Abstract
Fluorescent small molecules are powerful tools for exploring cellular biology. As a more hydrophobic, photostable, and less pH sensitive alternative to fluorescein, we synthesized Pennsylvania Green, a bright, monoanionic fluorophore related to Oregon Green and Tokyo Green. Comparison of membrane probes comprising N-alkyl-3 -cholesterylamine linked to 4-carboxy-Tokyo Green (pKa ~ 6.2) and 4-carboxy-Pennsylvania Green (pKa ~ 4.8)revealed that only Pennsylvania Green was highly fluorescent in acidic early and recycling endosomes within living mammalian cells.
Molecular probes derived from fluorescein (1, Figure 1) are widely used as tools for studies of cellular biology. This green fluorophore is particularly suited for cellular analysis by confocal laser scanning microscopy and flow cytometry due to its excitation maximum at 490 nm, closely matching the 488 nm spectral line of the argon-ion laser. In addition, fluorescein has a high molar absorptivity and excellent quantum yield (0.92 at pH 9). Under physiological conditions (pH 7.4), fluorescein is predominantly a highly hydrophilic dianion. However, the monoanionic form of fluorescein exhibits the relatively high pKa of 6.5, rendering this dye much less fluorescent in acidic solutions.1 Fluorescein is also relatively susceptible to photobleaching, which is thought to involve reactions with molecular oxygen and proximity-induced reactions of the dye.2
Figure 1.
Structures of known (1–3) and novel (4–6) fluorescent dyes in their anionic forms.
Oregon Green, a more acidic 2’, 7’-difluoro derivative of fluorescein (2, Figure 1), was developed as a less pH-sensitive fluorophore.3 The appended fluorine atoms reduce the pKa of this dye to 4.8, substantially improving fluorescence at low pH. This compound is also significantly more photostable than fluorescein. However, the high cost and high polarity of Oregon Green limits its utility as a building block for hydrophobic molecular probes.
As a more hydrophobic alternative to fluorescein, recent pioneering work by Urano, Nagano, and coworkers replaced the carboxylate of fluorescein with a methyl group.4 This structural modification yielded a highly fluorescent monoanionic fluorophore termed Tokyo Green (3, Figure 1). This analogue of fluorescein provides a new platform for the design of fluorescent probes.
We report here the synthesis of a novel fluorophore termed Pennsylvania Green (4). This fluorophore melds the pH-insensitivity and photostability of Oregon Green with the hydrophobicity of Tokyo Green. To demonstrate the utility of the Pennsylvania Green fluorophore, we compared cellular membrane probes derived from 4-carboxy-Tokyo Green (5) and 4-carboxy-Pennsylvania green (6). The lower pKa of the Pennysylvania Green-derived probe enables visualization of early / recycling endosomes within living mammalian cells, and this fluorophore provides a usful tool for analysis of these and related acidic intracellular compartments.
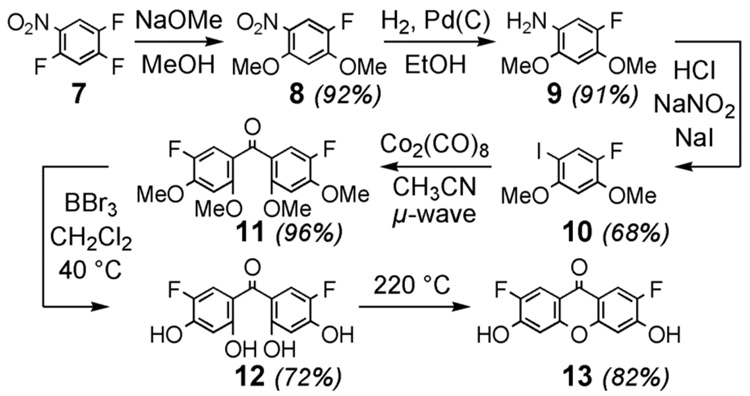
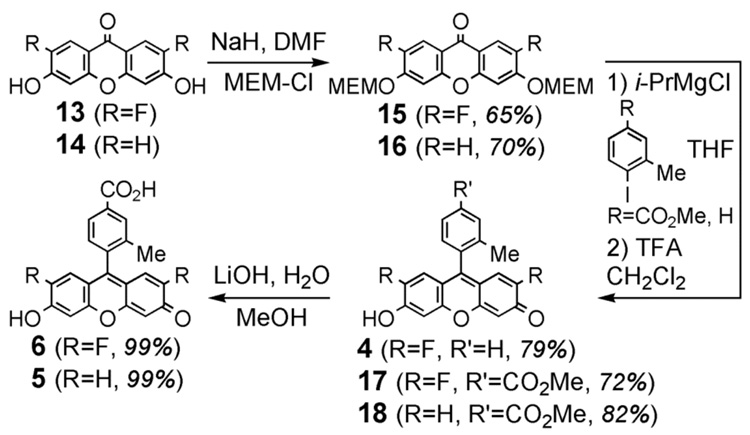
The synthesis of 4-carboxy-Pennsylvania Green (6) was accomplished in 10-steps from commercially available 1,2,4-trifluoro-5-nitrobenzene (7). As shown in Scheme 1, 1-amino-2,4-dimethoxy-5-fluorobenzene (9) was prepared via 8 by a previously reported route.3 Subjection of 9 to Sandmeyer conditions afforded the novel iodoarene 10. This compound (10) was converted to the known5 benzophenone 12 in excellent yield using Larhed’s recently reported6 microwave and cobalt octacarbonyl-mediated synthesis of diaryl ketones followed by demethylation with boron tribromide. The five-step route to 12 shown in Scheme 1 is significantly more rapid and efficient (40% overall yield) than the previously reported5 convergent 8-step synthesis of this compound. Cyclization at elevated temperature under precedented conditions afforded the known xanthone 13.5 As shown in Scheme 2, this xanthone (13) and commercially available xanthone 14 were converted into the novel fluorophores 4-carboxy- Pennsylvania Green (6) and 4-carboxy-Tokyo Green (5). Halogen metal exchange with i-PrMgCl7 proved to be an efficient method for installation of the methyl benzoate moiety of 17 and 18.
Scheme 1.
An improved synthesis of xanthone 13.
Scheme 2.
Synthesis of fluorophores.
To examine the effects of substitution with fluorine, pKa values of 4-carboxy-Pennsylvania Green methyl ester (17) and 4-carboxy-Tokyo Green methyl ester (18) were determined by absorbance vs. pH titrations. As shown in Figure 2, these experiments revealed that the Pennsylvania Green derivative is identical in pKa to Oregon Green (pKa = 4.8) and 1.4 pKa units more acidic than the corresponding Tokyo Green derivative (pKa = 6.2).
Figure 2.
Absorbance vs. pH titrations of 17 (Panel A) and 18 (Panel B) used to determine pKa values (Panel C). Overlaid emission spectra (Panels A and B) are in arbitrary intensity units.
As listed in Table 1, relative fluorescence quantum yields of fluorophores 17 and 18 were determined at pH 9.0 and pH 5.0 by the method of Williams et al. (data provided in Figure S1 of the supporting information).8 Fluorescein (1) and 5-carboxyfluorescein were used as standards in these experiments. At pH 9.0, both the Pennsylvania Green (17) and Tokyo Green (18) fluorophores were very similar with quantum yields of 0.91 and 0.93 respectively. However, as predicted by the differences in pKa, at pH 5.0 the more acidic Pennsylvania Green derivative (17) was substantially brighter with a quantum yield of 0.68 compared with 0.39 for the the Tokyo Green (18) derivative.
Table 1.
Comparison of physicochemical properties of known (1–3)3,4 and novel (17–18) fluorophores. Q.Y.: Quantum yield.
| compound | abs / em (nm) | pKa | Q.Y. (pH) |
|---|---|---|---|
| Fluorescein (1) | 490 / 514 | 6.5 | 0.92 (9) 0.37 (5.4) |
| Oregon Green (2) | 490 / 514 | 4.8 | 0.97 (9) |
| Tokyo Green (3) | 491 / 510 | 6.2 | 0.85 (13) 0.32 (3.4) |
| 4-Carboxy-Pennsylvania Green methyl ester (17) | 496 / 517 | 4.8 | 0.91 (9) 0.68 (5) |
| 4-Carboxy-Tokyo Green methyl ester (18) | 496 / 517 | 6.2 | 0.93 (9) 0.39 (5) |
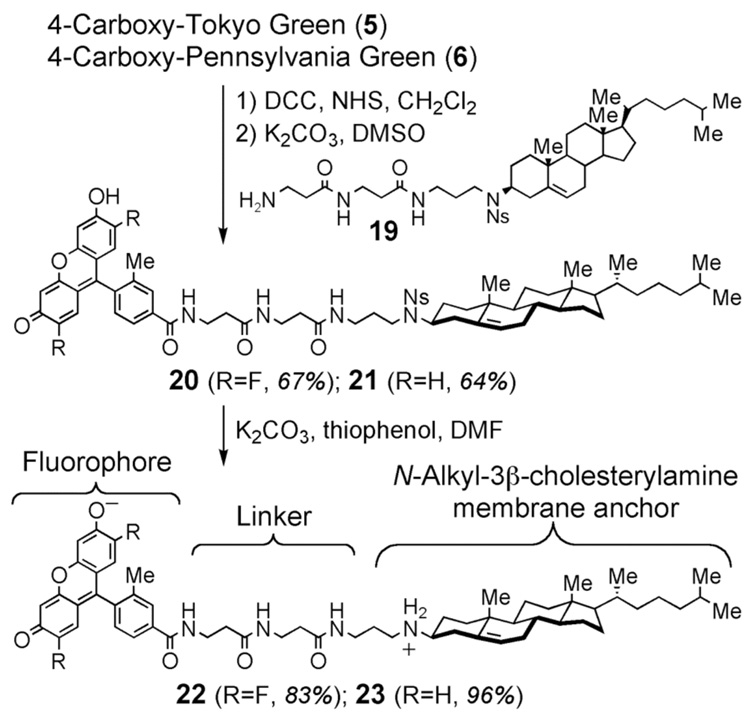
Two novel membrane probes (22 and 23) were synthesized to compare Pennsylvania Green and Tokyo Green in a cellular environment. These probes were prepared by coupling fluorophores 5 and 6 to the known9 3β-cholesterylamine derivative 19 to afford 20 and 21, followed by removal of 3β-nosyl protecting groups (Scheme 3). Related compounds comprising fluorophores linked to N-alkyl-3β-cholesterylamines have previously been shown to localize on the exofacial leaflet of plasma membranes and within acidic intracellular endosomes of living mammalian cells.9 N-Alkyl-3β-cholesterylamines can rapidly cycle between these two cellular destinations, similar to many natural cell surface receptors.10
Scheme 3.
Synthesis of molecular probes of mammalian plasma membranes and intracellular endosomes.
Living Jurkat lymphocytes treated with molecular probes 22 and 23 were examined by confocal laser scanning microscopy. This human T-cell line was treated with the compounds for 1 h, cells were gently centrifuged and washed to remove unincorporated probes, and cellular fluorescence was imaged as shown in Figure 3. These experiments revealed that both compounds could be observed on the cellular plasma membrane and provide novel markers that define the cell surface. However, only the Pennsylvania Green probe (22) exhibited bright fluorescence in intracellular compartments (compare panels A and B in Figure 3). These compartments were identified as early and recycling endosomes by colocalization with internalized red fluorescent transferrin protein (data provided in the supporting information).11 The higher pKa of Tokyo Green results in substantial fluorescence quenching of 23 in the acidic environment of endosomes (pH ≤ 6.5).12 This was confirmed by treatment with 23 and the specific vacuolar H+ ATPase inhibitor Bafilomycin A1,13 which by blocking acidification of endosomes, increased the intracellular fluorescence of 23 (Figure 3, panel C).
Figure 3.
Confocal laser scanning (left) and differential interference contrast (right) micrographs of living Jurkat lymphocytes. Cells were treated with probes 22 and 23 (10 µM) in RPMI media for 1 h at 37 °C and washed with fresh media prior to analysis by microscopy. In panel C, cells were treated with 23 (10 µM) and the vacuolar H+ ATPase inhibitor Bafilomycin A1 (1 µM) to prevent acidification of endosomes. White arrows illustrate intracellular endosomal fluorescence. Scale bar = 10 µm.
In addition to its lower pKa, a second advantage of Oregon Green compared with fluorescein relates to enhanced photostability conferred by fluorination.3 To examine whether Pennsylvania Green is more photostable than Tokyo Green, Jurkat lymphocytes treated with probes 22 and 23 were subjected to continuous irradiation with the Ar-ion laser (488 nm) of a confocal microscope. As shown in Figure 4, the fluorescence decay of individual cells due to photobleaching was quantified (examples are provided in the supporting information), and application of a single exponential function allowed calculation of the half-lives of these fluorescent probes. This analysis revealed that the Pennsylvania Green probe 22 is substantially more photostable (t1/2 = 49 min) compared to the Tokyo Green probe 23 (t1/2 = 29 min). The Pennsylvania Green fluorophore thus has the potential to provide a valuable new tool for the construction of molecular and cellular probes.
Figure 4.
Analysis of photobleaching rates of molecular probes added to living Jurkat lymphocytes. Cells were treated with 22 or 23 (10 µM, 1 h, 37 °C), washed with fresh media, and imaged by confocal microscopy while subjected to continuous irradiation at 488 nm with a 25 mW argon-ion laser at 1% laser power. At specific time points, the fluorescence intensity of five individual cells was quantified and averaged. Fluorescence half-lives were calculated with a one-site exponential decay model.
Supplementary Material
Supporting figures, experimental procedures and characterization data for new compounds. This material is available free of charge via the internet at http://pubs.acs.org.
Acknowledgment
We thank the National Institutes of Health (R01-CA83831) for financial support. We thank Prof. Mary Beth Williams for the use of her spectrometers.
References
- 1.Sjoback R, Nygren J, Kubista M. Spectrochim. Acta A. 1995;51:L7–L21. [Google Scholar]
- 2.Song LL, Hennink EJ, Young IT, Tanke HJ. Biophys. J. 1995;68:2588–2600. doi: 10.1016/S0006-3495(95)80442-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun WC, Gee KR, Klaubert DH, Haugland RP. J. Org. Chem. 1997;62:6469–6475. [Google Scholar]
- 4.Urano Y, Kamiya M, Kanda K, Ueno T, Hirose K, Nagano T. J. Am. Chem. Soc. 2005;127:4888–4894. doi: 10.1021/ja043919h. [DOI] [PubMed] [Google Scholar]
- 5.Chen CA, Yeh RH, Lawrence DS. J. Am. Chem. Soc. 2002;124:3840–3841. doi: 10.1021/ja017530v. [DOI] [PubMed] [Google Scholar]
- 6.Enquist P-A, Nilsson P, Larhed M. Org. Lett. 2003;5:4875–4878. doi: 10.1021/ol036091x. [DOI] [PubMed] [Google Scholar]
- 7.Knochel P, Dohle W, Gommermann N, Kneisel FF, Kopp F, Korn T, Sapountzis I, Vu VA. Angew. Chem. Int. Ed. 2003;42:4302–4320. doi: 10.1002/anie.200300579. [DOI] [PubMed] [Google Scholar]
- 8.Williams AT, Winfield SA. Analyst. 1983;108:1067–1071. [Google Scholar]
- 9.Boonyarattanakalin S, Martin SE, Dykstra SA, Peterson BR. J. Am. Chem. Soc. 2004;126:16379–16386. doi: 10.1021/ja046663o. [DOI] [PubMed] [Google Scholar]
- 10.Peterson BR. Org. Biomol. Chem. 2005;3:3607–3612. doi: 10.1039/b509866a. [DOI] [PubMed] [Google Scholar]
- 11.Sheff D, Pelletier L, O'Connell CB, Warren G, Mellman I. J. Cell. Biol. 2002;156:797–804. doi: 10.1083/jcb.20111048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maxfield FR, McGraw TE. Nat. Rev. Mol. Cell. Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. J. Biol. Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting figures, experimental procedures and characterization data for new compounds. This material is available free of charge via the internet at http://pubs.acs.org.