Abstract
As in humans and monkeys, lutein [(3R,3′R,6′R)-βε, -carotene-3,3′-diol] and zeaxanthin [a mixture of (3R,3′R)-β,β-carotene-3,3′diol and (3R,3′S-meso)-β,β-carotene-3,3′-diol] are found in substantial amounts in the retina of the Japanese quail Coturnix japonica. This makes the quail retina an excellent non-primate small animal model for studying the metabolic transformations of these important macular carotenoids that are thought to play an integral role in protection against light-induced oxidative damage such as that found in age-related macular degeneration (AMD). In this study, we first identified the array of carotenoids present in the quail retina using C30-HPLC coupled with in-line mass-spectral and photodiode-array detectors. In addition to dietary lutein (2.1%) and zeaxanthin (11.8%), we identified adonirubin (5.4%), 3′-oxolutein (3.8%), meso-zeaxanthin (3.0%), astaxanthin (28.2%), galloxanthin (12.2%), ε,ε-carotene (18.5%), and β-apo-2′-carotenol (9.5%) as major ocular carotenoids. We next used deuterium-labeled lutein and zeaxanthin as dietary supplements to study the pharmacokinetics and metabolic transformations of these two ocular pigments in serum and ocular tissues. We then detected and quantitated labeled carotenoids in ocular tissue using both HPLC-coupled mass spectrometry and non-invasive resonance Raman spectroscopy. Results indicated that dietary zeaxanthin is the precursor of 3′-oxolutein, β-apo-2′-carotenol, adonirubin, astaxanthin, galloxanthin, and ε, ε-carotene, whereas dietary lutein is the precursor for meso-zeaxanthin. Studies also revealed that the pharmacokinetic patterns of uptake, carotenoid absorption, and transport from serum into ocular tissues were similar to results observed in most human clinical studies.
Keywords: Carotenoid, Retina, Japanese quail, Lutein, Zeaxanthin, HPLC, APCI-MS
Epidemiological and clinical studies in humans have generally demonstrated that there is an inverse relationship between nutritional consumption of antioxidants and prevalence of old age ocular disorders such as age-related macular degeneration (AMD) and cataract (1, 2). The carotenoids lutein and zeaxanthin are notable examples because they are specifically concentrated in ocular tissues at high concentrations, and they are excellent absorbers of phototoxic blue light and reactive oxygen species (3,4). Studies using data from the Eye Disease Case Control (EDCC) Study Group have demonstrated that individuals with high blood levels of lutein and zeaxanthin and high consumption of foods rich in these same carotenoids had significantly lower risk of exudative AMD, and several follow up studies including the Age-Related Eye Disease Study (AREDS) have confirmed these findings (5–8, and personal communication from Dr. Emily Chew, AREDS principal investigator).
In the human retina, dietary lutein [(3R,3′R,6′R)-β,ε-carotene-3,3′-diol] and zeaxanthin [(3R,3′R)-β,β-carotene-3,3′-diol] are unevenly distributed in the retina, with millimolar concentrations found at the fovea with a lutein:zeaxanthin ratio of 1:2 and a one-hundred-fold lower concentration in the periphery with a 3:1 lutein: zeaxanthin ratio (9–11). As would be expected in a metabolically active, oxidatively stressed tissue, a number of important metabolites are also present. Previously, we and others have reported the presence of several non-dietary metabolites such as (3R,3′S-meso)-zeaxanthin, (3R,3′S,6′R)-lutein (3′-epilutein), 3-hydroxy-β,ε-carotene-3′-one (3′-oxolutein), and 3-methoxyzeaxanthin (3-MZ) in the human cadaver retina using HPLC and spectroscopic detection methods (10–15), but understanding the biochemical mechanisms of these biotransformations generally requires living animal models. Several living monkey studies have been reported recently, but primate studies are expensive and cumbersome to carry out, so an appropriate non-primate small animal model could also have great utility (16–18).
Although several previous studies have reported carotenoid distributions in chickens and finches (19–21), the Japanese quail Coturnix japonica has emerged in recent years as a very useful small animal model for ocular carotenoid metabolism and physiology studies. Quail have high endogenous levels of lutein and zeaxanthin and their metabolites in ocular tissues, these levels can be augmented in response to dietary supplementation, and supplemented carotenoids can act as photoprotectants in light damage models (13,22–24). This is in contrast to non-primate mammals, which typically have low carotenoid levels in the eye and poor ocular response to supplementation. In this work, we have used C30-HPLC coupled with in-line photodiode-array and mass-spectral detection for the comprehensive identification of dietary carotenoids and their metabolites from retinal tissues of Japanese quail. We then studied metabolic transformations of deuterium-labeled lutein and zeaxanthin in serum and ocular tissues and demonstrated that resonance Raman spectroscopy is a useful noninvasive method to monitor ocular uptake. Results of these studies provide improved insights into the biochemical processes that may protect against age-related macular degeneration in humans.
EXPERIMENTAL PROCEDURES
Chemicals
Standards of lutein, zeaxanthin, meso-zeaxanthin, astaxanthin, canthaxanthin, cryptoxanthin, lycopene, echininone, and 3′-oxolutein were generous gifts from carotenoid manufacturers such as Kemin Health (Des Moines, Iowa), DSM (Kaiseraugst, Switzerland), and BASF (Ludwigshafen, Germany). Organic solvents were HPLC-grade from Fisher Scientific (Hampton, NH).
Preparation of deuterated carotenoids
Lutein
The freshwater alga Chlorella protothecoides CS 41 was procured from the microalgae supply service, CSIRO, Hobart, Australia. It was cultivated in Woods Hole MBL media in 50 ml of medium in 250 ml Erlenmeyer flasks and incubated at 20 ± 1 °C under continuous shaking (180 rpm) in dark/light alternating cycles on a rotary shaker at 180 rpm for 240 h (22). The media components were dissolved in deuterated water (70%, Spectra Stable Isotopes, Columbia, MD) to obtain deuterated lutein.
Zeaxanthin
Flavobacterium multivorum ATCC 55238 (American Type Culture Collection, Manassas, VA ) was grown on liquid growth medium which contained 25 g/l glucose (autoclaved separately), 10 g/l yeast extract, and 10 g/l peptone at pH 7.0. A 5% (v v−1) inoculum of Flavobacterium multivorum cultivated in the growth medium in the logarithmic phase (12 h, A500 nm 0.56) was used throughout the studies. All experiments were done in 50 ml of medium in 250 ml Erlenmeyer flasks and incubated at 28 ± 1 °C on a rotary shaker at 250 rpm for 60 h. The media components were dissolved in deuterated water (100%, Spectra Stable Isotopes, Columbia, MD)to obtain deuterated zeaxanthin (26).
Xanthophyll supplementation studies in Japanese quail
We chose to focus on only female quails because Toyoda et al. reported higher levels of lutein and zeaxanthin in the serum and ocular tissues in comparison to males (22). The use of quail was approved by the University of Utah’s Animal Care and Use Committee, and the investigations reported in this study adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Adult female Japanese quail raised on a basal turkey diet (8 weeks old, Coturnix japonica, Pharaoh XLD1 strain) were procured from B&D Farms (Harrah, Oklahoma) and fed 10–20 grams per bird per day of a basal turkey diet confirmed to be low in carotenoids (1–10 ng/g, Purina Mills, St. Louis, Missouri). Two experimental groups were orally fed five times per week with deuterated lutein or zeaxanthin (0.25 mg of carotenoid in 0.5 ml olive oil per day) prepared from the microbial sources mentioned above for 16 weeks. Control birds were given 0.5 ml olive oil vehicle five times per week. The birds were sacrificed in groups of four experimental birds and two control birds every four weeks, and the carotenoids of the serum and retina were measured by HPLC/MS. Eyes were enucleated, and ocular tissues were dissected with the aid of a dissecting microscope. Ocular tissues were stored in separate tubes at −70°C.
Extraction and saponification of carotenoids from quail retina
Animal tissues were weighed and extracted three times with tetrahydrofuran (THF, 3 × 10 mL) containing 0.1% butylated hydroxytoluene (BHT) by water bath sonication at 5°C to 10°C for 30 minutes each time. The combined extracts were evaporated to dryness on a rotary evaporator under reduced pressure. Unless otherwise noted, the residue was redissolved in hexane (0.9 ml) and subjected to saponification with 0.1 ml of 20% (w/v) methanolic potassium hydroxide (KOH) for two hours with constant stirring at room temperature. The final concentration of the KOH in the saponification solution was 0.5 M, and control experiments confirmed that complete hydrolysis of xanthophyll esters with minimal degradation was achieved. After saponification, the samples were washed with water until the aqueous extracts were found to be neutral after spotting on pH paper strips. The vials were centrifuged at approximately 2000 g to remove the minor amounts of whitish insoluble solid particles. The solution was evaporated to dryness on a rotary evaporator under reduced pressure at below 40°C, reconstituted in the appropriate HPLC solvents, and centrifuged as above before analysis. The residual samples were analyzed by LC-MS for presence of carotenoids.
Extraction of carotenoids from serum
Serum (0.5 ml) was treated with ethanol (0.5 ml) and hexane (0.5 ml) containing 0.1% BHT and centrifuged to remove the proteins. The aqueous phase was re-extracted with hexane (1.5 ml), and the organic extracts were evaporated to dryness on a rotary evaporator under reduced pressure at below 40°C. After evaporation of the solvent, the residue was reconstituted in the appropriate HPLC solvents and centrifuged at 2000 g before analysis
HPLC conditions
The dried pigments were re-dissolved in one ml of HPLC mobile phase. The samples were divided into two parts for two HPLC systems. System 1 (for general analysis of ocular carotenoids): HPLC separation was carried out at a flow rate of 1.0 ml/min on a C30 column (YMC Europe GmbH, Dinslaken, Germany, 25 cm length × 4.6 mm id) by a linear gradient of methanol and methylene chloride (% methanol@min: 99 @0; 90 @10; 70 @20; 0 @30; 99 @35; 99 @40). System 2 (for separation of zeaxanthin stereoisomers): Mobile phase containing hexane: iso-propanol (95:5 v/v); HPLC separation was carried out at a flow rate of 0.7 ml/min on a chiral column (ChiralPak AD, 25 cm length × 4.6 mm id, Chiral Technologies, Exton, PA). The columns were maintained at room temperature, and the HPLC detector was operated at 450 nm. Peak identities were confirmed by photodiode-array (PDA) spectra, by mass spectra, and by co-elution with authentic standards as necessary. Standard solutions of each individual carotenoid with known concentrations calculated spectroscopically using published extinction coefficients (27–28) were injected in different volumes so as to achieve final injected amounts ranging from 0.1 to 100 ng. The carotenoid content was quantitated using standard curves of available standards. β,ε-Carotene was quantitated by using a standard curve of β-carotene.Adonirubin, and galloxanthin were quantitated using astaxanthin and 10′-apocarotenol as surrogates, respectively.
Mass spectrometry equipment and conditions
Mass spectral (MS) analysis was performed using a Thermo Scientific MSQ single quadrupole mass spectrometer (San Jose, CA), equipped with an atmospheric pressure chemical ionization (APCI) source. The molecular ions were initially acquired in full-scan mode from 400–650 nm with 0.2 step size and 2 ms dwell time. Typical conditions were: corona discharge current 5 μA, RF lens bias voltage 0.1V, cone voltage 80V, and heater temperature 500°C. The ion source and tuning lens parameters were optimized automatically by infusing carotenoid standards via the built-in injector. Selected ion monitoring (SIM) was performed using dwell time of 200 ms for each channel with channels set to distinguish between unlabeled and deuterated carotenoids.
For routine quantitative analysis, the Thermo Scientific MSQ QUAN BROWSER (Xcaliber) was used. The software provides a peak integration of the ion intensity for each of the programmed mass ranges. For calibration curves, standard solutions were injected in different volumes so as to achieve final injected concentrations ranging from 1–8000 pg as previously described (29). Analysis was performed in triplicate, and the calibration curves were obtained by plotting concentration against area.
Resonance Raman measurement of ocular carotenoids in quail
We used a previously developed resonance Raman instrument designed for noninvasive quantitative measurement of human macular carotenoid pigments in the living eye (30). Monkey studies have confirmed the linearity of in vivo Raman measurements and HPLC analyses performed after enucleation (30). Prior to Raman measurements, birds were anesthetized with 2 μL/g intramuscular injection of Ketamine (50 mg/mL ) and their pupils were dilated with 1% tropicamide and 2.5% phenylephrine (Bausch and Lomb, Tampa, FL). The bird’s eye was aligned with the optical axis of the optical sampling module of the Raman setup. For a typical measurement, 1 mW argon laser light (488 nm) was directed as a 1 mm diameter spot onto the retina surface for 250 msec. Raman back-scattered light was collected with the sampling module and delivered to the Raman spectrograph via a fiber-optic bundle. Raman spectra were detected with a cooled silicon 2D-CCD array. The peak heights at the carotenoid C=C stretch frequencies of 1524 cm−1 (undeuterated) or 1504 cm−1 (deuterated) were quantified after subtraction of background fluorescence using specialized Windows-based software for data collection and processing.
Statistical Tests
Student t-tests, Fisher exact tests, and linear regressions were performed using SigmaStat software (Jandel Scientific, San Rafael, CA).
RESULTS
Identification of carotenoids in the quail retina
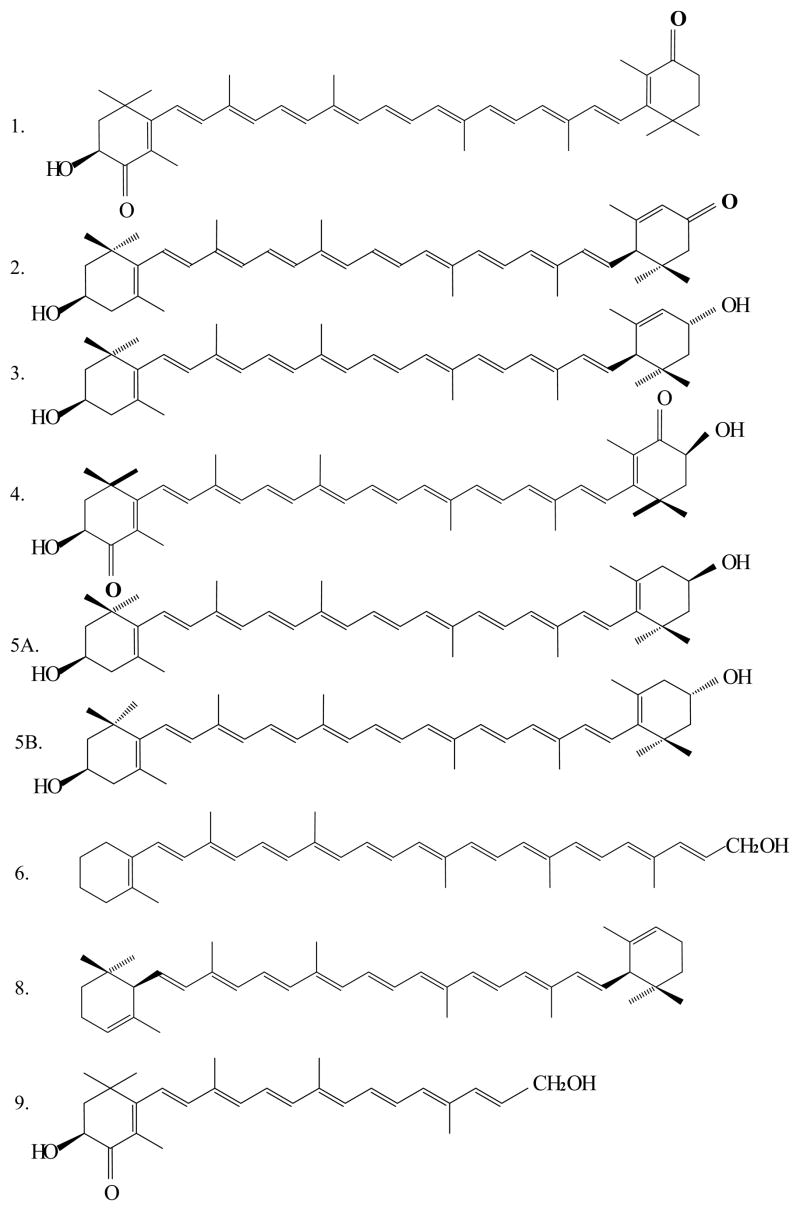
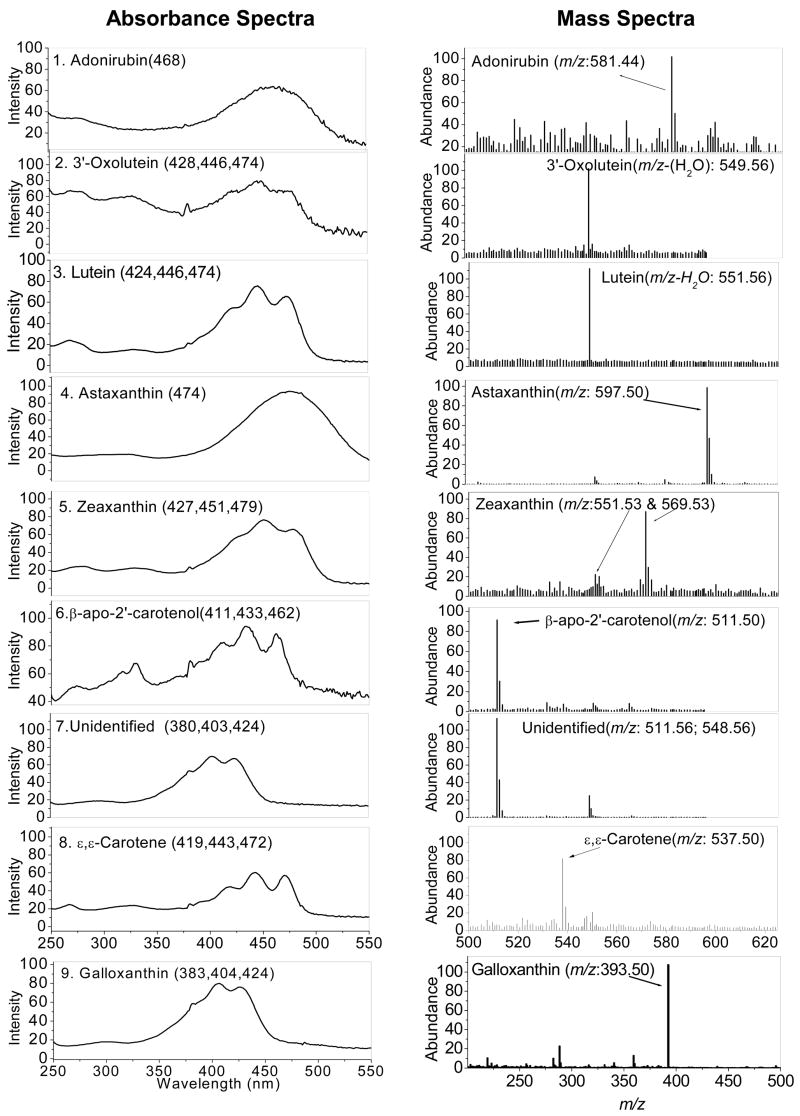
After saponification of ocular carotenoid extracts, a total of nine major carotenoids were resolved from retina of Japanese quail on the C30 column (Figure 1). If the saponification step is omitted, the unesterified carotenoid peak pattern is altered, and new late eluting peaks are present corresponding to xanthophyll esters. Peak identity assignments were made by examination of photodiode-array (PDA) spectra and mass spectra in comparison to the published literature (27–29). Authentic standards were available for lutein, zeaxanthin, astaxanthin, and 3′-oxolutein to confirm retention times. The rest of the ocular carotenoid identifications for which standards were not available (adonirubin, galloxanthin, ε,ε-carotene, and β-apo-2′-carotenol) are consistent with the previously published avian literature (31–34). The chemical structures, PDA spectra, and mass spectra of the major carotenoids are shown in Figures 2 and 3. Trace amounts of amounts of canthaxanthin, echinenone, and several other carotenoids were also detected. The zeaxanthin peak seen on the C30 column was a 4:1 mixture of dietary (3R, 3′R) zeaxanthin and (3R,3′S-meso)-zeaxanthin which could be resolved after collection and re-analysis on a chiral column.
Figure 1.
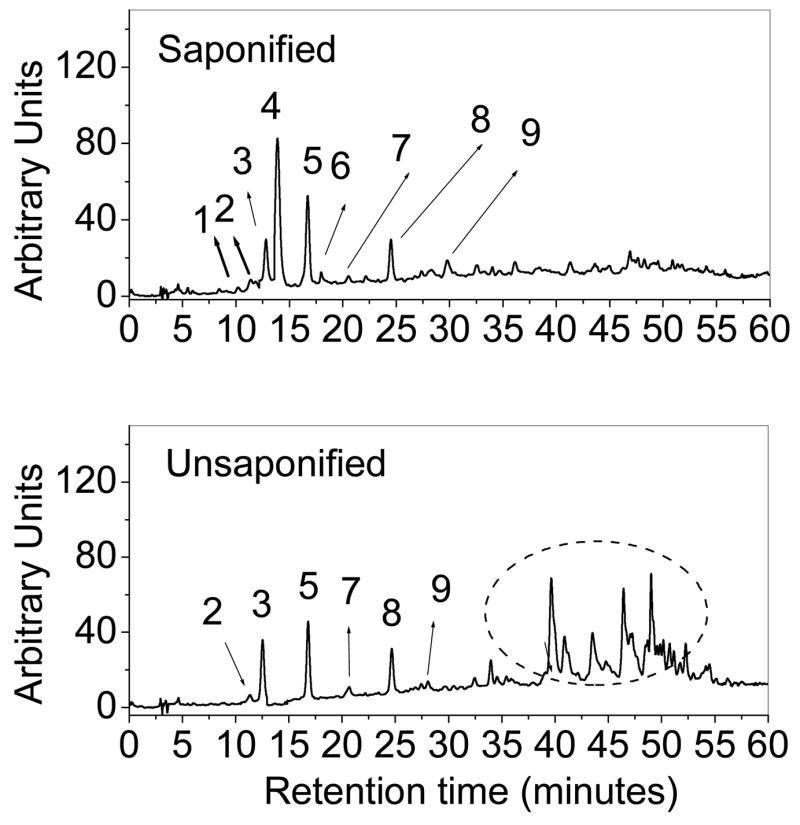
HPLC chromatogram of saponified and unsaponified retinal extracts from Japanese quail with detection at 450 nm (A). Major identified carotenoids include 1. Adonirubin, 2. 3ε-Oxolutein, 3. Lutein, 4. Astaxanthin, 5. Zeaxanthin and meso-Zeaxanthin, 6. β-apo-2′-Carotenol, 7. Unidentified peak, 8. ε,ε-Carotene, and 9. Galloxanthin. The circled region in the unsaponified chromatogram indicates the presence of esterified carotenoids.
Figure 2.
Structures of major identified carotenoids shown in figure 2: 1. Adonirubin, 2 3′-Oxolutein, 3. Lutein, 4. Astaxanthin, 5A. Zeaxanthin, 5B. meso-Zeaxanthin 6. β-apo-2′-Carotenol, 8. ε,ε-Carotene, and 9. Galloxanthin.
Figure 3.
Absorbance and mass spectra of major identified carotenoids from saponified preparations: 1. Adonirubin, 2. 3′-Oxolutein, 3. Lutein, 4. Astaxanthin, 5. Zeaxanthins, 6. β-apo-2′-Carotenol, 7. Unidentified peak, 8.ε,ε-Carotene, and 9. Galloxanthin.
HPLC/MS in SIM mode proved very sensitive, permitting detection limits of as low as 10 picograms with a signal-to-noise (S/N) criterion of 20. The method was 75–100 times more sensitive than HPLC with a commonly used PDA detector, permitting quantitation of even minor metabolites such as 3′-oxolutein and β-apo-2′-carotenol. Absolute and relative amounts of quail ocular carotenoids and degrees of esterification determined in parallel analyses with and without saponification are given in Table 1.
Table 1.
Typical carotenoid content of the retina from Japanese quail at the beginning of the experiments (8–12 weeks old) separated on a C30 column.
| Peak Number | Carotenoid | ng/retina
(n=4; ± SD) |
% of total carotenoidsb | % Esterificationc |
|---|---|---|---|---|
| 1 | Adonirubin | 5.4 ±5.1 | 5.6 | 100 |
| 2 | 3′-Oxolutein | 3.8 ±3.2 | 3.9 | 75 |
| 3 | (3R,3′R,6′R)-Lutein | 2.1±3.2 | 2.1 | 68 |
| 4 | Astaxanthin | 28.2±2.5 | 28.9 | 100 |
| 5 | (3R,3′R)-Zeaxanthin and (3R,3′S-meso)-Zeaxanthina | 14.8±5.1 | 15.2 | 49 |
| 6 | β-apo-2′-Carotenol | 9.5±4.5 | 9.8 | 100 |
| 7 | Unidentified | 2.9±2.2 | 2.9 | 53 |
| 8 | ε,ε-Carotene | 18.5±6.3 | 18.9 | 0 |
| 9 | Galloxanthin | 12.2±6.1 | 12.7 | 91 |
Ratio of (3R,3′R)-Zeaxanthin and (3R,3′S-meso)-Zeaxanthin was 4:1.
Total carotenoid level in the basal group was 97.4 ng/retinal tissue.
Percent esterification was determined in parallel analyses with and without saponification.
Effect of deuterated xanthophyll supplementation
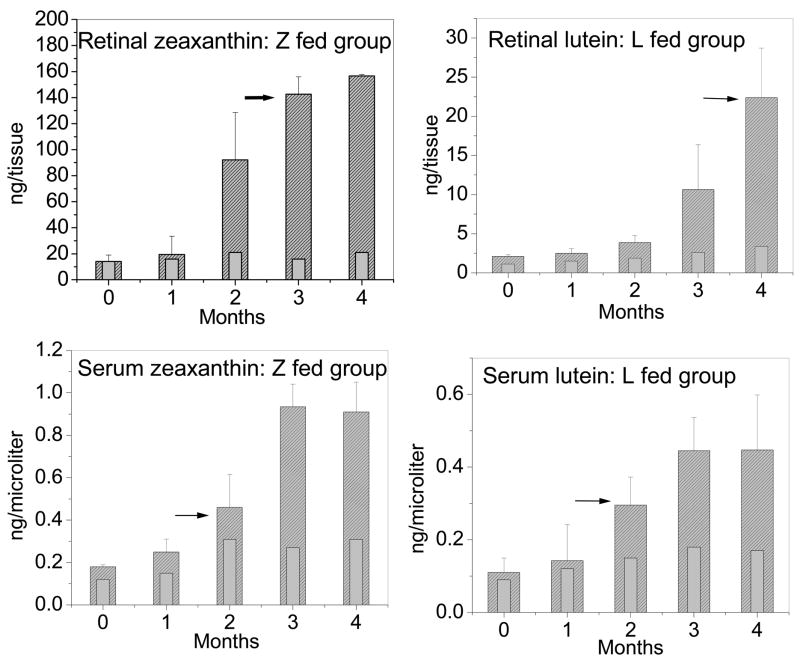
The basal levels of zeaxanthin in the serum and retina of quail were 0.18 ±0.05 ng/μl and 14.2 ±5.1 ng/tissue respectively. After daily supplementation with deuterated zeaxanthin, the serum levels rose six-fold in 3 months, and retinal levels increased by a factor of 11 (157.5 ±13.8 ng/tissue, P < 0.001) (Figure 4). Mass spectral analysis demonstrated that retinal zeaxanthin was 94% deuterated at 12 weeks of supplementation (Figure 5, Table 2). Although we did not observe any appreciable increase in the levels of the other ocular carotenoids relative to the values shown in Table 1, adonirubin, 3′-oxolutein, astaxanthin, β-apo-2′-carotenol, ε,ε-carotene, and galloxanthin had detectable deuteration. Only lutein, meso-zeaxanthin, and an unidentified carotenoid remained undeuterated in response to deuterated zeaxanthin supplementation (Table 2).
Figure 4.
Increase and saturation of retinal and serum carotenoids in the supplemented groups (n=4) from 0 to 16 weeks of feeding. Light gray bars with no shading indicate levels of carotenoids in control birds (n=2) at that time point. The difference between control and experimental groups was statistically significant (P < 0.001) after the second month of feeding for all groups except for retinal levels in lutein fed group, which was statistically significant (P < 0.001) after the third month of feeding. L and Z fed groups represent deuterated lutein and zeaxanthin supplemented groups respectively. Black arrows indicate initial detection of deuterated activity in the serum and/or retina.
Figure 5.
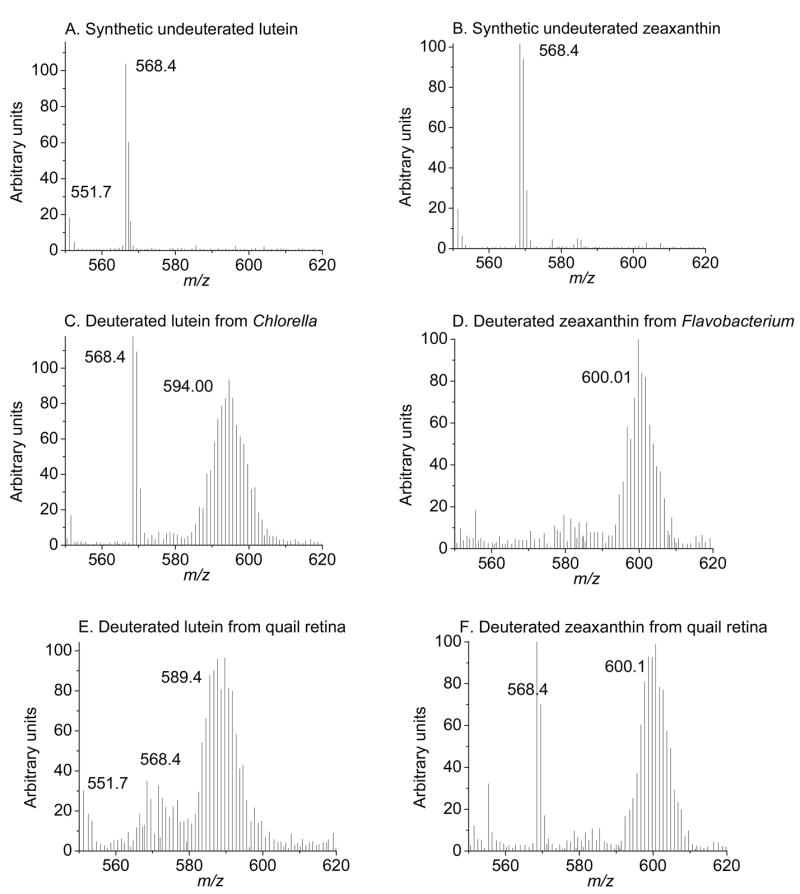
Mass spectra of undeuterated and deuterated lutein and zeaxanthin from microbial sources and from retinas of quail fed with deuterated carotenoids for 16 weeks. Deuterated lutein and zeaxanthin display isotopomer distribution between m/z 580–605; major mass peaks are labeled in the graphs.
Table 2.
Average degree of deuteration of ocular carotenoids in quail supplemented with deuterated zeaxanthin or lutein for 16 weeksa.
| Peak Number | Carotenoids | Zeaxanthin Supplemented | Lutein Supplemented |
|---|---|---|---|
| 1 | Adonirubin | 48% | <10% |
| 2 | 3′-Oxolutein | 32% | <10% |
| 3 | (3R,3′R,6′R)-Lutein | <10% | 83% |
| 4 | Astaxanthin | 28% | <10% |
| 5A | (3R,3′R)-Zeaxanthin | 94% | <10% |
| 5B | (3R,3′S-meso)-Zeaxanthin | <10% | 42% |
| 6 | β-apo-2′-Carotenol | 51% | <10% |
| 7 | Unidentified | <10% | <10% |
| 8 | ε,ε-Carotene | 48% | <10% |
| 9 | Galloxanthin | 58% | <10% |
The basal levels of lutein in the serum and retina of quail were 0.11 ± 0.04 ng/μl and 2.1 ±2.2 ng/tissue, respectively. After supplementation with deuterated algal lutein, the serum levels rose 4-fold in 3 months, and retinal levels increased by a factor of 10 in four months (22.1 ±5.7 ng/tissue, P < 0.001). We did not observe any appreciable increase in the levels of any other retinal carotenoids during lutein supplementation, and mass spectral analysis indicated that only lutein (83%) and meso-zeaxanthin (42%) had detectable deuteration (Figure 5 and Table 2).
Resonance Raman measurements
In separate experiments, we investigated whether or not resonance Raman spectrosocopy could be used to monitor uptake of supplemented carotenoids noninvasively in our quail. Deuteration of carotenoid standards leads to an alteration of the C=C peak from 1524 cm−1 to 1504 cm−1 due to a change of the bond vibration frequency induced by the heavier atoms incorporated into the polyene backbone (Figure 6). An identical alteration of the C=C stretch vibration was observed in birds fed a deuterated zeaxanthin diet for 4 months relative to undeuterated control birds. Quantitatively, Raman counts were linearly related to total carotenoids measured by HPLC (r = 0.88; P < 0.0001; n=20), and the 2.8-fold increase in the C=C stretch intensity with supplementation matched well with the 3.6-fold increase in total carotenoid levels measured by HPLC in this batch of birds (P < 0.001 for both, Table 3).
Figure 6.
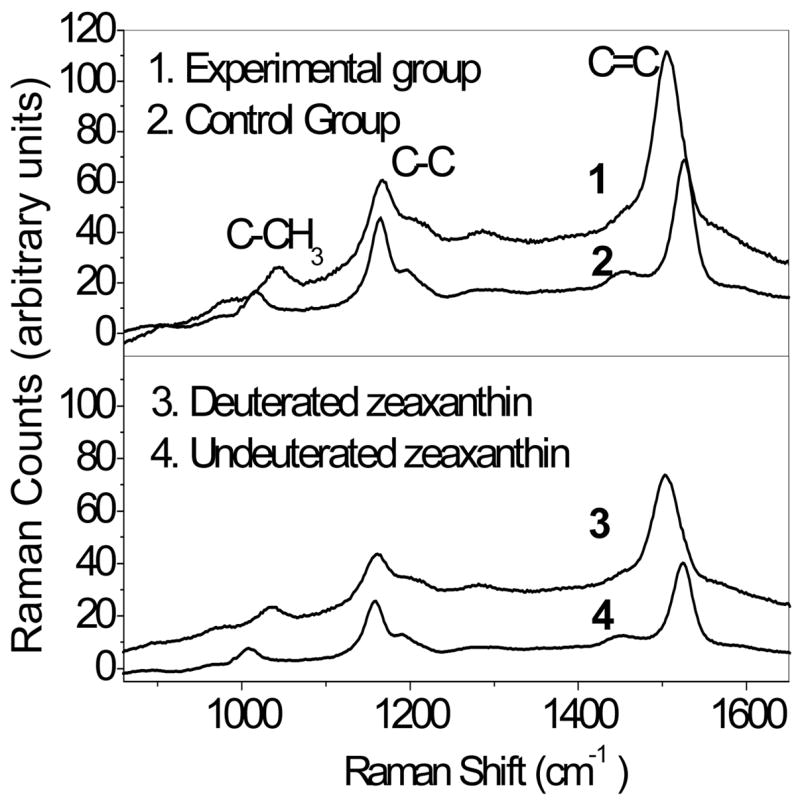
Normalized resonance Raman spectra recorded from living quail eyes. The upper panel shows a spectrum from a bird fed deuterated zeaxanthin for 12 weeks (1) in comparison to a bird fed an undeuterated control diet (2). The lower panel shows spectra of deuterated (3) and undeuterated (4) standards. Note the 20 cm−1 displacement of the C=C stretch peak with deuteration in the standards and in the living eye. See Table 3 for quantitative analysis of the C=C peak height in comparison to HPLC analysis.
Table 3.
Correlation between resonance Raman counts and total carotenoid levels quantitated by HPLC.
| Groups | C=C Peak Height
(Raman counts) (mean ± SD) |
Total Carotenoids
(ng/retina) (mean ± SD) |
|---|---|---|
| Control ( n=12) | 1161 ± 407 | 37 ± 31 |
| Zeaxanthin Supplemented (n=8) | 3211 ± 261 | 132 ± 73 |
DISCUSSION
The macula of the human eye specifically accumulates high levels of lutein, zeaxanthin, and their metabolites where they are likely to act as photoprotective antioxidants. Among mammals, this specific ocular accumulation of carotenoids appears to be unique to primates which is why elucidation of the biochemical mechanisms of carotenoid accumulation and metabolism has proven to be quite challenging. On the other hand, birds such as the Japanese quail are considered to be an attractive surrogate model system for studying ocular physiology of carotenoids due to the fact that they exhibit selective ocular xanthophyll accumulation similar to humans (13,21–24). In addition, Japanese quail are small and domesticated, they age rapidly, and their retinas exhibit age-related loss of photoreceptors and accumulation of RPE lipofuscin (24, 33). Khachik et al. in 2002 pointed out that there are limitations to the quail model, however. These include their higher diversity of ocular carotenoids and their unusually high level of ocular carotenoid esterification to long-chain fatty acids (13).
Considering the complexity of carotenoids present in Japanese quail retinas, we used a C30-HPLC column to resolve a greater range of carotenoids than had previously been achieved. Absorbance spectroscopy and mass spectrometry were used as major tools for identification of ocular carotenoids. In quails, carotenoids are known to be distributed in discrete oil droplets in the cone inner segments. We observed variable levels of xanthophyll esterification ranging from 100% to less than 50% depending on the carotenoid studied which indicates that at least a portion of the quail’s ocular carotenoids are likely to be readily available as local antioxidants, not just light screening pigments. As expected, dietary lutein and zeaxanthin were among the major ocular carotenoids, but astaxanthin which was not present in the quails’ diet on the farm or in the laboratory was also present at comparable levels. We also detected lower levels of other carotenoids not found in their diet such as adonirubin, galloxanthin, ε,ε-carotene, 3′-oxolutein, meso-zeaxanthin, and β-apo-2′-carotenol. Many, but not all, of these non-dietary carotenoids have been reported in quail and/or chicken retinas in the past. Unlike previous reports (13,34) we did not detect any cryptoxanthin or lycopene in the quail retina.
While supplementation with lutein and/or zeaxanthin is actively promoted to individuals at risk for visual loss from macular degeneration, clinical studies have shown that humans exhibit very slow and variable macular increases in response to long-term supplementation. Only recently has there been a retrospective cadaver tissue study that confirms that ocular levels do indeed rise three- to ten-fold in response to long-term lutein supplementation (15). Our current study in Japanese quail corroborates that high dose dietary supplementation can increase the ocular levels of carotenoids over a period of many weeks, a finding that correlates well with the time-course observed in most human clinical studies (1–2, 5–7, 35–38). We also demonstrated that resonance Raman spectroscopy can be used for rapid noninvasive qualitative and quantitative analysis of carotenoids from ocular tissue of the Japanese quail and that this method could noninvasively distinguish between deuterated and undeuterated molecules of carotenoids. With further optimization, this noninvasive method could prove useful in future pharmacokinetic supplementation studies in birds and even primates especially if a 13C label is also introduced to shift the C=C bond vibration frequency even further relative to deuterium alone. We are currently developing an imaging mode of this technology which would greatly improve its utility in laboratory animal studies by allowing spatial delineation of changes of carotenoid distributions in response to dietary interventions.
The retina’s high levels of polyunsaturated fatty acids make retinal membrane lipids vulnerable to cellular damage caused by reactive oxygen intermediates. Lutein and zeaxanthin are believed to limit mammalian retinal oxidative damage by absorbing incoming blue light and/or by quenching reactive oxygen intermediates, but this antioxidant function may generate several metabolites such as meso-zeaxanthin and 3′-oxolutein, and it has been proposed that these may be biomarkers of aging and oxidative stress (13, 32,39). The biochemical pathways for ocular carotenoid interconversions are poorly understood, however. Previously, Johnson et al. reported in 2005 that carotenoid deficient monkeys had significant serum and retinal responses to lutein and zeaxanthin supplementation, that dietary lutein appeared to be the precursor to ocular meso-zeaxanthin, and that 3′-oxolutein may arise from dietary zeaxanthin (17). In this report using deuterium-labeled precursors, we are able to confirm directly in the quail that dietary lutein and zeaxanthin are the precursors for meso-zeaxanthin and 3′-oxolutein, respectively. Furthermore, we were able to demonstrate that dietary zeaxanthin is the precursor for the diverse array of carotenoids present in the avian retinal oil droplets, although the biochemical mechanisms responsible for these rather complex conversions remain to be determined. Retinal carotenoids maintain their dynamic equilibrium in the eye through interrelated regulation mechanisms driven by binding proteins, enzymes and/or photochemical alterations. Our observation that high degrees of deuteration are found in the quail retina only after three to four months of carotenoid supplementation indicates that the endogenous carotenoid pool is quite stable and only slowly exchangeable, consistent with previously reported carotenoid depletion studies in monkeys and chickens (17–18, 40).
In summary, these studies on the Japanese quail demonstrate that this small animal model can provide important insights into the physiology of the ocular carotenoids that can be applied to the eye of humans and other primates. Since these birds also accumulate AMD-associated toxic aging pigments such as A2E and other lipofuscin constituents at levels comparable to humans (unpublished data), Japanese quail are likely to be a very useful model system to study the potential ability of carotenoids to modify the course of age-related macular degeneration.
Acknowledgments
The technical assistance of Igor Ermakov, PhD, Department of Physics, University of Utah with resonance Raman measurements of quail eyes is greatly appreciated.
ABBREVIATIONS
- AMD
Age-related macular degeneration
- MS
Mass spectra
- THF
Tetrahydrofuran
- HPLC
High-performance liquid chromatography
- APCI
Atmospheric pressure chemical ionization
- SIM
Selected ion monitoring
- RPE
Retinal pigment epithelium
- BHT
Butylated hydroxytoluene
Footnotes
This work was supported by National Institute of Health Grant EY-11600 and by Research to Prevent Blindness, Inc. (New York, NY).
References
- 1.Mares-Perlman JA, Millen AE, Ficek TL, Hankinson SE. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. Overview, J Nutr. 2002;132:518S–524S. doi: 10.1093/jn/132.3.518S. [DOI] [PubMed] [Google Scholar]
- 2.Gale CR, Hall NF, Phillips DI, Martyn CN. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2003;44:2461–2465. doi: 10.1167/iovs.02-0929. [DOI] [PubMed] [Google Scholar]
- 3.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 5.Seddon JMAU, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272:1413–1420. [PubMed] [Google Scholar]
- 6.Eye Disease Case-Control Study Group. Antioxidant status and neovascular age-related macular degeneration. Arch Ophthalmol. 1993;111:104–109. doi: 10.1001/archopht.1993.01090010108035. [DOI] [PubMed] [Google Scholar]
- 7.Bressler NM, Bressler SB, Congdon NG, Ferris FL, 3, Friedman DS, Klein R, Lindblad AS, Milton RC, Seddon JM. Age-Related Eye Disease Study Research Group. Potential public health impact of Age-Related Eye Disease Study results: AREDS report no 11. Arch Ophthalmol. 2003;121:621–624. doi: 10.1001/archopht.121.11.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bone RA, Landrum JT, Friedes LM, Gomez CM, Kilburn MD, Menendez E, Vidal I, Wang W. Distribution of Lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res. 1997;64:211–218. doi: 10.1006/exer.1996.0210. [DOI] [PubMed] [Google Scholar]
- 10.Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29:843–849. [PubMed] [Google Scholar]
- 11.Handelman GJ, Dratz EA, Reay CC, van Kuijk JG. Carotenoids in the human macula and whole retina. Invest, Ophthalmol, Vis, Sci. 1988;29:850–855. [PubMed] [Google Scholar]
- 12.Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001;72:215–223. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- 13.Khachik F, de Moura F, Zhao DY, Aebischer CP, Bernstein PS. Transformations of selected carotenoids in plasma, liver, and ocular tissues of humans and in nonprimate animal models. Invest Ophthalmol Vis Sci. 2002;43:3383–3392. [PubMed] [Google Scholar]
- 14.Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci. 1997;38:1802–1811. [PubMed] [Google Scholar]
- 15.Bhosale P, Zhao DY, Bernstein PS. HPLC measurement of ocular carotenoid levels in human donor eyes in the lutein supplementation era. Invest Ophthalmol Vis Sci. 2007;48:543–549. doi: 10.1167/iovs.06-0558. [DOI] [PubMed] [Google Scholar]
- 16.Khachik F, London E, de Moura FF, Johnson M, Steidl S, Detolla L, Shipley S, Sanchez R, Chen XQ, Flaws J, Lutty G, McLeod S, Fowler B. Chronic ingestion of (3R,3′R,6′R)-lutein and (3R,3′R)-zeaxanthin in the female rhesus macaque. Invest Ophthalmol Vis Sci. 2006;47:5476–5486. doi: 10.1167/iovs.06-0194. [DOI] [PubMed] [Google Scholar]
- 17.Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2005;46:692–702. doi: 10.1167/iovs.02-1192. [DOI] [PubMed] [Google Scholar]
- 18.Neuringer M, Sandstrom MM, Johnson EJ, Snodderly DM. Nutritional manipulation of primate retinas, I: effects of lutein or zeaxanthin supplements on serum and macular pigment in xanthophyll-free rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:3234–3243. doi: 10.1167/iovs.02-1243. [DOI] [PubMed] [Google Scholar]
- 19.Stransky H, Schulze I. Carotenoids in Gallus domesticus: comparative analysis of blood and retina of chickens and of egg yolk. J Comp Physiol B Meta Transp Funct. 1977;114:265–277. [Google Scholar]
- 20.Tyczkowski JK, Schaeffer JLC, Parkhurst Hamilton PB. 3′-Oxolutein, a metabolite of lutein in chickens. Poult Sci. 1986;65:2135–2141. doi: 10.3382/ps.0652135. [DOI] [PubMed] [Google Scholar]
- 21.McGraw KJ, Adkins-Regan E, Parker RS. Anhydrolutein in the zebra finch: a new, metabolically derived carotenoid in birds. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:811–818. doi: 10.1016/s1096-4959(02)00100-8. [DOI] [PubMed] [Google Scholar]
- 22.Toyoda Y, Thomson LR, Langner A, Craft NE, Garnett KM, Nichols CR, Cheng KM, Dorey CK. Effect of dietary zeaxanthin on tissue distribution of zeaxanthin and lutein in quail. Invest Ophthalmol Vis Sci. 2002;43:1210–1221. [PubMed] [Google Scholar]
- 23.Thomson LR, Toyoda Y, Delori FC, Garnett KM, Wong ZY, Nichols CR, Cheng KM, Craft NE, Dorey CK. Long term dietary supplementation with zeaxanthin reduces photoreceptor death in light-damaged Japanese quail. Exp Eye Res. 2002;75:529–542. doi: 10.1006/exer.2002.2050. [DOI] [PubMed] [Google Scholar]
- 24.Karadas F, Surai P, Grammenidis E, Sparks NH, Acamovic T. Supplementation of the maternal diet with tomato powder and marigold extract: effects on the antioxidant system of the developing quail. Br Poult Sci. 2006;47:200–208. doi: 10.1080/00071660600611003. [DOI] [PubMed] [Google Scholar]
- 25.Bhosale P, Serban B, Bernstein PS. Production of deuterated lutein by Chlorella protothecoides and its detection by mass spectrometric methods. Biotechnol Lett. 2006;28:1371–1375. doi: 10.1007/s10529-006-9105-8. [DOI] [PubMed] [Google Scholar]
- 26.Bhosale P, Teredesai PV, Lihong J, Ermakov IV, Gellermann W, Bernstein PS. Production of deuterated zeaxanthin by Flavobacterium multivorum and its detection by resonance Raman and mass spectrometric methods. Biotechnol Lett. 2005;27:1719–1723. doi: 10.1007/s10529-005-2737-2. [DOI] [PubMed] [Google Scholar]
- 27.Britton G. UV/Visible Spectroscopy. In: Britton G, Liaaen-Jenson S, Pfander H, editors. Carotenoids. 1B. Birkhaeuser; Basel, Switzerland: 1995. pp. 13–62. [Google Scholar]
- 28.Pfander H. Methods in Enzymology. Vol. 213. New York: Academic Press; 1992. Carotenoid: An overview; pp. 3–13. [Google Scholar]
- 29.Bhosale P, Bernstein PS. Quantitative measurement of 3′-oxolutein from human retina by normal-phase high-performance liquid chromatography coupled to atmospheric pressure chemical ionization mass spectrometry. Anal, Biochem. 2005;345:296–301. doi: 10.1016/j.ab.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Gellermann W, Ermakov IV, Ermakova MR, McClane RW, Zhao DY, Bernstein PS. In vivo resonant Raman measurement of macular carotenoid pigments in the young and the aging human retina. J Opt Soc Am A Opt Image Sci Vis. 2002;19:1172–1186. doi: 10.1364/josaa.19.001172. [DOI] [PubMed] [Google Scholar]
- 31.Tyczkowski JK, Schaeffer JL, Parkhurst C, Hamilton PB. 3′-Oxolutein, a metabolite of lutein in chickens. Poult Sci. 1986;65:2135–2141. doi: 10.3382/ps.0652135. [DOI] [PubMed] [Google Scholar]
- 32.Goodwin TW. Metabolism, nutrition, and function of carotenoids. Annu Rev Nutr. 1986;6:273–297. doi: 10.1146/annurev.nu.06.070186.001421. [DOI] [PubMed] [Google Scholar]
- 33.Fite KV, Bengston L, Donaghey B. Experimental light damage increases lipofuscin in the retinal pigment epithelium of Japanese quail (Coturnix japonica) Exp Eye Res. 1993;57:449–460. doi: 10.1006/exer.1993.1147. [DOI] [PubMed] [Google Scholar]
- 34.Goldsmith TH, Collins JS, Licht S. The cone oil droplets of avian retinas. Vision Res. 1984;24:1661–1671. doi: 10.1016/0042-6989(84)90324-9. [DOI] [PubMed] [Google Scholar]
- 35.Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, Pei K, Tsipursky M, Nyland J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial) Optometry. 2004;75:216–230. doi: 10.1016/s1529-1839(04)70049-4. [DOI] [PubMed] [Google Scholar]
- 36.Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: The effect of 140 days of a lutein supplement. Exp Eye Res. 1997;65:57–62. doi: 10.1006/exer.1997.0309. [DOI] [PubMed] [Google Scholar]
- 37.Hammond BR, Jr, Johnson EJ, Russell RM, Krinsky NI, Yeum KJ, Edwards RB, Snodderly DM. Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci. 1997;38:1795–1801. [PubMed] [Google Scholar]
- 38.Berendschot TT, Goldbohm RA, Klopping WA, van de Kraats J, van Norel J, van Norren D. Influence of lutein supplementation on macular pigment, assessed with two objective techniques. Invest Ophthalmol Vis Sci. 2000;41:3322–3326. [PubMed] [Google Scholar]
- 39.Bhosale P, Zhao DY, Serban B, Bernstein PS. Identification of 3-methoxyzeaxanthin as a novel age-related carotenoid metabolite in the human macula. Invest Ophthalmol Vis Sci. 2007;48:1435–1440. doi: 10.1167/iovs.06-1046. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Connor SL, Wang W, Johnson EJ, Connor WE. The selective retention of lutein, meso-zeaxanthin and zeaxanthin in the retina of chicks fed a xanthophyll-free diet. Exp Eye Res. 2007;84:591–598. doi: 10.1016/j.exer.2006.11.013. [DOI] [PubMed] [Google Scholar]