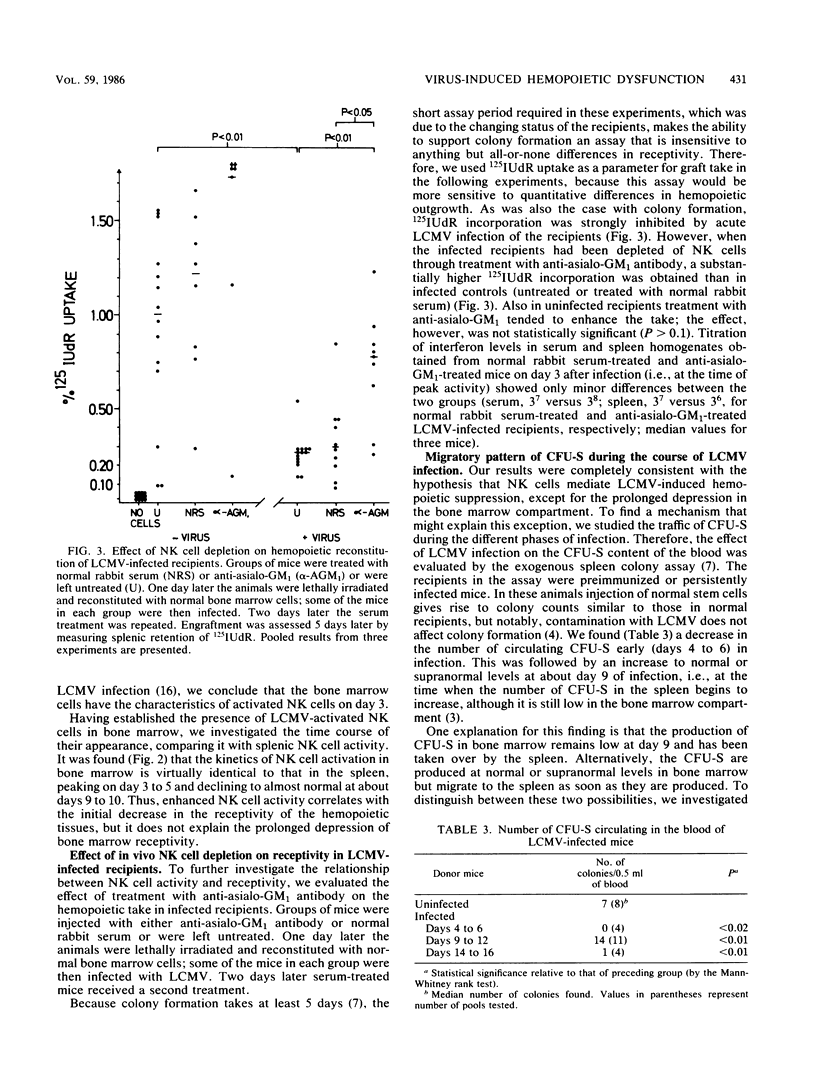
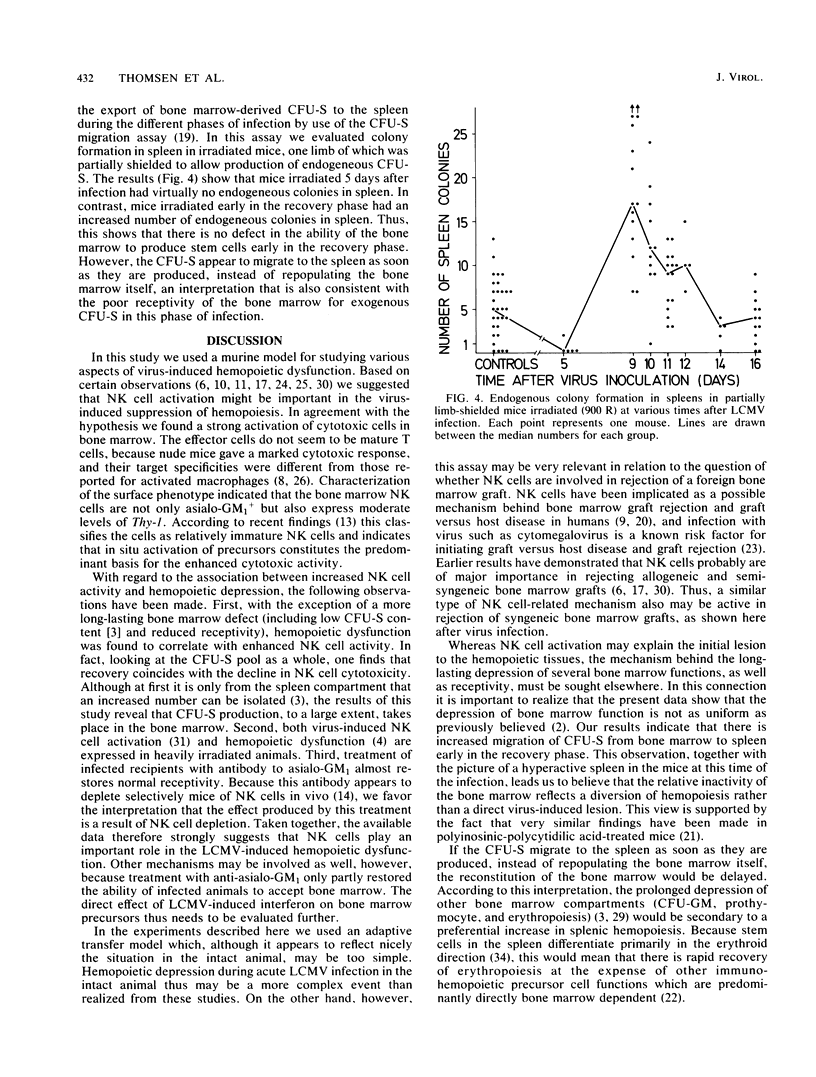
Results of this study showed that lymphocytic choriomeningitis virus infection causes a marked activation of natural killer (NK) cells not only in the spleen but also in the bone marrow. This activity reached its peak at about day 3 of infection and declined after days 6 to 7. Enhanced NK cell activity was found to correlate with decreased receptivity for syngeneic stem cells in bone marrow and spleen, with the notable exception that decreased receptivity persisted longer in bone marrow. Treatment of infected recipients with anti-asialo GM1 (ganglio-N-tetraosylceramide) significantly increased the receptivity for syngeneic hemopoietic cells. These findings are consistent with the hypothesis that NK cell activation causes rejection of syngeneic stem cells, thus resulting in hemopoietic depression. To understand the mechanisms behind the prolonged decrease in bone marrow receptivity (and bone marrow function in the intact mouse) mentioned above, we followed the changes in the number of pluripotential stem cells (CFU-S) circulating in the peripheral blood and in endogenous spleen colonies in irradiated mice, the limbs of which were partially shielded. It was found that following a marked early decline, both parameters increased to normal or supranormal levels at about day 9 after infection. Because the bone marrow pool of CFU-S is only about 20% of normal at this time after infection, a marked tendency for CFU-S at this stage in the infection to migrate from the bone marrow to the spleen is suggested. It seems, therefore, that as NK cell activity declines, the spleen regains the ability to support growth of hemopoietic cells and the bone marrow resumes an elevated export of stem cells to the spleen. This diversion of hemopoiesis could explain both the long-standing deficiencies of the bone marrow compartment and the prolonged decrease in the receptivity of this organ.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bro-Jorgensen K., Knudtzon S. Changes in hemopoiesis during the course of acute LCM virus infection in mice. Blood. 1977 Jan;49(1):47–57. [PubMed] [Google Scholar]
- Bro-Jorgensen K., Volkert M. Defects in the immune system of mice infected with lymphocytic choriomeningitis virus. Infect Immun. 1974 Apr;9(4):605–614. doi: 10.1128/iai.9.4.605-614.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bro-Jorgensen K., Volkert M. Haemopoietic defects in mice infected with lymphocytic choriomeningitis virus. 2. The viral effect upon the function of colony-forming stem cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(6):853–862. [PubMed] [Google Scholar]
- Bro-Jørgensen K. The interplay between lymphocytic choriomeningitis virus, immune function, and hemopoiesis in mice. Adv Virus Res. 1978;22:327–369. doi: 10.1016/s0065-3527(08)60777-0. [DOI] [PubMed] [Google Scholar]
- Cudkowicz G., Hochman P. S. Do natural killer cells engage in regulated reactions against self to ensure homeostasis? Immunol Rev. 1979;44:13–41. doi: 10.1111/j.1600-065x.1979.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Curry J. L., Trentin J. J. Hemopoietic spleen colony studies. I. Growth and differentiation. Dev Biol. 1967 May;15(5):395–413. doi: 10.1016/0012-1606(67)90034-6. [DOI] [PubMed] [Google Scholar]
- Doe W. F., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. I. Cytolytic effect on tumor target cells. J Exp Med. 1978 Aug 1;148(2):544–556. doi: 10.1084/jem.148.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokhelar M. C., Wiels J., Lipinski M., Tetaud C., Devergie A., Gluckman E., Tursz T. Natural killer cell activity in human bone marrow recipients: early reappearance of peripheral natural killer activity in graft-versus-host disease. Transplantation. 1981 Jan;31(1):61–65. doi: 10.1097/00007890-198101000-00014. [DOI] [PubMed] [Google Scholar]
- Hansson M., Beran M., Andersson B., Kiessling R. Inhibition of in vitro granulopoiesis by autologous allogeneic human NK cells. J Immunol. 1982 Jul;129(1):126–132. [PubMed] [Google Scholar]
- Hansson M., Kiessling R., Andersson B. Human fetal thymus and bone marrow contain target cells for natural killer cells. Eur J Immunol. 1981 Jan;11(1):8–12. doi: 10.1002/eji.1830110103. [DOI] [PubMed] [Google Scholar]
- Hansson M., Kärre K., Kiessling R., Roder J., Andersson B., Häyry P. Natural NK-cell targets in the mouse thymus: characteristics of the sensitive cell population. J Immunol. 1979 Aug;123(2):765–771. [PubMed] [Google Scholar]
- Hurme M., Sihvola M. Natural killer (NK) cell activity during lymphatic regeneration: early appearance of Thy-1+ NK cells and highly interleukin 2-(IL 2) receptive, Thy-1- cells. J Immunol. 1983 Aug;131(2):658–661. [PubMed] [Google Scholar]
- Kasai M., Yoneda T., Habu S., Maruyama Y., Okumura K., Tokunaga T. In vivo effect of anti-asialo GM1 antibody on natural killer activity. Nature. 1981 May 28;291(5813):334–335. doi: 10.1038/291334a0. [DOI] [PubMed] [Google Scholar]
- Kelleher J. F., Luban N. L., Mortimer P. P., Kamimura T. Human serum "parvovirus": a specific cause of aplastic crisis in children with hereditary spherocytosis. J Pediatr. 1983 May;102(5):720–722. doi: 10.1016/s0022-3476(83)80243-1. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Eriksson E., Hallenbeck L. A., Welsh R. M. A comparative analysis of the cell surface properties of activated vs endogenous mouse natural killer cells. J Immunol. 1980 Oct;125(4):1551–1557. [PubMed] [Google Scholar]
- Kiessling R., Hochman P. S., Haller O., Shearer G. M., Wigzell H., Cudkowicz G. Evidence for a similar or common mechanism for natural killer cell activity and resistance to hemopoietic grafts. Eur J Immunol. 1977 Sep;7(9):655–663. doi: 10.1002/eji.1830070915. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Martelly I., Jullien P. Effect of repeated injections of polyriboinosinic-polyribocytidylic acid on mouse hematopoietic stem cells. J Natl Cancer Inst. 1974 Oct;53(4):1021–1025. doi: 10.1093/jnci/53.4.1021. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The effect of bleeding on the number of in vitro colony-forming cells in the bone marrow. Br J Haematol. 1969 Apr;16(4):397–407. doi: 10.1111/j.1365-2141.1969.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Miller S. C. Genetic resistance to transplantation of xenogeneic bone marrow in mice of various strains: influence of an interferon inducer and age. Scand J Immunol. 1981 Nov;14(5):515–522. [PubMed] [Google Scholar]
- Riccardi C., Santoni A., Barlozzari T., Herberman R. B. In vivo reactivity of mouse natural killer (NK) cells against normal bone marrow cells. Cell Immunol. 1981 May 1;60(1):136–143. doi: 10.1016/0008-8749(81)90254-9. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Lohmann-Matthes M. L., Domzig W., Kiessling R., Haller O. A functional comparison of tumor cell killing by activated macrophages and natural killer cells. Eur J Immunol. 1979 Apr;9(4):283–288. doi: 10.1002/eji.1830090407. [DOI] [PubMed] [Google Scholar]
- Serjeant G. R., Topley J. M., Mason K., Serjeant B. E., Pattison J. R., Jones S. E., Mohamed R. Outbreak of aplastic crises in sickle cell anaemia associated with parvovirus-like agent. Lancet. 1981 Sep 19;2(8247):595–597. doi: 10.1016/s0140-6736(81)92739-2. [DOI] [PubMed] [Google Scholar]
- Thomsen A. R., Bro-Jørgensen K., Jensen B. L. Lymphocytic choriomeningitis virus-induced immunosuppression: evidence for viral interference with T-cell maturation. Infect Immun. 1982 Sep;37(3):981–986. doi: 10.1128/iai.37.3.981-986.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen A. R., Jensen B. L. Concanavalin A-mediated in vitro activation of a secondary cytotoxic T-cell response in virus-primed splenocytes. Scand J Immunol. 1980;12(2):109–118. doi: 10.1111/j.1365-3083.1980.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Warner J. F., Dennert G. Effects of a cloned cell line with NK activity on bone marrow transplants, tumour development and metastasis in vivo. Nature. 1982 Nov 4;300(5887):31–34. doi: 10.1038/300031a0. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med. 1978 Jul 1;148(1):163–181. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Doe W. F. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice: natural killer cell activity in cultured spleen leukocytes concomitant with T-cell-dependent immune interferon production. Infect Immun. 1980 Nov;30(2):473–483. doi: 10.1128/iai.30.2.473-483.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Zinkernagel R. M., Hallenbeck L. A. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. II. "Specificities" of the natural killer cells. J Immunol. 1979 Feb;122(2):475–481. [PubMed] [Google Scholar]
- Wolf N. S., Trentin J. J. Hemopoietic colony studies. V. Effect of hemopoietic organ stroma on differentiation of pluripotent stem cells. J Exp Med. 1968 Jan 1;127(1):205–214. doi: 10.1084/jem.127.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. T lymphocyte interaction with viruses and virus-infected tissues. Prog Med Virol. 1975;19:120–160. [PubMed] [Google Scholar]
- Young N. Aplastic anemia: research themes and clinical issues. Prog Hematol. 1981;12:227–273. [PubMed] [Google Scholar]


