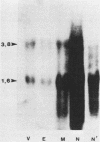
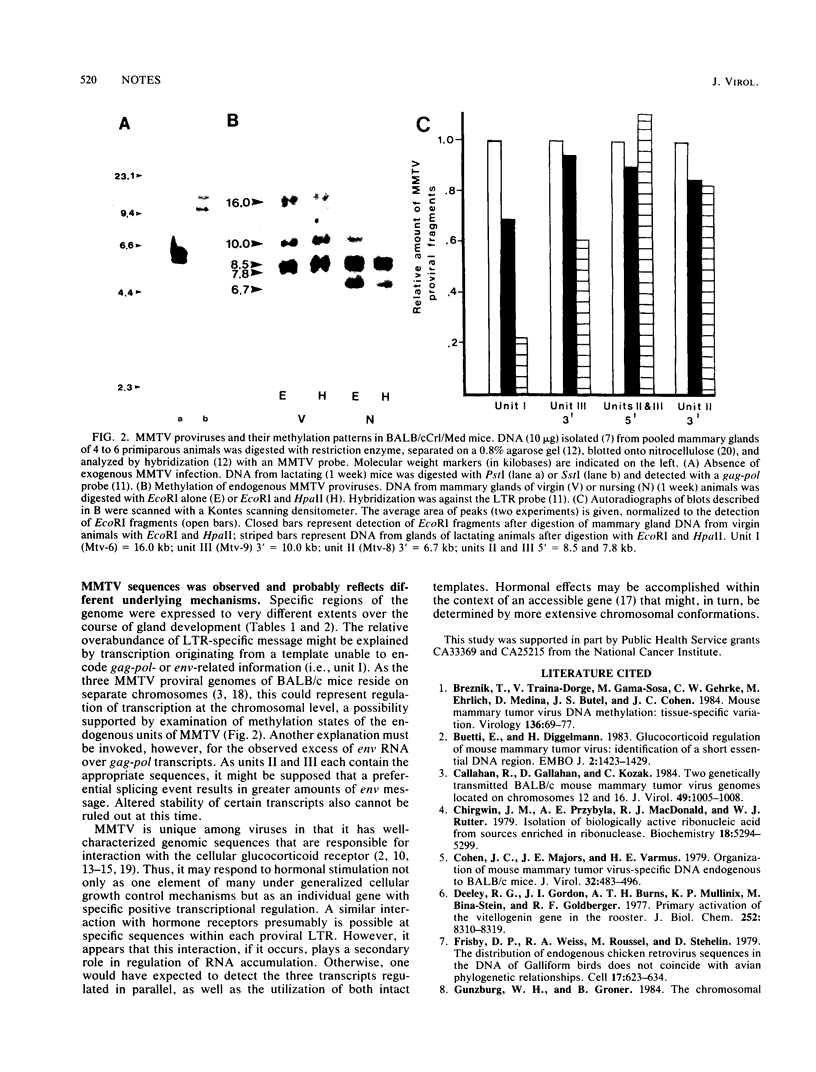
Abstract
Expression of endogenous mouse mammary tumor virus sequences varied over the course of development of the mammary gland during primary pregnancy and lactation in virus-free BALB/c mice. Although RNA from all regions of the genome was detected, both the level and temporal regulation of expression were different for long terminal repeat-, env-, and gag-pol-specific RNAs. Analysis of the methylation status of proviral DNA indicated differential accessibility of the three endogenous units during development. The results demonstrated noncoordinate regulation of mouse mammary tumor virus expression with respect to provirus template utilized and specific transcripts accumulated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breznik T., Traina-Dorge V., Gama-Sosa M., Gehrke C. W., Ehrlich M., Medina D., Butel J. S., Cohen J. C. Mouse mammary tumor virus DNA methylation: tissue-specific variation. Virology. 1984 Jul 15;136(1):69–77. doi: 10.1016/0042-6822(84)90248-4. [DOI] [PubMed] [Google Scholar]
- Buetti E., Diggelmann H. Glucocorticoid regulation of mouse mammary tumor virus: identification of a short essential DNA region. EMBO J. 1983;2(8):1423–1429. doi: 10.1002/j.1460-2075.1983.tb01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Gallahan D., Kozak C. Two genetically transmitted BALB/c mouse mammary tumor virus genomes located on chromosomes 12 and 16. J Virol. 1984 Mar;49(3):1005–1008. doi: 10.1128/jvi.49.3.1005-1008.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Majors J. E., Varmus H. E. Organization of mouse mammary tumor virus-specific DNA endogenous to BALB/c mice. J Virol. 1979 Nov;32(2):483–496. doi: 10.1128/jvi.32.2.483-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Frisby D. P., Weiss R. A., Roussel M., Stehelin D. The distribution of endogenous chicken retrovirus sequences in the DNA of galliform birds does not coincide with avian phylogenetic relationships. Cell. 1979 Jul;17(3):623–634. doi: 10.1016/0092-8674(79)90270-8. [DOI] [PubMed] [Google Scholar]
- Günzburg W. H., Groner B. The chromosomal integration site determines the tissue-specific methylation of mouse mammary tumour virus proviral genes. EMBO J. 1984 May;3(5):1129–1135. doi: 10.1002/j.1460-2075.1984.tb01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- Majors J., Varmus H. E. A small region of the mouse mammary tumor virus long terminal repeat confers glucocorticoid hormone regulation on a linked heterologous gene. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5866–5870. doi: 10.1073/pnas.80.19.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. A., Ostrowski M. C., Hager G. L., Simons S. S., Jr Covalent and noncovalent receptor-glucocorticoid complexes preferentially bind to the same regions of the long terminal repeat of murine mammary tumor virus proviral DNA. Biochemistry. 1984 Dec 18;23(26):6883–6889. doi: 10.1021/bi00321a093. [DOI] [PubMed] [Google Scholar]
- Pfahl M., McGinnis D., Hendricks M., Groner B., Hynes N. E. Correlation of glucocorticoid receptor binding sites on MMTV proviral DNA with hormone inducible transcription. Science. 1983 Dec 23;222(4630):1341–1343. doi: 10.1126/science.6318311. [DOI] [PubMed] [Google Scholar]
- Ponta H., Kennedy N., Skroch P., Hynes N. E., Groner B. Hormonal response region in the mouse mammary tumor virus long terminal repeat can be dissociated from the proviral promoter and has enhancer properties. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1020–1024. doi: 10.1073/pnas.82.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popko B. J., Pauley R. J. Organization and expression of mouse mammary tumor virus sequences in normal and neoplastic C3Hf/HeSed mouse tissues. J Virol. 1984 Nov;52(2):328–335. doi: 10.1128/jvi.52.2.328-335.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M. Regulation of mouse mammary tumor virus gene expression by glucocorticoid hormones. Curr Top Microbiol Immunol. 1983;106:79–103. doi: 10.1007/978-3-642-69357-1_4. [DOI] [PubMed] [Google Scholar]
- Robbins J. M., Gallahan D., Hogg E., Kozak C., Callahan R. An endogenous mouse mammary tumor virus genome common in inbred mouse strains is located on chromosome 6. J Virol. 1986 Feb;57(2):709–713. doi: 10.1128/jvi.57.2.709-713.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Beato M. Contacts between hormone receptor and DNA double helix within a glucocorticoid regulatory element of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1984 May;81(10):3029–3033. doi: 10.1073/pnas.81.10.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Teramoto Y. A., Medina D., McGrath C., Schlom J. Noncoordinate expression of murine mammary tumor virus gene products. Virology. 1980 Dec;107(2):345–353. doi: 10.1016/0042-6822(80)90302-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Medeiros E., Bishop J. M., Nowinski R. C., Sarkar N. H. Transcription of mouse mammary tumor virus genes in tissues from high and low tumor incidence mouse strains. J Mol Biol. 1973 Oct 5;79(4):663–679. doi: 10.1016/0022-2836(73)90070-3. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Ringold G., Yamamoto K. R. Regulation of mouse mammary tumor virus gene expression by glucocorticoid hormones. Monogr Endocrinol. 1979;12:253–278. doi: 10.1007/978-3-642-81265-1_14. [DOI] [PubMed] [Google Scholar]
- Wheeler D. A., Butel J. S., Medina D., Cardiff R. D., Hager G. L. Transcription of mouse mammary tumor virus: identification of a candidate mRNA for the long terminal repeat gene product. J Virol. 1983 Apr;46(1):42–49. doi: 10.1128/jvi.46.1.42-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooyen A. J., Michalides R. J., Nusse R. Structural analysis of a 1.7-kilobase mouse mammary tumor virus-specific RNA. J Virol. 1983 May;46(2):362–370. doi: 10.1128/jvi.46.2.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]