Abstract
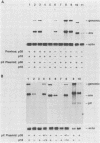
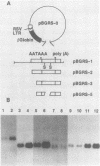
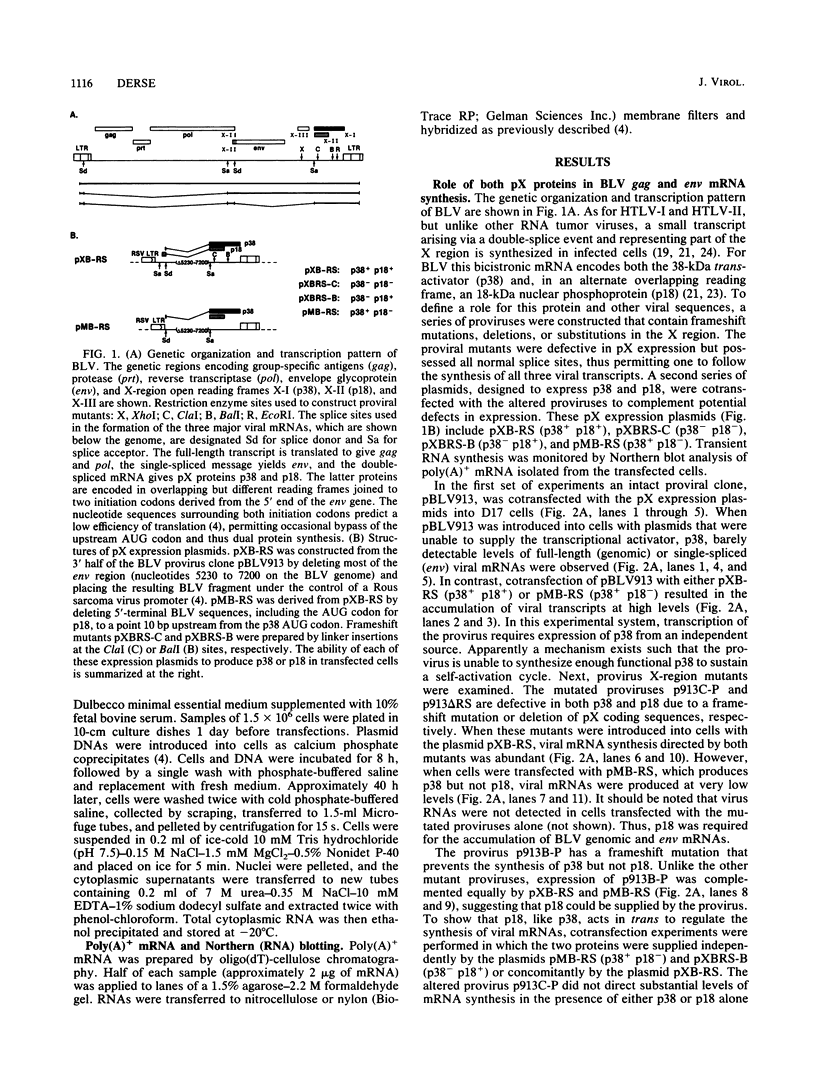
Bovine leukemia virus (BLV) and the human T-cell leukemia virus types I and II comprise a unique retrovirus subfamily which has evolved complex strategies for the regulation of gene expression. A transcriptional control circuit has been characterized in both human and bovine systems in which cis-acting promoter control elements are responsive to trans-acting factors encoded in the pX region of the virus. The BLV pX mRNA encoding the transcriptional trans-acting factor is translated in an alternate reading frame to produce an 18-kilodalton nuclear phosphoprotein, p18. A function for this protein was revealed in cotransfection experiments using mutated BLV proviruses in combination with pX expression plasmids. These experiments indicated that p18 was required for the accumulation of viral mRNAs representing full-length (genomic) and single-spliced (env) transcripts. In contrast, synthesis of the double-spliced pX mRNA was not influenced by p18 expression. Large regional deletions and substitutions of provirus sequences localized elements essential for p18 regulation to the 3' long terminal repeat. Furthermore, sequences within a 250-nucleotide region between the AATAAA signal and poly(A) site were found to be essential for efficient virus mRNA 3'-end processing and response to p18 regulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Bothwell A. L., Knapp M., Siden E., Mather E., Koshland M., Baltimore D. Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3' ends. Cell. 1980 Jun;20(2):293–301. doi: 10.1016/0092-8674(80)90615-7. [DOI] [PubMed] [Google Scholar]
- Chen I. S., Slamon D. J., Rosenblatt J. D., Shah N. P., Quan S. G., Wachsman W. The x gene is essential for HTLV replication. Science. 1985 Jul 5;229(4708):54–58. doi: 10.1126/science.2990037. [DOI] [PubMed] [Google Scholar]
- Derse D. Bovine leukemia virus transcription is controlled by a virus-encoded trans-acting factor and by cis-acting response elements. J Virol. 1987 Aug;61(8):2462–2471. doi: 10.1128/jvi.61.8.2462-2471.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derse D., Caradonna S. J., Casey J. W. Bovine leukemia virus long terminal repeat: a cell type-specific promoter. Science. 1985 Jan 18;227(4684):317–320. doi: 10.1126/science.2981431. [DOI] [PubMed] [Google Scholar]
- Derse D., Casey J. W. Two elements in the bovine leukemia virus long terminal repeat that regulate gene expression. Science. 1986 Mar 21;231(4744):1437–1440. doi: 10.1126/science.3006241. [DOI] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Paskalis H., Kleinman-Ewing C., Wong-Staal F., Pavlakis G. N. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985 Aug 16;229(4714):675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- Franchini G., Wong-Staal F., Gallo R. C. Human T-cell leukemia virus (HTLV-I) transcripts in fresh and cultured cells of patients with adult T-cell leukemia. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6207–6211. doi: 10.1073/pnas.81.19.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysdael J., Bruck C., Kettmann R., Burny A. Bovine leukemia virus. Curr Top Microbiol Immunol. 1984;112:1–19. doi: 10.1007/978-3-642-69677-0_1. [DOI] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Inoue J., Seiki M., Yoshida M. The second pX product p27 chi-III of HTLV-1 is required for gag gene expression. FEBS Lett. 1986 Dec 15;209(2):187–190. doi: 10.1016/0014-5793(86)81108-5. [DOI] [PubMed] [Google Scholar]
- Inoue J., Yoshida M., Seiki M. Transcriptional (p40x) and post-transcriptional (p27x-III) regulators are required for the expression and replication of human T-cell leukemia virus type I genes. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3653–3657. doi: 10.1073/pnas.84.11.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh I., Yoshinaka Y., Sagata N., Ikawa Y. The bovine leukemia virus X region encodes a trans-activator of its long terminal repeat. Jpn J Cancer Res. 1987 Feb;78(2):93–98. [PubMed] [Google Scholar]
- Kettmann R., Deschamps J., Cleuter Y., Couez D., Burny A., Marbaix G. Leukemogenesis by bovine leukemia virus: proviral DNA integration and lack of RNA expression of viral long terminal repeat and 3' proximate cellular sequences. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2465–2469. doi: 10.1073/pnas.79.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Marbaix G., Cleuter Y., Portetelle D., Mammerickx M., Burny A. Genomic integration of bovine leukemia provirus and lack of viral RNA expression in the target cells of cattle with different responses to BLV infection. Leuk Res. 1980;4(6):509–519. doi: 10.1016/0145-2126(80)90062-4. [DOI] [PubMed] [Google Scholar]
- Kiyokawa T., Seiki M., Iwashita S., Imagawa K., Shimizu F., Yoshida M. p27x-III and p21x-III, proteins encoded by the pX sequence of human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8359–8363. doi: 10.1073/pnas.82.24.8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamoun R. Z., Astier-Gin T., Kettmann R., Deschamps J., Rebeyrotte N., Guillemain B. J. The pX region of the bovine leukemia virus is transcribed as a 2.1-kilobase mRNA. J Virol. 1985 May;54(2):625–629. doi: 10.1128/jvi.54.2.625-629.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Wilson M. C. Regulation of adenovirus-2 gene expression at the level of transcriptional termination and RNA processing. Nature. 1981 Mar 12;290(5802):113–118. doi: 10.1038/290113a0. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Simek S. L., Dubois G. C., Showalter S. D., Gilden R. V., Stephens R. M. Expression of the bovine leukemia virus X region in virus-infected cells. J Virol. 1987 May;61(5):1577–1585. doi: 10.1128/jvi.61.5.1577-1585.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Kettman R., Burny A., Haseltine W. A. Trans activation of the bovine leukemia virus long terminal repeat in BLV-infected cells. Science. 1985 Jan 18;227(4684):320–322. doi: 10.1126/science.2981432. [DOI] [PubMed] [Google Scholar]
- Sagata N., Tsuzuku-Kawamura J., Nagayoshi-Aida M., Shimizu F., Imagawa K., Ikawa Y. Identification and some biochemical properties of the major XBL gene product of bovine leukemia virus. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7879–7883. doi: 10.1073/pnas.82.23.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Ikawa Y. Two distinct polypeptides may be translated from a single spliced mRNA of the X genes of human T-cell leukemia and bovine leukemia viruses. FEBS Lett. 1985 Nov 11;192(1):37–42. doi: 10.1016/0014-5793(85)80038-7. [DOI] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Ogawa Y., Tsuzuku-Kawamura J., Ikawa Y. Bovine leukemia virus: unique structural features of its long terminal repeats and its evolutionary relationship to human T-cell leukemia virus. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4741–4745. doi: 10.1073/pnas.81.15.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Inoue J., Takeda T., Yoshida M. Direct evidence that p40x of human T-cell leukemia virus type I is a trans-acting transcriptional activator. EMBO J. 1986 Mar;5(3):561–565. doi: 10.1002/j.1460-2075.1986.tb04247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Dayton A., Terwilliger E., Haseltine W. A second post-transcriptional trans-activator gene required for HTLV-III replication. Nature. 1986 May 22;321(6068):412–417. doi: 10.1038/321412a0. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Trus M., Perkins D., Patarca R., Wong-Staal F., Gelmann E., Gallo R., Haseltine W. A. Repetitive structure in the long-terminal-repeat element of a type II human T-cell leukemia virus. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4617–4621. doi: 10.1073/pnas.81.15.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]