Abstract
Traumatic brain injury (TBI) is a major cause of disability in the pediatric population and can result in abnormal development. Experimental studies conducted in animals have revealed impaired plasticity following developmental TBI, even in the absence of significant anatomical damage. The N-methyl-D-aspartate receptor (NMDAR) is clearly involved in both normal development and in the pathophysiology of TBI. Following lateral fluid percussion injury in postnatal day (PND) 19 rats, we tested the hypothesis that TBI sustained at an early age would result in impaired NMDAR expression. Using immunoblotting and reverse transcriptase-polymerase chain reaction (RT-PCR), protein and RNA levels of NMDAR subunits were measured in the cerebral cortex and hippocampus on post-injury days (PID) 1, 2, 4, and 7 (though the PID7 analysis was only for protein) and compared with age-matched shams. Significant effects of hemisphere (analysis of variance [ANOVA], p < 0.01), and interactions between hemisphere and injury (ANOVA, p < 0.05) and hemisphere and PID (ANOVA, p < 0.05) were found for synaptic protein levels of the NR2A subunit in hippocampus. Specifically, within the ipsilateral hippocampus, NR2A was reduced by 9.9%, 47.9%, 40.8%, and 6.3% on PID1, PID2, PID4, and PID7, respectively. Within the cortex, there was a significant effect of injury (ANOVA, p < 0.05) without any hemispheric differences. These bilateral cortical reductions measured 30.5%, 3.2%, 5.7%, and 13.4% at the same timepoints after injury. Injury had no significant main effect on NR1 or NR2B protein levels. RT-PCR analysis showed no significant changes in NR1, NR2A, or NR2B gene expression; however, as a positive control, hsp70 was induced more than twofold in ipsilateral cortex and hippocampus on PID1. It is known that NR2A expression levels increase during normal development, and in response to environmental stimuli. Our data suggest that injury-induced reduction in the expression of NR2A is one likely mechanism for the impaired experience-dependent neuroplasticity seen following traumatic injury to the immature brain.
Keywords: glutamate, immature, pediatric, plasticity, synapse, synaptoneurosomes, traumatic brain injury
INTRODUCTION
Pediatric Traumatic Brain Injury (TBI) results in 3,000-7,000 deaths and an estimated 100,000 hospitalizations in the United States annually, making it the major cause of mortality and morbidity in this age group (Weiner and Weinberg, 2000). Traumatic injury to the immature brain can result in abnormal development and lead to persistent cognitive and behavioral problems (Bloom et al., 2001; Ewing-Cobbs et al., 1998; Fineman et al., 2000; (Giza et al., 2005; Levin, 2003; Max et al., 1997; Taylor and Alden, 1997).
N-Methyl-D-aspartate receptor (NMDAR) activation has an important role in traumatic brain injury (Faden et al., 1989; (Yoshino et al., 1992) and is a necessary component of experience-dependent plasticity and normal development (Carroll and Zukin, 2002; Kind and Neumann, 2001; (Perez-Otano and Ehlers, 2005). N-methyl-D-aspartate receptor-mediated neurotransmission is regulated by the number of receptors expressed at the synapse and the subunit composition of these receptors (Chen et al., 1999; Scheetz and Constantine-Paton, 1994). Changes in NMDAR-mediated neurotransmission lead to alterations in synaptic plasticity (Jablonska et al., 1995; Kleinschmidt et al., 1987; Maletic-Savatic et al., 1999; Quinlan et al., 1999b). Structurally, the NMDAR is composed of two major subunits, NR1 and NR2. The NR1 subunit is required for the function of the ion channel, but does not confer substantial glutamate sensitivity. Rather, differential glutamate sensitivity is conferred by the NR2 subunit, with four subtypes designated A-D. NR2A subunit-predominant receptors are less sensitive to glutamate, generate lower peak currents, and remain open for a shorter time than those with predominantly NR2B subunits (Scheetz and Constantine-Paton, 1994).
While both NR2A and NR2B are found extensively in neocortex and hippocampus and both increase during development, they are differentially expressed as the brain matures. In the neonatal period, NR2B predominates and there is little NR2A expression, but with cerebral maturation, NR2A increases much more rapidly than NR2B (Monyer et al., 1994; Sheng et al., 1994). This preferential increase in NR2A results in a developmental increase in the relative ratio of NR2A/NR2B expression (as measured by mRNA or protein). In visual cortex, this change in the subunit ratio of NR2A/NR2B occurs at the time of ocular dominance column formation (in ferret; Roberts and Ramoa, 1999) and is altered by visual experience (in rat; Quinlan et al., 1999a,b). Similar changes in NR2A/NR2B ratio can be seen as somatosensory cortex matures, that is, an increased NR2A/NR2B ratio is associated with a change in NMDAR function as measured by duration of N-methyl-D-aspartate (NMDA)-mediated currents (Flint et al., 1997). The remaining NR2 subtypes—NR2C and NR2D—are not predominantly expressed in our regions of interest (cortex and hippocampus; Scheetz and Constantine-Paton, 1994), nor have they been as closely tied to experience-dependent developmental plasticity.
The consequences of NMDAR manipulation in the developing brain are distinct from similar responses in the mature brain. For example, while overstimulation of NMDARs in both the developing and adult brain results in excitotoxicity, NMDAR antagonists are neuroprotective preferentially in the adult. In fact, administration of NMDAR antagonists early in development (before postnatal day 7) has been shown to result in significant cell death via apoptotic mechanisms (Bittigau et al., 1999; Ikonomidou et al., 1999; Ikonomidou and Turski, 1996; Pohl et al., 1999).
One of the neurochemical hallmarks of TBI is the indiscriminate release of glutamate (Faden et al., 1989; (Katayama et al., 1990), which results in ionic fluxes and is associated with altered cerebral glucose metabolism (Yoshino et al., 1991). Preventing NMDAR activation using pharmacological antagonists or by lesioning glutamatergic pathways mitigates this post-traumatic ionic flux (Katayama et al., 1990) and elevated glucose uptake (Kawamata et al., 1992; Yoshino et al., 1992). Other studies have shown evidence for NMDAR dysfunction following experimental TBI in adult animals, including decreased NMDAR binding within 24 h of injury (Miller et al., 1990) and impaired induction of long-term potentiation up to 15 days post-injury (D’Ambrosio et al., 1998; Reeves et al., 1995; Sick et al., 1998).
To induce cerebral trauma without significant cell death, we utilized a model of concussive brain injury in the preweanling rat. This fluid percussion (FP) model induces a biomechanical injury to the brain without extensive histological damage (Fineman et al., 2000; Gurkoff et al., 2006; Prins et al., 1996). Within a day after injury, rat pups subjected to a moderate FP injury demonstrated no deficits in open field behavior or Morris Water Maze acquisition when compared with age-matched shams (Fineman et al., 2000; Prins and Hovda, 1998). However, when postnatal day (PND) 19 pups were reared in an enriched environment (EE) after FP, the FP/EE rats failed to demonstrate increases in cortical thickness and improvements in Morris Water Maze performance seen in sham-injured/EE-reared controls (Fineman et al., 2000; Giza et al., 2005). Therefore, while developmental FP injury alone does not lead to gross histological or behavioral deficits, it does impair experience-dependent neuroplasticity (and concomitant anatomical changes) induced by EE rearing. This, combined with the abovementioned evidence of altered glutamatergic neurotransmission following adult TBI, led to our hypothesis that TBI during development would induce molecular changes in the NMDAR, either by a reduction in number or by alteration of subunit composition.
METHODS
Subjects
Animals studied (n = 80) were all male Sprague-Dawley rat pups that underwent sham or FP injury on PND19. For protein studies, at each time point, animals were randomly divided into two groups: age-matched sham (n = 7) and FP-injured (n = 7) animals. For gene expression studies, at each time point, animals were also randomly divided into the same two groups: age-matched sham (n = 4) and FP injured (n = 4). Tissue harvesting time points for protein were post-injury day (PID) 1, 2, 4, and 7. Tissue for RNA was harvested on PID1, PID2, and PID4. A total of 94 animals underwent surgery, with 12 excluded for mild injury and two that died after injury. All pups for all experiments were housed as litters with access to mothers until weaning on PND20, 1 day following surgery. An additional six naive animals were used to characterize the synaptoneurosome preparation. All procedures were approved by the UCLA Chancellor’s Animal Research Committee.
Fluid Percussion Injury
PND19 Sprague-Dawley male pups were anesthetized with 1-2 mL/min isoflurane in 100% O2 using a mask. After reaching a surgical level of anesthesia (complete loss of response to painful stimuli), the animals were secured in a stereotaxic frame, where the body temperature was kept constant (37-38°C) by a thermostatic heating pad. The skin was sterilely prepped with betadine and ethanol, and a sagittal incision was made in the scalp, exposing the pericranium. A 3.0-mm-diameter craniotomy was made 3.0-mm posterior to bregma and 6.0-mm lateral (left) to the midline, using a high-speed drill (Dremel). A plastic injury cap was secured over the craniotomy using silicone adhesive, cyanoacrylate, and dental cement. When the dental cement completely hardened, the injury cap was filled with saline. Anesthesia was then removed, and the animal was attached to the FP device (Dixon et al., 1987). At the first sign of hindpaw withdrawal reflex, a moderate fluid pulse was administered (2.65-2.7 atm). Apnea and loss of consciousness (LOC) times were recorded, and were determined by the resumption of spontaneous respiration and by responsiveness to toe pinch, respectively. If no spontaneous respirations were evident by 40 sec post-injury, resuscitation with 100% O2 was administered until spontaneous respirations returned. For the level of injury severity used in this study, all animals required some resuscitation, as more mildly injured animals (LOC < 120 sec) were excluded. After recovery of the hindpaw withdrawal reflex, the animal was re-anesthetized for injury cap removal and closure of the surgical incision. Local infiltration of marcaine (0.25%) and topical application of antibiotic ointment completed the procedure. Sham animals underwent the similar preparation as above, up to and including craniotomy, but without attachment of the injury cap or administration of the fluid pulse.
Tissue Harvesting and Synaptoneurosome Preparation
For protein analysis, brains of the FP-injured pups and their age-matched shams were harvested on PID1, PID2, PID4, and PID7. To enrich the samples for synaptic proteins and reduce variability in total protein content, synaptoneurosomes were prepared following standard protocols (Johnson et al., 1997; Quinlan et al., 1999a,b; Scheetz et al., 2000). The brains were immediately placed in ice-cold homogenization buffer (HB; 0.137 M NaCl, 2.7 mM KCl, 0.44 mM KH2PO4, 4.2 mM NaHCO3, 10 mM HEPES, 10 mM glucose, pH 7.4). The brains were divided into 3-mm sections using a brain slicer. Ipsilateral and contralateral parietal cortex and hippocampus were dissected and then homogenized in 1.0 mL of HB. Following this, each sample was diluted with an additional 1.8 mL of ice-cold HB. The mixture was loaded into a 3.0-mL syringe attached to a 13-mm-diameter Millipore syringe filter holder, forced through a 100-μm nylon mesh filter, and collected in a polystyrene round bottom tube. The filtered homogenate was then loaded into another 3.0-mL syringe and sequentially filtered through two, pre-wetted 5-μm nitrocellulose membranes. The homogenate was kept on ice at all times. The filtrate was then centrifuged at 1000 × g for 20 min. The supernatant was removed, and the remaining pellet was resuspended in 1.0 mL of homogenization buffer and spun at 1000 × g for 10 min. After the second supernatant was removed, the remaining pellet (synaptoneurosome fraction) was resuspended in 150 μL of homogenization buffer and stored at −70°C for immunoblot analysis.
Protein Quantitation and Immunoblotting
Total protein of each brain synaptoneurosome fraction was determined using the BioRad DC assay. Synaptoneurosome samples (10 μg protein each) were resolved on a 7.5% Tris-HCl ready-made minigel (Bio-Rad, 2006) at 160 V for 40 min, then transferred onto nitrocellulose (Bio-Rad Criterion Transfer Apparatus) at 100 V for 60 min. The nitrocellulose membrane was stained for protein using the Bio-Rad Sypro Ruby Protein Stain solution and imaged using the Bio-Rad Fluor-S MultiImager.
After imaging for total protein, the nitrocellulose was blocked in 7% milk in Tris-buffered saline with 1% Tween-20 (TTBS) for 60 min and then incubated overnight with various primary antibodies diluted in TTBS. Primary antibodies (Chemicon, Temecula, CA) were used at the following dilutions: NR1, 1:100; and NR2A and NR2B, 1:750. This was followed by peroxidase-conjugated secondary antibody incubation, diluted 1:10,000 in 1% milk in TTBS. Bands on the Western blots were then visualized using Supersignal Chemiluminescent substrate (Pierce product no. 34080) and detected with the Bio-Rad Fluor-S MultiImager.
Tissue Harvesting and mRNA Preparation
At each time point—i.e., PID1, PID2, or PID4—rats were quickly decapitated and their brain regions were isolated and dissected at 4°C in a manner similar to that of the protein samples above, without the synaptoneurosome preparation. The dissected brain regions were stored at —70°C until used for RNA isolation. Total RNA was isolated from each sample using the RNA-STAT-60 solution (Tel-Test Inc.) and purified by the RNeasy Mini Kit spin columns (Qiagen).
Real-Time Reverse Transcription-Polymerase Chain Reaction and Analysis
Relative expression of mRNA for the genes encoding the NMDA subunits NR1, NR2A, and NR2B was performed by the Comparative CT (ddCT) Method (Perkin-Elmer) using the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene as control. A validation experiment was performed for each gene of interest and its control in order to determine the conditions for optimal concentration of primers and probes. The threshold cycle CT of each gene was recorded by a real-time polymerase chain reaction (PCR) machine (iCycler iQ Detector, Bio-Rad). The normalized CT (dCT) was obtained by subtraction of the CT for GAPDH from the CT for the gene of interest. The difference between the dCT for experimental and control samples gave rise to the ddCT value that was used for the calculation of the relative mRNA expression using a formula 2-ddCT as modified by Pfaffl (2001). The relative mRNA level is expressed as fold change in injured samples over sham.
The following primer pairs were used for each gene of interest: NR 1: Forward primer: 5′-GTT CTT CCG CTC AGG CTT TG-3′; Reverse primer: 5′-AGG GAA ACG TTC TGC TTC CA-3′; Probe: 5′-CGG CAT GCG CAA GGA CAG CC-3′.
NR2A: Forward primer: 5′-AGC CCC CTT CGT CAT CGT A-3′; Reverse primer: 5′-GAC AGG GCA CCG TGT TCC T-3′; Probe: 5′-AGG ACA TAG ACC CCC TGA CTG AGA CCT GTG-3′. NR2B: Forward primer: 5′-AGC TGG TAG CCA TGA AC-3′; Reverse primer: 5′-GAT CTT CCG GTC AGA CAT-3′; Probe: 5′-CTG ACC CAA AGA GCA TCA TCA CCC G-3′. GAPDH: Forward primer: 5′-TGC ACC ACC AAC TGC TTA-3′; Reverse primer: 5′-GGA TGC AGG GAT GAT GTT-3′; Probe: 5′-CAG AAG ACT GTG GAT GGC CCC TC-3′. HSP70: Forward primer: 5′-AGG TTG CAT GTT CTT TGC G-3′; Reverse primer: 5′-AAA CTG TAC ACA GGG TGG C-3′; Probe: 5′-CTT CCT GCG AAC ACC TCA GCA CT-3′.
Immunoblot Analysis
Once the Sypro Ruby stain protein image was acquired, lane length and width were defined for signal quantification using the Bio-Rad Quantity One software. The software identifies and subtracts background within each lane using the mathematical rolling disc method. Trace quantity values of intensity count per unit length were generated and used as a measure of total protein detected on the nitrocellulose membrane post-transfer (Bio-Rad, Sypro Ruby Instruction Manual) (Berggren et al., 2002).
After antibody reaction, volumetric analyses were performed on the immunoblot bands using Quantity One software. Volume values of intensity count per unit area are generated for each band and used as a measure of the specific subunit being detected (Bio-Rad, Quantity One Software Manual). The immunoblot signal values were then normalized to the total protein signal (Sypro Ruby stain) from the same lane.
Statistical Analysis
At each postnatal age/PID, the average of protein-normalized sham values was then set at 100%. This was necessary to control for age-specific changes in NMDAR protein levels, and also to allow comparisons of sham versus injured differences between blots. Each immunoblot thus contained sham and injured samples for a given postnatal age/PID. Individual percent values for each sample were calculated for both sham and injured specimens. These individual percent values of the normalized immunoblot signals were then subjected to analysis of variance (ANOVA) by brain region using group (sham or injured) and time point (PID1, PID2, PID4, PID7) as between-subject variables and hemisphere (ipsilateral or contralateral) as a within-subject variable. If a main effect or interaction was detected by ANOVA in the overall model and since separate animals were analyzed for each individual PID, descriptive statistics using t-tests were performed between sham and injured samples on individual PIDs to illustrate the timecourse differences. All analyses were performed using SPSS v11.0 software. All values are presented as mean ± standard error of the mean.
RESULTS
Subject and Injury Characteristics
Characteristics for all groups are summarized in Table 1. The average apnea time for all injured animals was 213 ± 17 sec. There were no significant differences in apnea time across all post-injury time points (PID1 220 ± 38 sec, PID2 266 ± 44 sec, PID4 165 ± 15 sec, PID7 195 ± 20 sec; ANOVA F3,27 = 1.8, NS). Duration of loss of consciousness (LOC) averaged 265 ± 18 sec for all injured animals. Unconsciousness times for each post-injury time point were as follows: PID1 280 ± 36 sec, PID2 307 ± 45 sec, PID4 202 ± 16 sec; PID7 273 ± 40 sec. No significant differences were found at any time point (ANOVA F3,27 = 2.1, NS). There was a trend toward shorter apnea and unconsciousness in the PID4 group; however, no animal in any group experienced unconsciousness of <120 sec. At the time of surgery, no significant weight differences were detected between injury groups (i.e., sham vs. FP; ANOVA F1,56 = 0.12, NS) or between post-injury time point groups (PID1, PID2, PID4, PID7; ANOVA F3,56 = 2.4, NS). The animals randomized into the PID4 and PID7 groups tended to weigh slightly more (Table 1).
TABLE 1.
Subject Characteristics
|
Post-injury day (PID)/ group |
Weight, start (g) |
Drop angle |
Apnea (sec) |
Duration of unconsciousness (sec) |
N (protein) |
N (RNA) |
|---|---|---|---|---|---|---|
| PID1 Sham | 46.7±1.4 | NA | NA | NA | 7 | 4 |
| FP | 48.0±2.0 | 14.9±0.4 | 220±38 | 280±36 | 7 | 4 |
| PID2 Sham | 45.6±1.1 | NA | NA | NA | 7 | 4 |
| FP | 46.6±1.5 | 15.5±0.1 | 266±44 | 307±45 | 7 | 4 |
| PID4 Sham | 50.1±1.4 | NA | NA | NA | 7 | 4 |
| FP | 48.1±1.9 | 15.6±0.1 | 165±15 | 202±16 | 7 | 4 |
| PID7 Sham | 51.7±2.0 | NA | NA | NA | 7 | — |
| FP | 50.4±1.8 | 15.4±0.1 | 195±20 | 273±40 | 7 | — |
Mean (±SEM) starting weight (in g) for all age-matched sham and FP-injured groups. Mean (±SEM) drop angle, apnea (in sec), and unconsciousness (in sec) following moderate-severe lateral FP injury. Groups were studied for both N-methyl-D-aspartate receptor subunit protein (n = 7) and mRNA (n = 4) characterization. Post-injury time points were 1, 2, 4, and 7 days, and all groups had the appropriate age-matched shams. There were no significant differences in starting weight, drop angle, apnea, or unconsciousness times between sham and injured groups, or between injured groups sacrificed at different time points (analysis of variance), although there was a trend towards shorter apnea and unconsciousness times in the animals randomized to the PID 4 group.
FP, fluid percussion.
Furthermore, no significant differences in apnea or LOC were detected when animals harvested for measurement of protein expression or gene expression were analyzed separately (apnea: ANOVA F1,40 = 0.37, NS; LOC: ANOVA F1,40 = 2.4, NS). There was a small but significant starting weight difference (4.4 g) between all animals harvested for protein studies (49.4 ± 0.7 g) compared with those used for gene expression analysis (45.0 ± 0.7 g; ANOVA F1,80 = 14.1, p < 0.001).
Synaptoneurosome Preparation
We utilized a well-described synaptoneurosome preparation to enhance the immunoblot signal of synaptic NMDA receptor subunit proteins and reduce sample variability (Johnson et al., 1997; Quinlan et al., 1999b; Scheetz et al., 2000). To demonstrate the benefits of the synaptoneurosome fraction in our hands, a representative set of naive control preparations (n = 6) was prepared for both crude protein homogenates and synaptoneurosomes. After normalizing for total protein content, crude homogenates and synaptoneurosome samples from identical cortical brain regions were immunoblotted for NR2A and NR2B in triplicate. On average, the synaptoneurosome fractions demonstrated a 181 ± 31% enrichment in NR2A antibody signal and a 280 ± 51% increase in NR2B signal when compared to crude homogenates. The NMDAR subunit signals were significantly higher in the synaptoneurosome fraction than in the crude homogenate for both subunits (one-tailed t-test; NR2A, p < 0.02; NR2B, p < 0.002). Furthermore, the coefficient of variance (CV) of the synaptoneurosome immunoblot signals was 3-6% lower than the crude homogenate, suggesting that analysis of the synaptoneurosome preparation would provide enhanced sensitivity to detect subunit changes.
Immunoblotting
NMDAR1
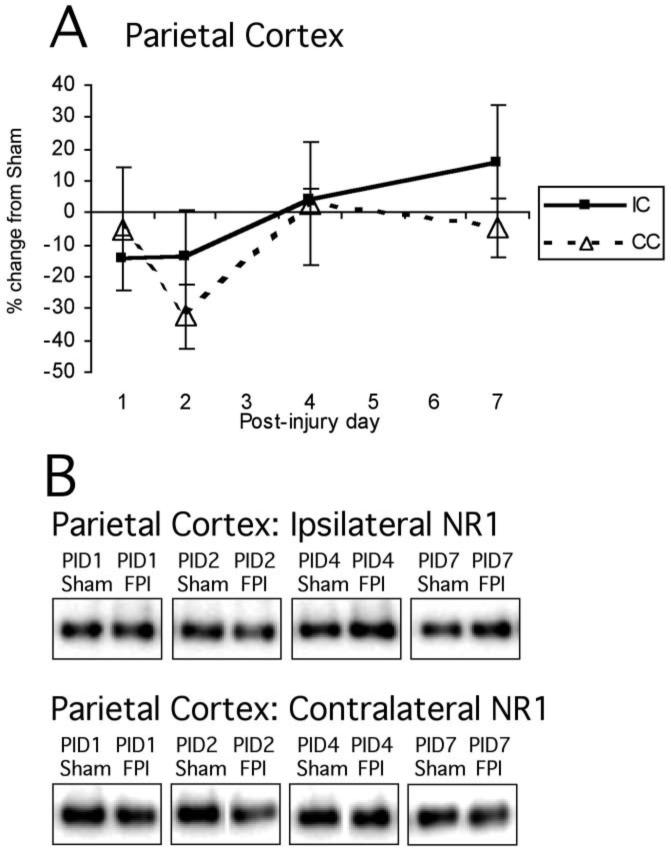
Immunoblotting for the NR1 subunit showed no significant overall effect of injury in any brain region (cortex: F1,47 = 0.79, NS; hippocampus F1,47 = 1.04, NS), nor was there any significant effect of hemisphere, PID, or interaction between these variables (Fig. 1A,B). In cortex, ipsilateral sham values were 100 ± 3.6, and ipsilateral FP were 98.0 ± 5.9; cortical contralateral sham signals averaged 100 ± 5.6, and contralateral FP averaged 91.0 ± 7.3.
FIG. 1.
Mean percent change (±SEM) of NR1 in lateral fluid percussion-injured (FPI) rats in parietal cortex (A) at post-injury day (PID) 1, 2, 4, and 7 days post-injury, as compared to age-matched shams (defined as 0%), with representative immunoblots of pooled sham and lateral FPI ipsilateral hippocampal synaptoneurosomes at PID1, PID2, PID4, and PID7 (B). No overall main effects of injury were detected for any region. IH, ipsilateral hippocampus; CH, contralateral hippocampus; IC, ipsilateral cortex; CC, contralateral cortex.
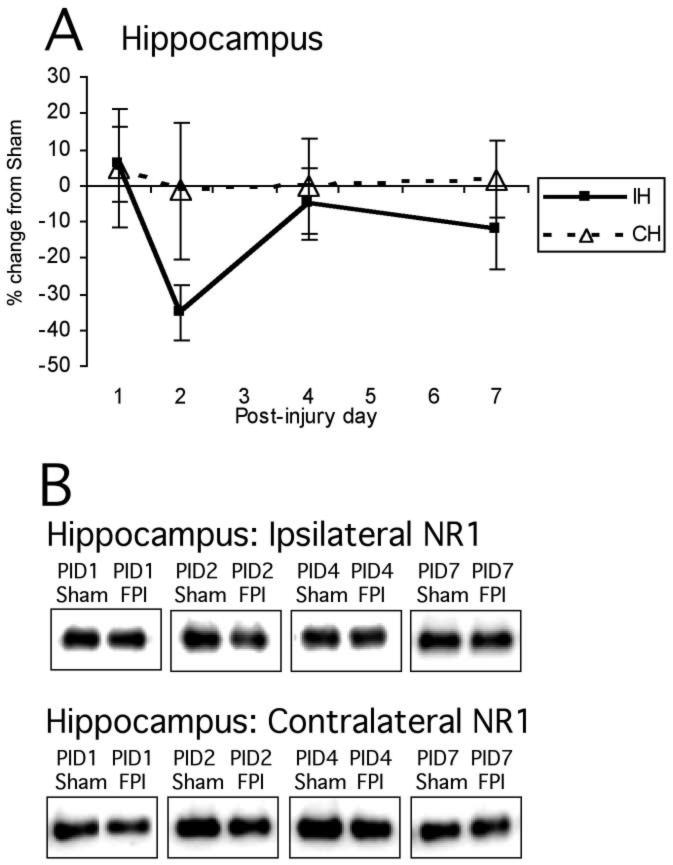
Normalized NR1 signals for hippocampus were as follows: ipsilateral sham 100 ± 5.7, ipsilateral FP 87.7 ± 5.2, contralateral sham 100 ± 6.4, and contralateral FP 100.5 ± 8.4. As in cortex, there was no overall effect of hemisphere, PID, or interaction between these variables (Fig. 2A,B).
FIG. 2.
Mean percent change (±SEM) of NR1 in lateral FPI rats in hippocampus (A) on post-injury day (PID) 1, 2, 4, and 7 as compared to age-matched shams (defined as 0%), with representative immunoblots of pooled sham and lateral FPI ipsilateral hippocampal synaptoneurosomes at PID1, PID2, PID4, and PID7 (B). No overall main effects of injury were detected for any region. For abbreviations, see legend to Figure 1.
NMDAR2A
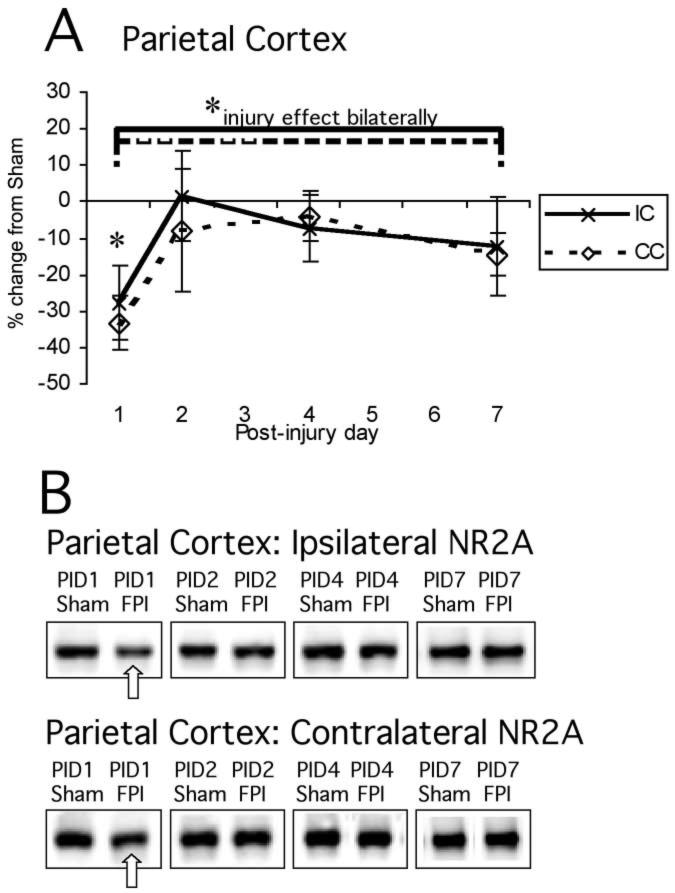
Normalized NR2A signal in cortex showed a significant main effect of injury (F1,48 = 4.74, p < 0.05; Fig. 3A,B). There was no significant main effect of hemisphere (F1,48 = 0.14, NS) or interaction between hemisphere and injury (F1,48 = 0.14, NS) or hemisphere and PID (F1,48 = 0.09, NS). Thus, there was clearly a post-injury reduction in cortical NR2A that was essentially similar bilaterally (Fig. 3A,B).
FIG. 3.
Mean percent change (±SEM) of NR2A in lateral FPI rats in the parietal cortex (A) at post-injury day (PID) 1, 2, 4, and 7 as compared to age-matched shams (defined as 0%). Representative immunoblots of pooled synaptoneurosomes from sham and FPI ipsilateral and contralateral cortices (B) are shown at the same time points. There were significant main effects of injury on NR2A levels (analysis of variance, p < 0.05), but no difference between hemispheres across all time points. When individual time points were tested, on PID1 there was a reduced level of NR2A in contralateral cortex (−33%, *p < 0.05) and a trend in ipsilateral cortex (−27%, p < 0.13). For abbreviations, see legend to Figure 1.
Descriptive analysis of each individual post-injury time point showed a 27.7% reduction of NR2A levels in ipsilateral cortex on PID1 that did not achieve statistical significance (p < 0.13); on PID2, PID4, and PID7, cortical NR2A in FP animals was indistinguishable from shams. Contralateral cortex also showed a reduction on PID1, and this decrease of 33.3% was significant at this time point (p < 0.01), before returning to age-matched sham levels on PID2, PID4, and PID7 (Fig. 3A,B).
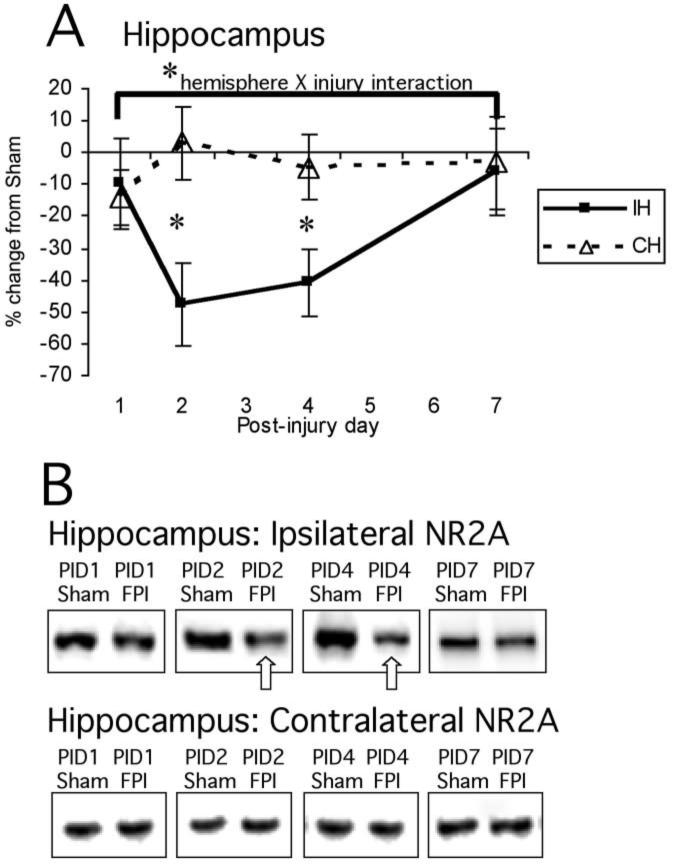
In hippocampus, NR2A protein signals showed a main effect of hemisphere (F1,46 = 8.5, p < 0.01) and significant interactions between hemisphere and injury (F1,46 = 5.2, p < 0.05) and hemisphere and PID (F1,46 = 3.0, p < 0.05). This indicates that NR2A levels change differentially in ipsilateral and contralateral hippocampus after FP, and that there is a significant effect of time post-injury on hippocampal NR2A levels. Even with both hemispheres included, there was a strong trend towards an overall effect of injury (F1,46 = 3.6, p = 0.06; Fig. 4A,B).
FIG. 4.
Mean percent change (±SEM) of NR2A in lateral FPI rats in the hippocampus (A) at post-injury day (PID) 1, 2, 4, and 7 as compared to age-matched shams (defined as 0%). Representative immunoblots of pooled synaptoneurosomes from sham and FPI ipsilateral and contralateral hippocampi (B) are shown at the same time points. There was a significant main effect of hemisphere (analysis of variance, p < 0.01), as well as significant interactions between hemisphere and injury (p < 0.05) and hemisphere and PID (p < 0.05). When individual time points were tested, NR2A showed a 47.9% reduction on PID2 (*p < 0.05) and a 40.8% reduction on PID4 (*p < 0.05). For abbreviations, see legend to Figure 1.
Descriptive analysis of each individual post-injury time point showed reductions in ipsilateral hippocampal NR2A at all times (−9.9% at PID1, −47.9% at PID2, −40.8% at PID4, −6.3% at PID7). The decreases were greatest at PID2 (sham 100 ± 17.6, FP 52.1 ± 11.9, p = 0.05) and PID4 (sham 100 ± 14.3, FP 59.2 ± 9.9, p < 0.05; Fig. 4A,B). Contralateral hippocampal NR2A did not differ between injured animals and age-matched shams at any post-injury time-point.
NMDAR2B
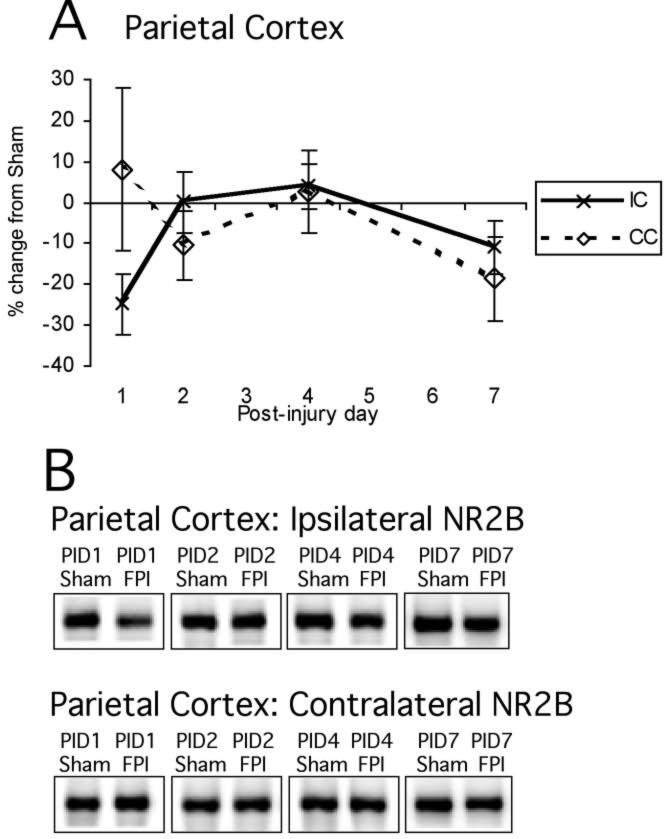
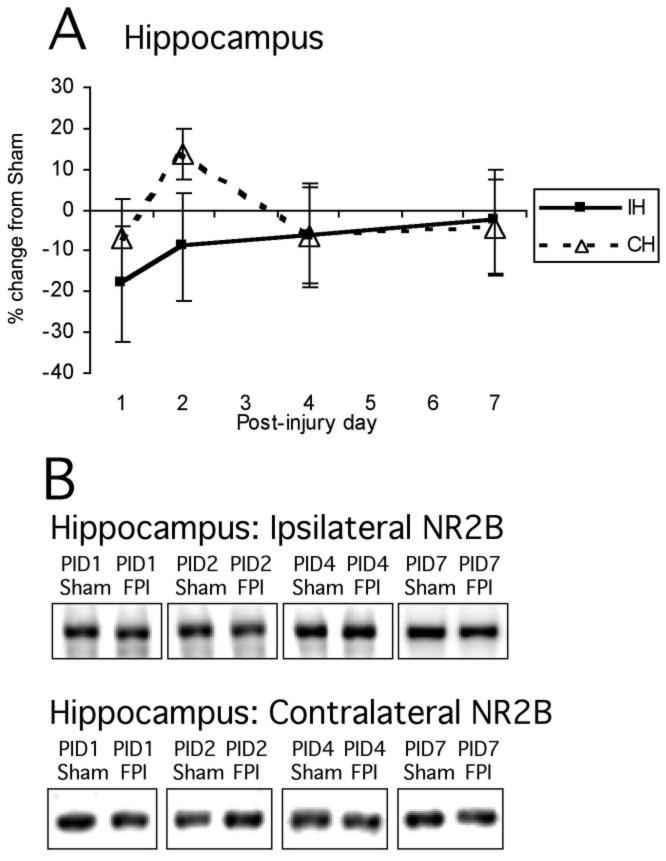
NR2B protein levels showed a distinctly different post-injury profile from NR2A (Figs. 5A,B and 6A,B). Normalized NR2B signal did not show any significant effect of injury in cortex (F1,48 = 1.23, NS) or hippocampus (F1,48 = 0.62, NS). There were no effects of hemisphere or interactions between hemisphere and injury, or hemisphere and PID in either cortex or hippocampus, so no descriptive statistics were performed. NR2B levels did not change significantly after FP injury in any brain region analyzed (cortex: sham 100 ± 4.7, FP 93.8 ± 4.8; hippocampus: sham 100 ± 5.7, FP 94.9 ± 5.4).
FIG. 5.
Mean percent change (±SEM) of NR2B in lateral FPI rats in parietal cortex (A) at post-injury day (PID) 1, 2, 4, and 7 as compared to age-matched shams (defined as 0%). Again, representative immunoblots of pooled synaptically enriched sham and FPI ipsilateral and contralateral cortices (B) are shown for PID1, PID2, PID4, and PID7. Time course of post-injury NR2B cortical protein expression shows no significant main effects. For abbreviations, see legend to Figure 1.
FIG. 6.
Mean percent change (±SEM) of NR2B in lateral FPI rats in hippocampus (A) at post-injury day (PID) 1, 2, 4, and 7 as compared to age-matched shams (defined as 0%). Again, representative immunoblots of pooled synaptically enriched sham and FPI ipsilateral and contralateral hippocampi (B) are shown for PID1, PID2, PID4, and PID7. Time course of post-injury NR2B hippocampal protein expression shows no significant changes. For abbreviations, see legend to Figure 1.
Real-Time RT-PCR
To determine whether alterations in gene expression level could be responsible for the reductions in NMDAR subunit protein expression detected after developmental TBI, real-time RT-PCR was performed on RNA harvested from a parallel group of animals. Analysis of relative gene expression using primers for each of the NMDAR subunits did not show any changes greater than twofold after developmental lateral FP injury (Table 2). However, as a positive control, RT-PCR for a gene known to be altered by traumatic brain injury, heat shock protein 70, did show a significant upregulation in both ipsilateral cortex and hippocampus at PID1 (2.56 and 2.05-fold induction, respectively).
TABLE 2.
Relative Gene Expression (Fold-Change of Injured Versus Sham) for Cortex And Hippocampus on Post-Injury Days 1, 2, And 4
| PID1 | PID2 | PID4 | |
|---|---|---|---|
| Hsp70 | |||
| IC | 2.56 | ||
| IH | 2.05 | ||
| NR1 | |||
| IC | −1.00 | −1.09 | 1.03 |
| CC | −1.30 | 1.11 | 1.00 |
| IH | −1.11 | −1.03 | −1.01 |
| CH | −1.30 | 1.22 | −1.03 |
| NR2A | |||
| IC | −1.27 | −1.24 | −1.08 |
| CC | −1.30 | −1.07 | −1.06 |
| IH | −1.42 | −1.02 | −1.08 |
| CH | −1.28 | 1.06 | −1.04 |
| NR2B | |||
| IC | 1.43 | −1.10 | 1.03 |
| CC | 1.08 | −1.21 | −1.18 |
| IH | −1.62 | 1.10 | 1.06 |
| CH | −1.26 | −1.17 | −1.14 |
Hsp 70 showed greater than twofold change in ipsilateral cortex and hippocampus, as a positive control. None of the N-methyl-D-aspartate receptor subunits (NR1, NR2A, NR2B) showed a twofold change (up or down).
DISCUSSION
The primary finding of this study is that developmental lateral FPI results in a preferential downregulation of the NMDAR subunit, NR2A, in a synaptic preparation. This effect was seen bilaterally in cortex, particularly early after injury (PID1); however, it was most profound in the ipsilateral hippocampus, where it was significantly reduced as late as PID4. NR1 and NR2B levels did not change significantly over the course of the first post-injury week. While these injury-induced changes were evident at the protein level, the relative levels of transcription of the NMDAR subunit mRNAs did not show consistent and significant reductions.
Functionally, increased NR2A levels allow for a more precise glutamatergic signal by altering kinetics of the NMDAR (Flint et al., 1997; Roberts and Ramoa, 1999). Enhanced NR2A gene expression during development occurs in response to environmental differences such as more nurturing maternal nursing and grooming behavior (Liu et al., 2000) and the change from dark rearing to light (Quinlan et al., 1999a,b). Increased NR2A also correlates with functional changes in the NMDAR complex in developing visual cortex (Roberts and Ramoa, 1999), parietal cortex (Flint et al., 1997; Kew et al., 1998), hippocampus (Gottmann et al., 1997), and cerebellum (Takahashi et al., 1996). It appears, then, that increases in NR2A may be a fundamental response to differential experience, and a crucial part of normal maturation of neuronal circuitry. Furthermore, it appears that NR2A expression in adults may also be responsive to environmental stimulation, as it is capable of being suppressed into adulthood by dark rearing (Quinlan et al., 1999a) or induced in adults by voluntary exercise (Molteni et al., 2002).
Previous studies of traumatic brain injury in developing animals have demonstrated a reduction in experience-dependent plasticity induced by rearing in an EE. PND19 rats subjected to lateral FP injury prior to rearing in EE do not show the cognitive enhancements manifest in sham-injured pups reared in EE (Fineman et al., 2000). Furthermore, increases in cortical depth and expansion of dendritic arbors seen in sham-injured/EE reared animals are mitigated when EE rearing follows early FP injury (Fineman et al., 2000; Ip et al., 2002).
Given the role played by NMDAR in normal and experience-dependent plasticity, and the fact that excessive NMDAR activation is a proven component of the pathophysiological cascade following TBI, it is reasonable to hypothesize a mechanism of inhibited plasticity after early TBI that involves alterations in the NMDAR. This could occur simply through a reduction in NMDAR numbers (and be manifest by diminished NR1 levels) or by a change in proportion of NR2 subunits.
Our results show no lasting change in synaptic NMDAR numbers by demonstrating no decline in protein levels of the NR1 subunit in cortex and hippocampus following PND19 lateral FP injury. This is corroborated by qualitative RT-PCR for NR1 expression, which also shows no significant change over PID1-4.
Besides reduction in numbers of NMDARs, NMDAR function may also be affected by changes in subunit composition. As discussed above, increased NR2A naturally occurs in response to various forms of environmental stimulation. The results presented here reveal substantial reductions of the NR2A subunit during the 1st week post-injury, particularly in ipsilateral hippocampus. It follows, then, that environment-induced increases in NR2A may be inhibited in this post-injury period. Further studies are ongoing to investigate the effects of EE rearing on NR2A levels in sham-injured and FP injured animals.
The selective loss of synaptic NR2A protein could occur in several ways. First, NR2A expression may be inhibited at the level of transcription. However, our results with RT-PCR suggest that this is not the case. Alternatively, synaptic NR2A protein turnover might increase following TBI. This could be mediated by an increase in NR2A subunit-specific turnover, with NR2A receptors either being selectively endocytosed for proteolysis or by specific translocation of NR2A-containing receptors away from the synapse (Carroll and Zukin, 2002). Since NR2A is rapidly being integrated into maturing synapses at this developmental stage, it follows that this process would be vulnerable to a diffuse pathophysiological process such as TBI. The apparent 1-2-day delay in the reduction of synaptic NR2A suggests that the release of NR2A-containing NMDAR from synapses may be a slower, multi-step process. Such a contention is supported by literature showing the relative stability of NR2A-containing NMDARs when localized in the post-synaptic density (Lavezzari et al., 2004).
An additional factor in interpreting NMDAR subunit changes after brain injury is the likelihood of significant cell death, perhaps with selective loss of NR2A-rich neurons. Adult FP injury leads to significant levels of neuronal loss (Conti et al., 1998). However, FP injury in immature animals is distinct from adult FP injury, in that concussed preweanling rat pups demonstrate minimal gross anatomic change or loss of neurons (Fineman et al., 2000; Gurkoff et al., 2006; Prins et al., 1996). Longstanding neurocognitive deficits in a setting of substantial cell death may be due both to the loss of cells as well as impaired function of the surviving cells. However, in this model of developmental FP injury, we report that significant dysfunction of an excitatory neurotransmitter system can occur with little or no cell death, and propose this as a primary mechanism for previously described deficits in experience-dependent plasticity after FP (Fineman et al., 2000; Giza et al., 2005; Ip et al., 2002).
The concept that a brain insult during development leads to chronic dysfunctional neurotransmission is also supported by studies of neonatal NMDA blockade. Chronic administration of the noncompetitive NMDA antagonist, MK-801 to rat pups results in deficits in glutamatergic and monoaminergic neurotransmission later in development (Bellinger et al., 2002; Gorter et al., 1992). Similarly, prenatal exposure to ethanol leads to decreased NR2A and NR2B expression in rat forebrain into the 3rd postnatal week (Hughes et al., 1998). Developmental exposure to lead is also associated with altered NMDAR subunit composition in hippocampus up to PND28 (Nihei and Guilarte, 1999). Interestingly, the lead-exposed rats demonstrated reduced NR2A protein levels in a setting of NR2B levels indistinguishable from age-matched controls (and thus a decreased 2A:2B ratio), and other studies clearly show cognitive deficits as a result of developmental lead exposure (Jett et al., 1997; Kuhlmann et al., 1997). This result is consistent with our findings following developmental brain injury, where disruption of neurotransmission during hippocampal maturation leads to reductions in NR2A subunit composition without significant alterations in NMDAR number (as determined by NR1 immunoreactivity) or NR2B expression.
This suggests that developmental deficits and impaired plasticity may occur when the NR2A/NR2B ratio deviates substantially from normal proportions. Alternative mechanisms of impairment include the possibility that NMDAR subunit types not normally seen in specific brain regions may be expressed after insults, such as the induction of NR2C following ischemia (Small et al., 1997), and that the effect of a change in NMDAR subunit composition may depend upon the relative developmental stage of the brain region at the time of injury. In this case, the structure undergoing more marked maturational change (hippocampus) may be more susceptible to derangement in neural activity and thus more likely to demonstrate persistent dysfunction.
In summary, we report that following developmental TBI there is a subunit-specific reduction in synaptic NR2A. These alterations of NR2 composition occur transiently in neocortex and persist at least for 4 days after injury in ipsilateral hippocampus. The changes in protein described do not appear to be mediated at the transcriptional level. We suggest that significant dysfunction of excitatory neurotransmission can occur after moderate traumatic injury to the immature brain, probably via decreased synaptic stability of existing NR2A-containing NMDARs. This is an appealing mechanism for post-traumatic deficits in plasticity and resulting neurocognitive impairment.
ACKNOWLEDGMENTS
Special thanks go to Yan Cai, Tad Kremen, and Niloofar Farmani for their excellent technical assistance. Additional thanks go to Dr. David McArthur for his statistical advice and input. This research was supported by NIH grants NS27544, NS02197, the UCLA Assistant Professor Initiative, and the UCLA Brain Injury Research Center.
Footnotes
Address reprint requests to: Christopher C. Giza, M.D. Room 18-218B NPI Mail Code 703919 Division of Neurosurgery Geffen School of Medicine at UCLA Los Angeles, CA 90095 E-mail:cgiza@mednet.ucla.edu
REFERENCES
- BELLINGER FP, WILCE PA, BEDI KS, WILSON P. Long-lasting synaptic modification in the rat hippocampus resulting from NMDA receptor blockade during development. Synapse. 2002;43:95–101. doi: 10.1002/syn.10020. [DOI] [PubMed] [Google Scholar]
- BERGGREN KN, SCHULENBERG B, LOPEZ MF. An improved formulation of SYPRO Ruby protein gel stain: comparison with the original formulation and with a ruthenium II tris (bathophenanthroline disulfonate) formulation. Proteomics. 2002;2:486–498. doi: 10.1002/1615-9861(200205)2:5<486::AID-PROT486>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- BIO-RAD Instruction Manual. Sypro Ruby Protein Stains, Rev. B.
- BIO-RAD Instruction Maunal. The Discovery Series: Quantity One Analysis Software. 2005.
- BITTIGAU P, SIFRINGER M, POHL D. Apoptotic neurodegeneration following trauma is markedly enhanced in the immature brain. Ann. Neurol. 1999;45:724–735. doi: 10.1002/1531-8249(199906)45:6<724::aid-ana6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- BLOOM DR, LEVIN HS, EWING-COBBS L. Lifetime and novel psychiatric disorders after pediatric traumatic brain injury. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:572–579. doi: 10.1097/00004583-200105000-00017. [DOI] [PubMed] [Google Scholar]
- CARROLL RC, ZUKIN RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25:571–577. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- CHEN N, LUO T, RAYMOND LA. Subtypedependence of NMDA receptor channel open probability. J. Neurosci. 1999;19:6844–6854. doi: 10.1523/JNEUROSCI.19-16-06844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONTI AC, RAGHUPATHI R, TROJANOWSKI JQ, McINTOSH TK. Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. J. Neurosci. 1998;18:5663–5672. doi: 10.1523/JNEUROSCI.18-15-05663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’AMBROSIO R, MARIS DO, GRADY MS, WINN HR, JANIGRO D. Selective loss of hippocampal long-term potentiation, but not depression, following fluid percussion injury. Brain Res. 1998;786:64–79. doi: 10.1016/s0006-8993(97)01412-1. [DOI] [PubMed] [Google Scholar]
- DIXON CE, LYETH BG, POVLISHOCK JT. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- EWING-COBBS L, FLETCHER JM, LEVIN HS, IOVINO I, MINER ME. Academic achievement and academic placement following traumatic brain injury in children and adolescents: a two-year longitudinal study. J. Clin. Exp. Neuropsychol. 1998;20:769–781. doi: 10.1076/jcen.20.6.769.1109. [DOI] [PubMed] [Google Scholar]
- FADEN AI, DEMEDIUK P, PANTER SS, VINK R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- FINEMAN I, GIZA CC, NAHED BV, LEE SM, HOVDA DA. Inhibition of neocortical plasticity during development by a moderate concussive brain injury. J. Neurotrauma. 2000;17:739–749. doi: 10.1089/neu.2000.17.739. [DOI] [PubMed] [Google Scholar]
- FLINT AC, MAISCH US, WEISHAUPT JH, KRIEGSTEIN AR, MONYER H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J. Neuroscience. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIZA CC, GRIESBACH GS, HOVDA DA. Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behav. Brain Res. 2005;157:11–22. doi: 10.1016/j.bbr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- GORTER JA, VEERMAN M, MIRMIRAN M. Hippocampal neuronal responsiveness to NMDA agonists and antagonists in the adult rat neonatally treated with MK-801. Brain Res. 1992;572:176–181. doi: 10.1016/0006-8993(92)90467-n. [DOI] [PubMed] [Google Scholar]
- GOTTMANN K, MEHRLE A, GISSELMANN G, HATT H. Presynaptic control of subunit composition of NMDA receptors mediating synaptic plasticity. J. Neurosci. 1997;17:2766–2774. doi: 10.1523/JNEUROSCI.17-08-02766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GURKOFF G, GIZA CC, HOVDA DA. Lateral fluid percussion injury in the developing rat causes an acute, mild behavioral dysfunction in the absence of significant cell death. Brain Res. 2006;1077:24–36. doi: 10.1016/j.brainres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- HUGHES PD, KIM YN, RANDALL PK, LESLIE SW. Effect of prenatal ethanol exposure on the developmental profile of the NMDA receptor subunits in rat forebrain and hippocampus. Alcohol Clin. Exp. Res. 1998;22:1255–1261. [PubMed] [Google Scholar]
- IKONOMIDOU C, BOSCH F, MIKSA M. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- IKONOMIDOU C, TURSKI L. Prevention of trauma-induced neurodegeneration in infant and adult rat brain: glutamate antagonists. Metab. Brain Dis. 1996;11:125–141. doi: 10.1007/BF02069500. [DOI] [PubMed] [Google Scholar]
- IP EY, GIZA CC, GRIESBACH GS, HOVDA DA. Effects of enriched environment and fluid percussion injury on dendritic arborization within the cerebral cortex of the developing rat. J. Neurotrauma. 2002;19:573–585. doi: 10.1089/089771502753754055. [DOI] [PubMed] [Google Scholar]
- JABLONSKA B, GIERDALSKI M, SIUCINSKA E, SKANGIEL-KRAMSKA J, KOSSUT M. Partial blocking of NMDA receptors restricts plastic changes in adult mouse barrel cortex. Behav. Brain Res. 1995;66:207–216. doi: 10.1016/0166-4328(94)00141-2. [DOI] [PubMed] [Google Scholar]
- JETT DA, KUHLMANN AC, FARMER SJ, GUILARTE TR. Age-dependent effects of developmental lead exposure on performance in the Morris water maze. Pharmacol. Biochem. Behav. 1997;57:271–279. doi: 10.1016/s0091-3057(96)00350-4. [DOI] [PubMed] [Google Scholar]
- JOHNSON MW, CHOTINER JK, WATSON JB. Isolation and characterization of synaptoneurosomes from single rat hippocampal slices. J. Neurosci Methods. 1977;77:151–156. doi: 10.1016/s0165-0270(97)00120-9. [DOI] [PubMed] [Google Scholar]
- KATAYAMA Y, BECKER DP, TAMURA T, HOVDA DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- KAWAMATA T, KATAYAMA Y, HOVDA DA, YOSHINO A, BECKER DP. Administration of excitatory amino acid antagonists via microdialysis attenuates the increase in glucose utilization seen following concussive brain injury. J. Cereb. Blood Flow Metab. 1992;12:12–24. doi: 10.1038/jcbfm.1992.3. [DOI] [PubMed] [Google Scholar]
- KEW JN, RICHARDS JG, MUTEL V, KEMP JA. Developmental changes in NMDA receptor glycine affinity and ifenprodil sensitivity reveal three distinct populations of NMDA receptors in individual rat cortical neurons. J. Neurosci. 1998;18:1935–1943. doi: 10.1523/JNEUROSCI.18-06-01935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIND PC, NEUMANN PE. Plasticity: downstream of glutamate. Trends Neurosci. 2001;24:553–555. doi: 10.1016/s0166-2236(00)01921-4. [DOI] [PubMed] [Google Scholar]
- KLEINSCHMIDT A, BEAR MF, SINGER W. Blockade of “NMDA” receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987;238:355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- KUHLMANN AC, McGLOTHAN JL, GUILARTE TR. Developmental lead exposure causes spatial learning deficits in adult rats. Neurosci. Lett. 1997;233:101–104. doi: 10.1016/s0304-3940(97)00633-2. [DOI] [PubMed] [Google Scholar]
- LAVEZZARI G, McCALLUM J, DEWEY CM, ROCHE KW. Subunit-specific regulation NMDA receptor endocytosis. J. Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN HS. Neuroplasticity following non-penetrating traumatic brain injury. Brain Inj. 2003;17:665–674. doi: 10.1080/0269905031000107151. [DOI] [PubMed] [Google Scholar]
- LIU D, DIORIO J, DAY JC, FRANCIS DD, MEANEY MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat. Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- MALETIC-SAVATIC M, MALINOW R, SVOBODA K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- MAX JE, LINDGREN SD, KNUTSON C, PEARSON CS, IHRIG D, WELBORN A. Child and adolescent traumatic brain injury: psychiatric findings from a paediatric outpatient specialty clinic. Brain Inj. 1997;11:699–711. doi: 10.1080/026990597123070. [DOI] [PubMed] [Google Scholar]
- MILLER LP, LYETH BG, JENKINS LW. Excitatory amino acid receptor subtype binding following traumatic brain injury. Brain Res. 1990;526:103–107. doi: 10.1016/0006-8993(90)90254-9. [DOI] [PubMed] [Google Scholar]
- MOLTENI R, YING Z, GOMEZ-PINILLA F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur. J. Neurosci. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- MONYER H, BURNASHEV N, LAURIE DJ, SAKMANN B, SEEBURG PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- NIHEI MK, GUILARTE TR. NMDAR-2A subunit protein expression is reduced in the hippocampus of rats exposed to Pb2 + during development. Brain Res. Mol. Brain Res. 1999;66:42–49. doi: 10.1016/s0169-328x(99)00005-4. [DOI] [PubMed] [Google Scholar]
- PEREZ-OTANO I, EHLERS MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28:229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- PFAFFL MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POHL D, BITTIGAU P, ISHIMARU MJ. N-methyl-D-aspartate antagonists and apoptotic cell death triggered by head trauma in developing rat brain. Proc. Natl. Acad. Sci. U.S.A. 1999;96:2508–2513. doi: 10.1073/pnas.96.5.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINS ML, HOVDA DA. Traumatic brain injury in the developing rat: effects of maturation on Morris water maze acquisition. J. Neurotrauma. 1998;15:799–811. doi: 10.1089/neu.1998.15.799. [DOI] [PubMed] [Google Scholar]
- PRINS ML, LEE SM, CHENG CL, BECKER DP, HOVDA DA. Fluid percussion brain injury in the developing and adult rat: a comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Brain Res. Dev. Brain Res. 1996;95:272–282. doi: 10.1016/0165-3806(96)00098-3. [DOI] [PubMed] [Google Scholar]
- QUINLAN EM, OLSTEIN DH, BEAR MF. Bidirectional, experience-dependent regulation of N-methyl-D- asparate receptor subunit composition in the rat visual cortex during postnatal development. Proc. Natl. Acad. Sci. U.S.A. 1999a;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUINLAN EM, PHILPOT BD, HUGANIR RL, BEAR MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat. Neurosci. 1999b;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- REEVES TM, LYETH BG, POVLISHOCK JT. Long-term potentiation deficits and excitability changes following traumatic brain injury. Exp. Brain Res. 1995;106:248–256. doi: 10.1007/BF00241120. [DOI] [PubMed] [Google Scholar]
- ROBERTS EB, RAMOA AS. Enhanced NR2A subunit expression and decreased NMDA receptor decay time at the onset of ocular dominance plasticity in the ferret. J. Neurophysiol. 1999;81:2587–2591. doi: 10.1152/jn.1999.81.5.2587. [DOI] [PubMed] [Google Scholar]
- SCHEETZ AJ, CONSTANTINE-PATON M. Modulation of NMDA receptor function: implications for vertebrate neural development. FASEB J. 1994;8:745–752. doi: 10.1096/fasebj.8.10.8050674. [DOI] [PubMed] [Google Scholar]
- SCHEETZ AJ, NAIRN AC, CONSTANTINE-PATON M. NMDA receptor-mediated control of protein synthesis at developing synapses. Nat. Neurosci. 2000;3:211–216. doi: 10.1038/72915. [DOI] [PubMed] [Google Scholar]
- SHENG M, CUMMINGS J, ROLDAN LA, JAN YN, JAN LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- SICK TJ, PEREZ-PINZON MA, FENG ZZ. Impaired expression of long-term potentiation in hippocampal slices 4 and 48 h following mild fluid-percussion brain injury in vivo. Brain Res. 1998;785:287–292. doi: 10.1016/s0006-8993(97)01418-2. [DOI] [PubMed] [Google Scholar]
- SMALL DL, POULTER MO, BUCHAN AM, MORLEY P. Alteration in NMDA receptor subunit mRNA expression in vulnerable and resistant regions of in vitro ischemic rat hippocampal slices. Neurosci. Lett. 1997;232:87–90. doi: 10.1016/s0304-3940(97)00592-2. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI T, FELDMEYER D, SUZUKI N. Functional correlation of NMDA receptor epsilon subunits expression with the properties of single-channel and synaptic current in the developing cerebellum. J. Neurosci. 1996;16:4376–4382. doi: 10.1523/JNEUROSCI.16-14-04376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR HG, ALDEN J. Age-related differences in outcomes following childhood brain insults: an introduction and overview. J. Int. Neuropsychol. Soc. 1997;3:555–567. [PubMed] [Google Scholar]
- WEINER HL, WEINBERG JS. Head injury in the pediatric age group. In: Cooper PR, Golfinos JG, editors. Head Injury. McGraw-Hill; San Francisco, pps.: 2000. pp. 419–456. [Google Scholar]
- YOSHINO A, HOVDA DA, KATAYAMA Y, KAWAMATA T, BECKER DP. Hippocampal CA3 lesion prevents postconcussive metabolic dysfunction in CA1. J. Cereb. Blood Flow Metab. 1992;12:996–1006. doi: 10.1038/jcbfm.1992.137. [DOI] [PubMed] [Google Scholar]
- YOSHINO A, HOVDA DA, KAWAMATA T, KATAYAMA Y, BECKER DP. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561:106–119. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]