Abstract
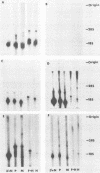
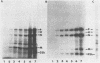
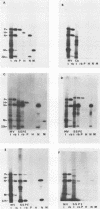
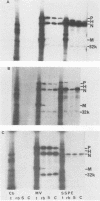
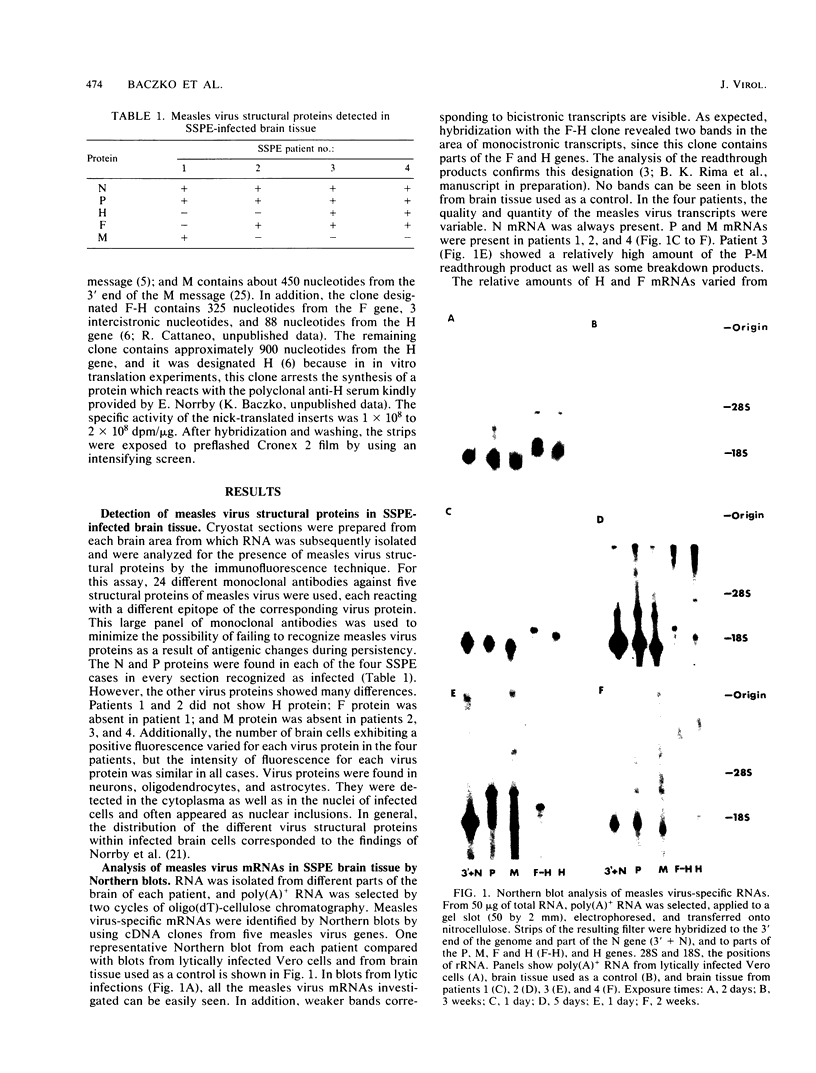
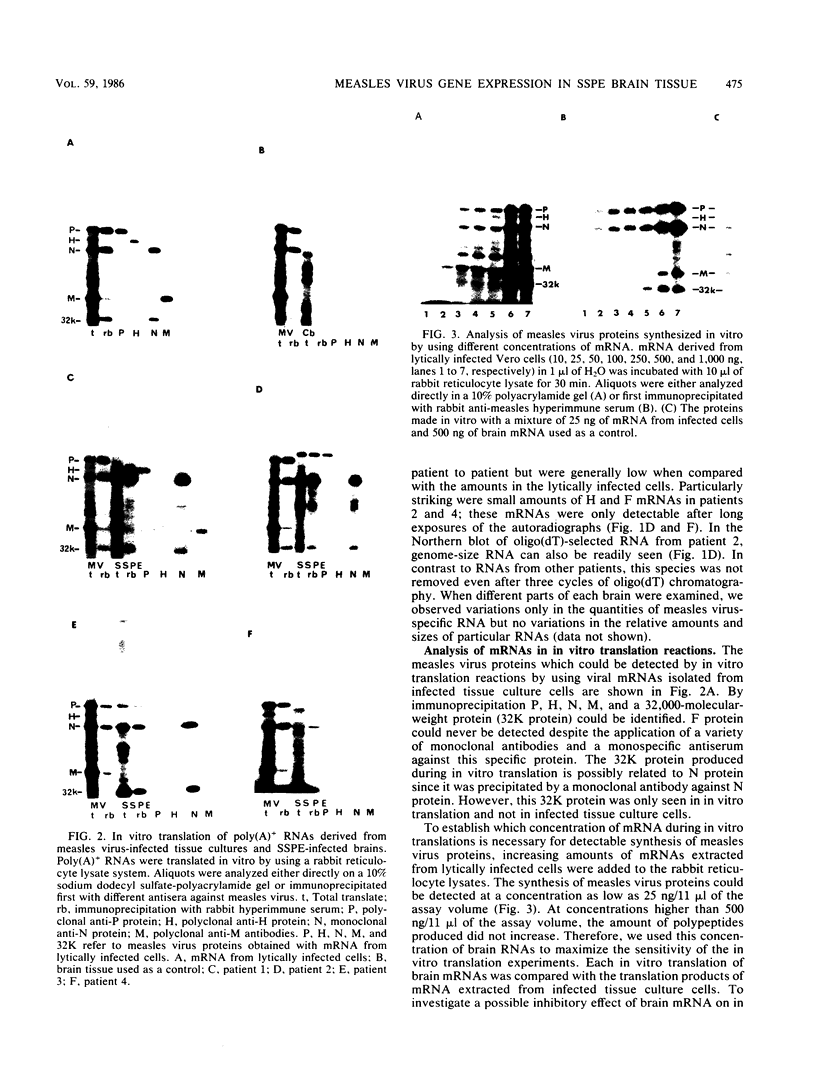
The persistence of measles virus in selected areas of the brains of four patients with subacute sclerosing panencephalitis (SSPE) was characterized by immunohistological and biochemical techniques. The five measles virus structural proteins were never simultaneously detectable in any of the brain sections. Nucleocapsid proteins and phosphoproteins were found in every diseased brain area, whereas hemagglutinin protein was detected in two cases, fusion protein was detected in three cases, and matrix protein was detected in only one case. Also, it could be shown that the amounts of measles virus RNA in the brains differed from patient to patient and in the different regions investigated. In all patients, plus-strand RNAs specific for these five viral genes could be detected. However, the amounts of fusion and hemagglutinin mRNAs were low compared with the amounts in lytically infected cells. The presence of particular measles virus RNAs in SSPE-infected brains did not always correlate with mRNA activity. In in vitro translations, the matrix protein was produced in only one case, and the hemagglutinin protein was produced in none. These results indicate that measles virus persistence in SSPE is correlated with different defects of several genes which probably prevent assembly of viral particles in SSPE-infected brain tissue.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baczko K., Carter M. J., Billeter M., ter Meulen V. Measles virus gene expression in subacute sclerosing panencephalitis. Virus Res. 1984 Oct;1(7):585–595. doi: 10.1016/0168-1702(84)90015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Richardson C. D., Rozenblatt S. Positive identification of a measles virus cDNA clone encoding a region of the phosphoprotein. J Virol. 1984 Jun;50(3):939–942. doi: 10.1128/jvi.50.3.939-942.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M. A., Baczko K., Schmid A., Ter Meulen V. Cloning of DNA corresponding to four different measles virus genomic regions. Virology. 1984 Jan 15;132(1):147–159. doi: 10.1016/0042-6822(84)90099-0. [DOI] [PubMed] [Google Scholar]
- Breschkin A. M., Morgan E. M., McKimm J., Rapp F. SSPE-BIKEN: a naturally arising hemagglutination-defective mutant of measles virus. J Med Virol. 1979;4(1):67–80. doi: 10.1002/jmv.1890040109. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Willcocks M. M., Löffler S., Ter Meulen V. Comparison of lytic and persistent measles virus matrix proteins by competition radioimmunoassay. J Gen Virol. 1983 Aug;64(Pt 8):1801–1805. doi: 10.1099/0022-1317-64-8-1801. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Willcocks M. M., Löffler S., ter Meulen V. Relationships between monoclonal antibody-binding sites on the measles virus haemagglutinin. J Gen Virol. 1982 Nov;63(Pt 1):113–120. doi: 10.1099/0022-1317-63-1-113. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Willcocks M. M., ter Meulen V. Defective translation of measles virus matrix protein in a subacute sclerosing panencephalitis cell line. Nature. 1983 Sep 8;305(5930):153–155. doi: 10.1038/305153a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Gantz D., Eble B., Walker D., Stowring L., Ventura P., Blum H., Wietgrefe S., Zupancic M., Tourtellotte W. Natural history of restricted synthesis and expression of measles virus genes in subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1985 May;82(9):3020–3024. doi: 10.1073/pnas.82.9.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Measles-virus proteins in the brain tissue of patients with subacute sclerosing panencephalitis: absence of the M protein. N Engl J Med. 1981 May 7;304(19):1152–1155. doi: 10.1056/NEJM198105073041906. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. Measles and subacute sclerosing panencephalitis virus proteins: lack of antibodies to the M protein in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2047–2051. doi: 10.1073/pnas.76.4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Norrby E., Swoveland P., Carrigan D. R. Expression of five viral antigens in cells infected with wild-type and SSPE strains of measles virus: correlation with cytopathic effects and productivity of infections. Arch Virol. 1982;73(3-4):255–262. doi: 10.1007/BF01318079. [DOI] [PubMed] [Google Scholar]
- Kimoto T., Baba K. Comparative studies on subacute sclerosing panencephalitis virus and a usual measles virus with special reference to their capacities to produce viral hemagglutinin in Vero cells. Biken J. 1975 Jun;18(2):123–133. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Hayes E. C., Gollobin S. D., Westfall L. K., Zweerink H. J. Antibodies against the measles matrix polypeptide after clinical infection and vaccination. Infect Immun. 1980 Mar;27(3):817–825. doi: 10.1128/iai.27.3.817-825.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E., Chen S. N., Togashi T., Shesberadaran H., Johnson K. P. Five measles virus antigens demonstrated by use of mouse hybridoma antibodies in productively infected tissue culture cells. Arch Virol. 1982;71(1):1–11. doi: 10.1007/BF01315171. [DOI] [PubMed] [Google Scholar]
- Norrby E., Kristensson K., Brzosko W. J., Kapsenberg J. G. Measles virus matrix protein detected by immune fluorescence with monoclonal antibodies in the brain of patients with subacute sclerosing panencephalitis. J Virol. 1985 Oct;56(1):337–340. doi: 10.1128/jvi.56.1.337-340.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rima B. K., Martin S. J., Gould E. A. A comparison of polypeptides in measles and SSPE virus strains. J Gen Virol. 1979 Mar;42(3):603–608. doi: 10.1099/0022-1317-42-3-603. [DOI] [PubMed] [Google Scholar]
- Roux L., Waldvogel F. A. Instability of the viral M protein in BHK-21 cells persistently infected with Sendai virus. Cell. 1982 Feb;28(2):293–302. doi: 10.1016/0092-8674(82)90347-6. [DOI] [PubMed] [Google Scholar]
- Rozenblatt S., Gesang C., Lavie V., Neumann F. S. Cloning and characterization of DNA complementary to the measles virus mRNA encoding hemagglutinin and matrix protein. J Virol. 1982 Jun;42(3):790–797. doi: 10.1128/jvi.42.3.790-797.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt S., Koch T., Pinhasi O., Bratosin S. Infective substructures of measles virus from acutely and persistently infected cells. J Virol. 1979 Oct;32(1):329–333. doi: 10.1128/jvi.32.1.329-333.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T. A., Fukuda A., Sugiura A. Characterization of major structural proteins of measles virus with monoclonal antibodies. J Gen Virol. 1985 Jul;66(Pt 7):1397–1409. doi: 10.1099/0022-1317-66-7-1397. [DOI] [PubMed] [Google Scholar]
- Sheppard R. D., Raine C. S., Bornstein M. B., Udem S. A. Measles virus matrix protein synthesized in a subacute sclerosing panencephalitis cell line. Science. 1985 Jun 7;228(4704):1219–1221. doi: 10.1126/science.4001938. [DOI] [PubMed] [Google Scholar]
- Sheshberadaran H., Chen S. N., Norrby E. Monoclonal antibodies against five structural components of measles virus. I. Characterization of antigenic determinants on nine strains of measles virus. Virology. 1983 Jul 30;128(2):341–353. doi: 10.1016/0042-6822(83)90261-1. [DOI] [PubMed] [Google Scholar]
- Young K. K., Heineke B. E., Wechsler S. L. M protein instability and lack of H protein processing associated with nonproductive persistent infection of HeLa cells by measles virus. Virology. 1985 Jun;143(2):536–545. doi: 10.1016/0042-6822(85)90392-7. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Löffler S., Carter M. J., Stephenson J. R. Antigenic characterization of measles and SSPE virus haemagglutinin by monoclonal antibodies. J Gen Virol. 1981 Dec;57(Pt 2):357–364. doi: 10.1099/0022-1317-57-2-357. [DOI] [PubMed] [Google Scholar]