Chlorofusin (1, Figure 1A) was isolated from the fungal strain Microdochium caespitosum and found to disrupt the MDM2-p53 interaction by binding to the N-terminal domain of MDM2.1 As such, chlorofusin represents an exciting lead for antineoplastic intervention that acts by a rare disruption of a protein-protein interaction.2 On the basis of spectroscopic and degradation studies, the structure was proposed to be composed of a densely functionalized chromophore linked through the terminal amine of ornithine to a nine residue cyclic peptide.1 Although the studies permitted the identification of the cyclic peptide structure and connectivity, the two asparagine residues were only established to have opposite stereochemistries (l and d) and their respective assignments were not possible. Previously, we reported the synthesis of the two cyclic peptide diastereomers bearing either the l-Asn3/d-Asn4 or d-Asn3/l-Asn4 stereochemistry and were able to correlate the former with the spectroscopic properties of the natural product.3
Figure 1.
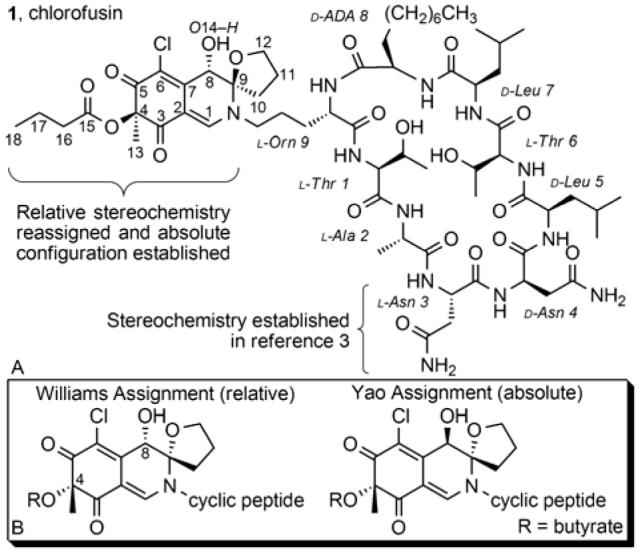
A. Chlorofusin. B. Previous chromophore assignments.
Similarly, the spectroscopic studies conducted by Williams provided an assigned relative stereochemistry for the chromophore, but did not permit an assignment of its absolute stereochemistry (Figure 1B). The relative stereochemistry was assigned using gradient 1D NOE studies albeit entailing a long range C4-Me/C8-H NOE observed only at very extended mixing times (500 ms).
Prompted by the recent disclosure of Yao that purports to have prepared chlorofusin,4 herein we report our independent reassignment of the chromophore relative stereochemistry that is still, but less obviously, consistent with the experimental NOEs reported by Williams, and an assignment of its absolute configuration that is opposite that disclosed by Yao. A total synthesis of this revised chlorofusin structure provided material displaying spectroscopic properties indistinguishable from that reported for the natural product confirming the new chromophore structural assignment and establishing the accuracy of our earlier l-Asn3/d-Asn4 cyclic peptide assignment.
Since the absolute configuration of the chromophore was unknown, the route pursued in our first generation synthesis permitted access to both enantiomeric series and to all possible diastereomers, albeit developed to access the Williams assigned diastereomer depicted in Figure 1B. The azaphilone 2 was prepared following established protocols5 and was chromatographically resolved (Daicel CHIRALCEL® OD column, 2 × 25 cm, 20% EtOH-hexanes, 7 mL/min, tR: 22.2 min (S)-2, 25.0 min (R)-2) into its enantiomers. Their absolute configurations were assigned based on the diagnostic sign of the longest wavelength (350-370 nm) Cotton effect in their CD spectra6a which, for such simple azaphilones, empirically has been shown to also correlate with the similarly diagnostic sign of their optical rotation.6b The Nδ-amine of ornithine in dipeptide 3, constituting the l-Orn-l-Thr segment of the cyclic peptide, was condensed with azaphilones (R)-2 or (S)-2 to provide 4 and 5 (Scheme 1). Subjection of each to a single-step oxidative spiroketalization (I2, AgNO3, H2O-DMSO) of the C8-C9 double bond provided all four diastereomers of the two enantiomeric series. Although space precludes a discussion of the development of this protocol, it is initiated by reversible iodonium ion formation and subsequent iodoetherification with N,O-ketal formation followed by Ag(I)-assisted displacement of the iodide by H2O-DMSO providing 6-13 directly. The major products, in which the C8 and C9 oxygen substituents are syn possessing the C8/C9-stereochemistry found in the Williams assignment, represent those that formally arise from a trans iodoetherification reaction followed by SN2 displacement of the iodide by water. Analogous to and extending unambiguous stereochemical assignments (x-ray and interconversion studies) made first with model benzylamine and n-butylamine azaphilone adducts (Supporting Information), the structures of all 8 diastereomers were fully assigned using COSY, HMQC, HMBC, and ROESY NMR. The two syn and two anti diastereomers in each enantiomeric series are readily distinguishable by diagnostic 1H NMR (C10-H, C12-H and C8-OH) and 13C NMR (C1, C2,7 C6,7 C10 and C12) chemical shifts, and the two anti diastereomers within each enantiomeric series are most readily distinguished by diagnostic C8-H, C13-H and C8-OH 1H NMR and C7 and C13 13C NMR chemical shifts. Supporting the assignments were N,O-ketal equilibration studies which relate syn/anti diastereomer pairs.
Scheme 1.
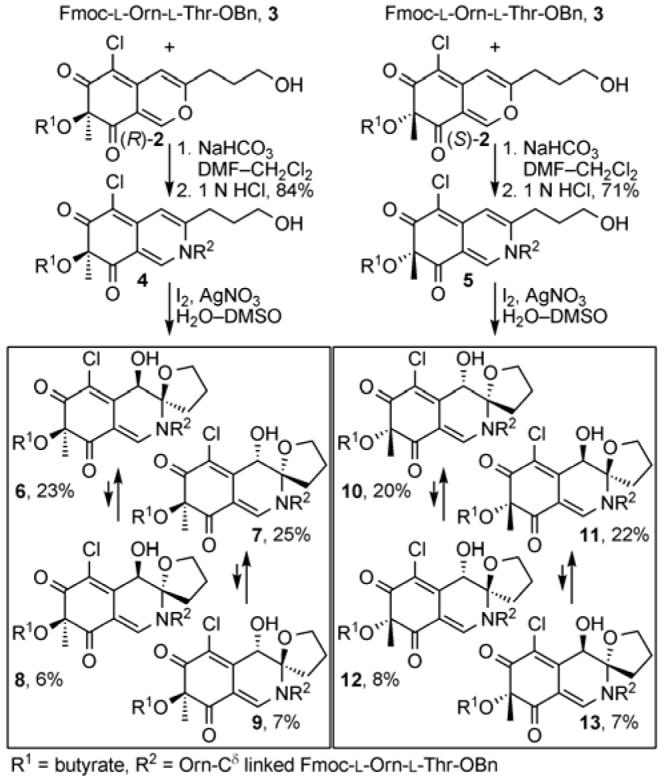
Out of this set of 8 diastereomers, the (4R,8S,9R)-diastereomer 9 provided a near perfect match with the spectroscopic properties reported for the chlorofusin chromophore, whereas the (4S,8R,9S)-diastereomer 13 (chromophore enantiomer) proved readily distinguishable by both the 1H NMR chemical shift and multiplicity of the ornithine CH2δ adjacent to the chromophore (δ 3.45, m, 2H for 9 vs δ 3.41 and 3.52, two m, 1H each for 13; chlorofusin = δ 3.42, t, 2H). This final multiplicity distinction allowed the absolute stereochemical assignment for the chromophore.
Accordingly, the (4R,8S,9R)-diastereomer 9 was incorporated into a total synthesis of chlorofusin. Fmoc deprotection (piperidine, CH2Cl2-DMF, 40 min) and coupling of the free amine 14 with the carboxylic acid of heptapeptide 15 cleanly provided 16 (EDCI, HOAt, NaHCO3, DMF, 0 to 23 °C, 24 h, 55%). Simultaneous benzyl ester deprotection and Cbz removal (H2, Pd/C, THF-DMF, 4 h) provided the corresponding amino acid 17 which was cyclized upon treatment with EDCI-HOAt (NaHCO3, DMF, 0 to 23 °C, 40 h, 60%) to provide material with spectroscopic properties indistinguishable from that reported for chlorofusin. In addition to natural chlorofusin, the (4R,8R,9R)-diastereomer proposed by Williams as well as the (4R,8S,9S) and (4R,8R,9S)-diastereomers of chlorofusin were also prepared by this route from 6-8 and the anticipated non-correlation of their spectroscopic properties with that reported for the natural product provided further support for the new structural assignment.
This (4R,8S,9R)-diastereomer (1) is clearly distinguishable from the diastereomer reported by Yao who correctly reassigns the chromophore relative stereochemistry, but which possesses the wrong the absolute stereochemistry. His (4S,8R,9S)-diastereomer (the chromophore enantiomer), even with his adjusted chemical shifts, exhibited the diagnostic ornithine CH2δ signal as two multiplets of 1H each (δ 3.42 and 3.50) analogous to 13 (vs δ 3.42, t, 2H for natural1 and synthetic chlorofusin) making it readily distinguishable from the data reported for the natural product.8
Supplementary Material
Scheme 2.
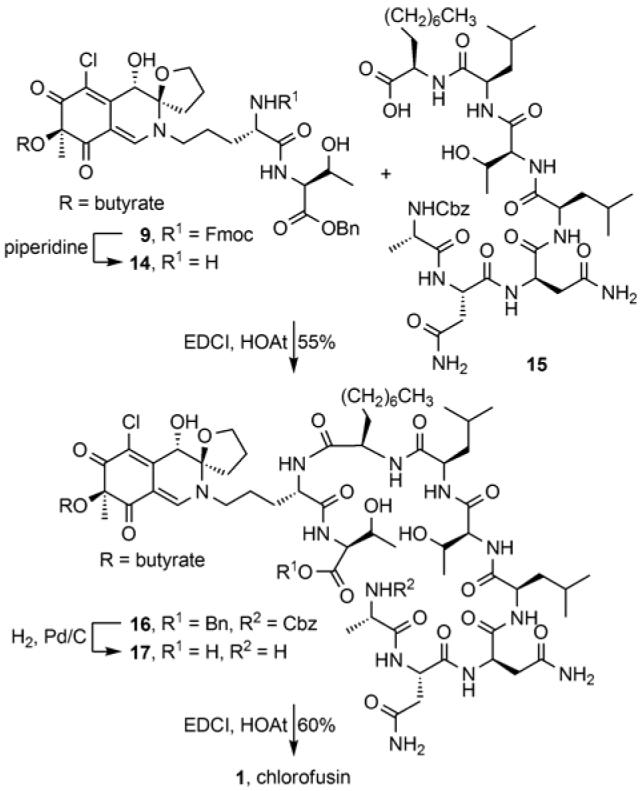
Synthesis of chlorofusin
Acknowledgment
We gratefully acknowledge the financial support of the National Institutes of Health (CA41101) and the Skaggs Institute for Chemical Biology. We especially thank Dr. S. S. Pfeiffer for initial studies on the chromophore synthesis and Dr. S. S. Pfeiffer and Dr. P. Desai for the original studies on the chlorofusin cyclic peptide.3 SYL and RCC are Skaggs Fellows.
References
- (1) (a).Duncan SJ, Grueschow S, Williams DH, McNicholas C, Purewal R, Hajek M, Gerlitz M, Martin S, Wrigley SK, Moore M. J. Am. Chem. Soc. 2001;123:554–560. doi: 10.1021/ja002940p. [DOI] [PubMed] [Google Scholar]; (b) Duncan SJ, Cooper MA, Williams DH. Chem. Commun. 2003:316–317. doi: 10.1039/b211889k. [DOI] [PubMed] [Google Scholar]
- (2).Boger DL, Desharnais J, Capps K. Angew. Chem., Intl. Ed. 2003;42:4138–4176. doi: 10.1002/anie.200300574. [DOI] [PubMed] [Google Scholar]
- (3) (a).Desai P, Pfeiffer SS, Boger DL. Org. Lett. 2003;5:5047–5050. doi: 10.1021/ol036083g.For additional preparations of this cyclic peptide, see:Malkinson JP, Zloh M, Kadom M, Errington R, Smith PJ, Searcey M. Org. Lett. 2003;5:5051–5054. doi: 10.1021/ol0360849.Tomonori M, Miyagi M, Suzuki K, Shibasaki M, Saikawa Y, Nakata M. Heterocycles. 2007;72:275–291.
- (4).Qian W-J, Wei W-G, Zhang Y-X, Yao Z-J. J. Am. Chem. Soc. 2007;129:6400–6401. doi: 10.1021/ja072225g. [DOI] [PubMed] [Google Scholar]
- (5).Chong R, King RR, Whalley WB. Chem. Commun. 1969:1512–1513.For additional studies on azaphilones related to the chlorofusin chromophore, see:Zhu J, Grigoriadis NP, Lee JP, Porco JA., Jr. J. Am. Chem. Soc. 2005;127:9342–9343. doi: 10.1021/ja052049g.Wei W-G, Qian W-J, Zhang Y-X, Yao Z-J. Tetrahedron Lett. 2006;47:4171–4174.
- (6) (a).Steyn PS, Vleggaar R. J. Chem. Soc., Perkin Trans. 1. 1976:204–206. [PubMed] [Google Scholar]; (b) Whalley WB, Ferguson G, Marsh WC, Restivo RJ. J. Chem. Soc., Perkin Trans. 1. 1976:1366–1369. [Google Scholar]
- (7).These two assignments (δ 115.2 for C2, 101.3 for C6 as reported by Williams), may be switched (δ 101.3 for C2, 115.2 for C6). This tentative reassignment is under continued investigation.
- (8).Subsequent to the web disclosure of ref. 4 as well as following the completion of our work, we re-examined a sample of authentic chlorofusin provided by Dr. Stephen Wrigley (2003, but aged and of unknown quality) that failed to provide a discernable 1H NMR spectrum at that time. With an intimate knowledge of the chromatographic and physical properties of such compounds in hand, the processing of the remaining material (<1 mg) provided a sample that exhibited a CD spectrum indistinguishable (sign and magnitude) from synthetic 1 confirming our absolute configuration assignment and an 1H NMR spectrum of sufficient quality to confirm that it represents the authentic natural product (see Supporting Information).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


