Abstract
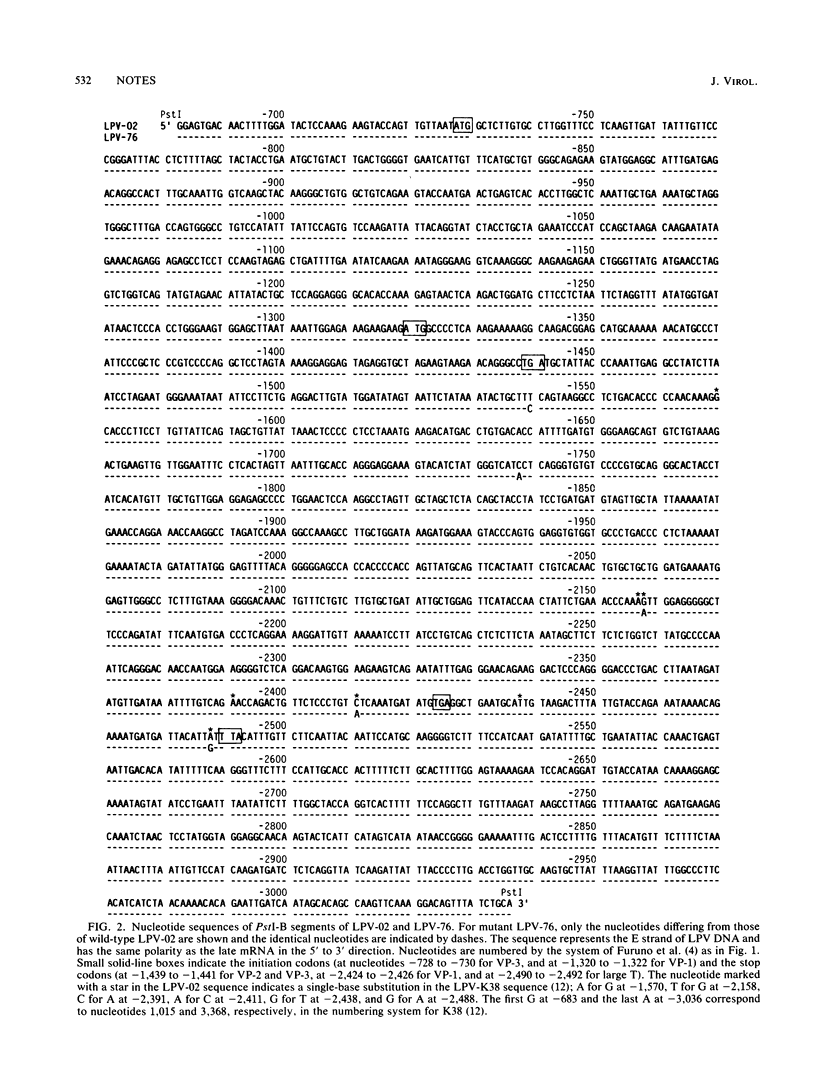
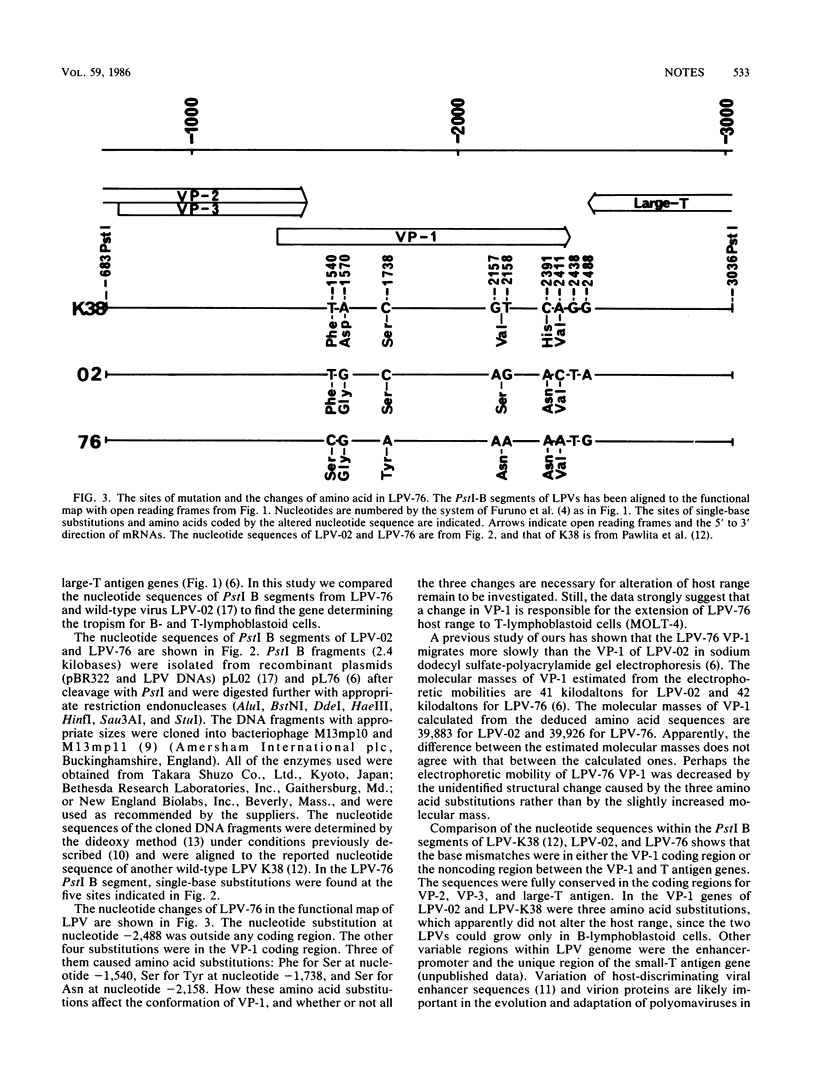
Monkey B-lymphotropic papovavirus (LPV) replicates only in B-lymphoblastoid cells, whereas the LPV mutant 76 (LPV-76) can grow in either B- or T-lymphoblastoid cells. The nucleotide sequence of the wild-type LPV PstI B segment was compared with that of LPV-76 PstI-B, within which the mutation responsible for the extended host range had been located. The VP-1 coding region of LPV-76 was found to have mutations, single-base substitutions causing three amino acid substitutions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brade L., Mueller-Lantzsch N., Kaiser S., Scharrer M. Biochemical studies on structural and nonstructural proteins of the African green monkey B-lymphotropic papovavirus (LPV). Virology. 1983 Jun;127(2):469–474. doi: 10.1016/0042-6822(83)90160-5. [DOI] [PubMed] [Google Scholar]
- Brade L., Müller-Lantzsch N., zur Hausen H. B-lymphotropic papovavirus and possibility of infections in humans. J Med Virol. 1981;6(4):301–308. doi: 10.1002/jmv.1890060405. [DOI] [PubMed] [Google Scholar]
- Brade L., Vogl W., Gissman L., zur Hausen H. Propagation of B-lymphotropic papovavirus (LPV) in human B-lymphoma cells and characterization of its DNA. Virology. 1981 Oct 15;114(1):228–235. doi: 10.1016/0042-6822(81)90268-3. [DOI] [PubMed] [Google Scholar]
- Furuno A., Miyamura T., Yoshiike K. Monkey B-lymphotropic papovavirus DNA: nucleotide sequence of the region around the origin of replication. J Virol. 1984 May;50(2):451–456. doi: 10.1128/jvi.50.2.451-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Matsumura Y., Nishihira T., Watanabe I., Ohi R., Kasai M., Kumagai K., Kamada N. Burkitt's lymphoma cell line bearing surface IgA and negative for nuclear antigen of Epstein-Barr virus (EBNA). Jpn J Exp Med. 1980 Dec;50(6):423–434. [PubMed] [Google Scholar]
- Kanda T., Takemoto K. K. Monkey B-lymphotropic papovavirus mutant capable of replicating in T-lymphoblastoid cells. J Virol. 1985 Jul;55(1):96–100. doi: 10.1128/jvi.55.1.96-100.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T., Yoshiike K., Takemoto K. K. Alignment of the genome of monkey B-lymphotropic papovavirus to the genomes of simian virus 40 and BK virus. J Virol. 1983 Apr;46(1):333–336. doi: 10.1128/jvi.46.1.333-336.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miyamura T., Furuno A., Yoshiike K. DNA rearrangement in the control region for early transcription in a human polyomavirus JC host range mutant capable of growing in human embryonic kidney cells. J Virol. 1985 Jun;54(3):750–756. doi: 10.1128/jvi.54.3.750-756.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosthaf L., Pawlita M., Gruss P. A viral enhancer element specifically active in human haematopoietic cells. Nature. 1985 Jun 13;315(6020):597–600. doi: 10.1038/315597a0. [DOI] [PubMed] [Google Scholar]
- Pawlita M., Clad A., zur Hausen H. Complete DNA sequence of lymphotropic papovavirus: prototype of a new species of the polyomavirus genus. Virology. 1985 May;143(1):196–211. doi: 10.1016/0042-6822(85)90108-4. [DOI] [PubMed] [Google Scholar]
- Sahai Srivastava B. I., Minowada J. Terminal deoxynucleotidyl transferase activity in a cell line (molt-4) derived from the peripheral blood of a patient with acute lymphoblastic leukemia. Biochem Biophys Res Commun. 1973 Apr 2;51(3):529–535. doi: 10.1016/0006-291x(73)91346-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa K., Takemoto K. K. Identification of B-lymphotropic papovavirus-coded proteins. J Virol. 1983 Feb;45(2):872–875. doi: 10.1128/jvi.45.2.872-875.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Furuno A., Kato K., Yoshiike K. Biological and biochemical studies of African green monkey lymphotropic papovavirus. J Virol. 1982 May;42(2):502–509. doi: 10.1128/jvi.42.2.502-509.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Kanda T. Lymphotropic papovavirus transformation of hamster embryo cells. J Virol. 1984 Apr;50(1):100–105. doi: 10.1128/jvi.50.1.100-105.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., Gissmann L. Lymphotropic papovaviruses isolated from African green monkey and human cells. Med Microbiol Immunol. 1979 Aug;167(3):137–153. doi: 10.1007/BF02121180. [DOI] [PubMed] [Google Scholar]


