Abstract
Experimentally induced fetal androgen excess induces polycystic ovary syndrome (PCOS)-like traits in adult female rhesus monkeys. Developmental changes leading to this endocrinopathy are not known. We therefore studied 15 time-mated, gravid female rhesus monkeys with known female fetuses. Nine dams received daily subcutaneous injections of 15 mg testosterone propionate (TP) and six received injections of oil vehicle (controls) from 40 through 80 days of gestation (term 165 [range: ±10] days), and all fetuses were delivered by Cesarean-section using established methods at term. Ultrasound-guided fetal blood sample collection and peripheral venous sample collection of dams and subsequent infants enabled determination of circulating levels of steroid hormones, LH and FSH.
TP injections elevated serum testosterone and androstenedione levels in the dams and prenatally androgenized (PA) fetuses. After cessation of TP injections, testosterone levels mostly normalized, while serum androstenedione levels in PA infants were elevated. TP injections did not increase estrogen levels in the dams, PA fetuses and infants, yet conjugated estrogen levels were elevated in the TP-injected dams. Serum levels of LH and FSH were elevated in late gestation PA fetuses, and LH levels were elevated in PA infants. These studies suggest that experimentally-induced fetal androgen excess increases gonadotropin secretion in PA female fetuses and infants, and elevates endogenous androgen levels in PA infants. Thus, in this nonhuman primate model, differential programming of the fetal hypothalamo-pituitary unit with concomitant hyperandrogenism provides evidence to suggest developmental origins of LH and androgen excess in adulthood.
Keywords: fetal programming, androgen excess, LH hypersecretion, LH negative feedback
Introduction
Experimental induction of androgen excess in female rhesus monkeys during early or late gestation has been shown to re-program reproductive and metabolic physiology resulting in polycystic ovary syndrome (PCOS) in adulthood (1). Such adult, prenatally androgenized (PA) female rhesus monkeys have been documented to exhibit intermittent or absent menstrual cycles (1, 2), ovarian hyperandrogenism (1, 3) and polycystic ovarian morphology (4), traits that confer the diagnosis of PCOS by either “NIH” (5) or “Rotterdam” (6) criteria, or currently proposed amendments (7; but see 8). Comparable to many PCOS women (7, 9, 10, 11), profound metabolic dysfunction has been shown to accompany the reproductive disorders of PA female rhesus monkeys (1).
Also, similar to many PCOS women, PA female monkeys exposed to androgen excess during early gestation exhibit LH excess and increased LH responsiveness to exogenous GnRH (monkeys: 1, 12; PCOS women: 13, 14), suggestive of enhanced hypothalamic GnRH release and/or increased pituitary sensitivity to GnRH. Diminished sex steroid negative feedback on LH release is found in both early and late gestation exposed PA female monkeys (1, 15, 16) and in PCOS women (17, 18), and may explain the increased pulsatile LH secretion observed in both species (PCOS women: 19; PA monkeys: 20). Conversely, circulating FSH levels have been reported to be low to normal in both PA female monkeys (12, 21) and PCOS women (14, 22), thereby elevating the serum LH:FSH ratio. It is has been proposed that LH hypersecretion in PCOS may originate during adolescence, initially caused by hyperandrogenism and then potentially contributing to peripubertal hyperandrogenism (as in some PCOS adolescents: 23, 24). Such neuroendocrine dysfunction, however, may originate in utero, from androgen/estrogen-induced changes in the fetal hypothalamo-pituitary axis (4, 25-28) prior to any subsequent peripubertal dysfunction.
Based on prior studies that have shown the importance of the rhesus monkey model of fetal androgen excess and its application to understanding the mechanism(s) associated with PCOS (1-4, 29), this study was designed to examine whether early gestation androgen excess during a defined window of development hormonally differentiates LH hypersecretion in PA female rhesus monkeys during fetal life and in early infancy and, if so, whether it is accompanied by endogenous hyperandrogenism during this period of development. Early gestation exposed PA monkeys were chosen for this study because adult female monkeys exposed to such fetal manipulation more clearly express LH dysfunction (increased basal levels, increased LH response to GnRH; decreased sensitivity to sex steroid negative feedback; 1, 15, 16) than female adults exposed during late gestation (decreased sensitivity to sex steroid negative feedback; 16). The overall intent was to determine whether androgen-induced reprogramming of the nonhuman primate fetal hypothalamo-pituitary-gonadal axis occurs in the early ontogeny of reproductive endocrine traits found in women with PCOS. Such excessive LH stimulation of the immature ovary could potentially contribute to the peri-pubertal onset of reproductive dysfunction found in women with hyperandrogenic disorders, including those with PCOS (28).
Materials and Methods
Animals
All animal procedures conformed to the requirements of the Animal Welfare Act and protocols were approved prior to implementation by the Institutional Animal Care and Use Committee (IACUC) at the University of California, Davis. Normally cycling, adult female rhesus monkeys (Macaca mulatta), housed at the California National Primate Research Center (CNPRC), with a history of prior pregnancy were time-mated and identified as pregnant, using established methods with limited time access of females to study males, thus ensuring an accuracy of ±2 gestation days (30). Pregnancy in rhesus monkeys is typically divided into trimesters by 55 day increments with 0 - 55 days gestation representing the first trimester, 56 -110 days gestation representing the second trimester, and 111 - 165 days gestation representing the third trimester (term 165 [range: ±10] days) (31). Once pregnancies are identified, they are routinely monitored sonographically to ensure normal fetal growth and development (30), but such assessment is not used to estimate gestational age.
Experimental design
Once time-mated gravid animals were identified for the study, dams were initially assessed sonographically to confirm normal growth and development, then monitored sonographically under ketamine (10 mg/kg, intramuscularly) every 7-10 days during gestation using standard protocols (30). Established biometrics were used to assess growth and development. At ∼30 days gestation, and after assessing viability of the conceptus, a 1 ml blood sample was collected from a peripheral vein of gravid animals to confirm that the conceptus was female using established PCR-based assays to demonstrate the absence of Y-chromosomal DNA in maternal blood (32). At 40 days gestation, 15 dams with confirmed female embryos were assigned on a rotational basis to receive consecutive daily subcutaneous injections of either 15 mg testosterone propionate in 100-200 μl of sesame oil (TP-injected dams; n=9) or sesame oil (oil-injected dams; n=6) with the last day of treatment on 80 days gestation (early second trimester). Treatment assignments were also balanced so that the two groups of dams were similar with regard to pre-conception age (TP-injected: 11.2±1.5 years of age; oil-injected controls: 8.4±2.0 years; mean±SEM) and body weight (TP-injected: 7.0±0.4 kg; oil-injected: 6.8±0.5 kg).
This early gestation exposure of female monkeys to TP-mediated androgen excess has been shown to result in a hyperandrogenic prenatal environment, equivalent to that found in early gestation males (33), and occurs during a gestational time period when multiple fetal organ systems, including those involved in reproduction and metabolism, are undergoing initial development (1). Elevating fetal female testosterone levels into the fetal male range provides a physiologically relevant test of our hypothesis for fetal androgen excess programming of PCOS, since in humans, congenital virilization of female fetuses commonly programs neuroendocrine and metabolic features of PCOS, leading to an increased incidence of PCOS (25-50% in classical congenital adrenal hyperplasia; 28) compared to that found in non-virilized women (6-7% PCOS; 34, 35, 36). Moreover, 40% of human fetal females have unbound (“free”) testosterone values during the early to mid-second trimester that are equivalent to those in normal human fetal males (37) indicating that our use of testosterone levels in this study equivalent to those of fetal males provided a relevant physiological challenge. The TP dose used in this study was also within the range of 5-15mg TP/day previously used to produce PA female rhesus monkeys with PCOS-like traits in adulthood (1, 2); this TP dose successfully overcomes the primate placenta's robust ability to aromatize and inactivate androgens that is considerably greater than that of non-primate species such as rats (38) and sheep (39). Sonographic studies were performed under ketamine, as noted above during gestation, then newborns were delivered by Cesarean-section at term (160 (range: ±2 days of gestation) using standard protocols (30). Cesarean-section was employed to provide a relatively consistent gestation length and to avoid birthing complications. All PA female fetuses displayed virilized external genitalia prenatally, when examined sonographically, which was confirmed grossly at the time of delivery, thus providing an effective biomarker of the effectiveness of early gestation androgen excess. Newborns were placed in incubators after delivery and were reared in the nursery using established protocols (30). At two months of age, infants were euthanized by an intravenous overdose of pentobarbital (60 mg/kg) for tissue procurement.
Blood sample collection
Fetal blood samples (1-3 ml) were collected by ultrasound-guided cardiocentesis using established protocols (40) at defined time points, as described below. Peripheral blood samples were collected from the dams (3-10ml) and infants (∼3ml) also at defined time points (see below). All blood samples for all age groups were collected between 0700-0900h, as shown in Figure 1. When multiple maternal or fetal blood samples were collected (80, 120 and 140 days of gestation), dams were sedated with an intramuscular injection of Telazol (5-8 mg/kg; Wyeth, Overland Park, KS). Infants were hand-held and not sedated for blood sampling. Due to maximal blood sample limits based upon body size, not all fetal and infant samples were available for all hormone determinations. These well developed blood sampling techniques provided unique insight into the simultaneous progression of fetal and maternal reproductive hormone levels within the same individual nonhuman primates during gestation without surgical intervention.
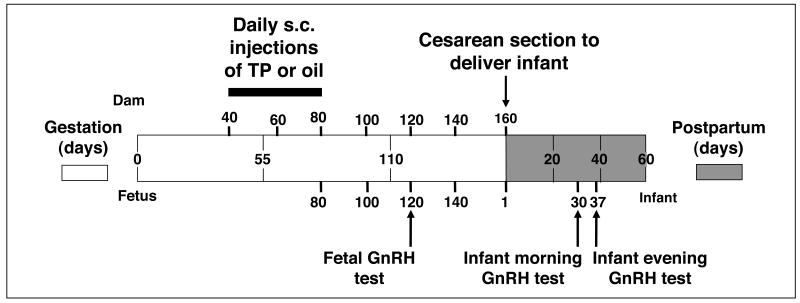
Figure 1.
Diagrammatic representation of the experimental design, illustrating days of maternal subcutaneous injections (15 mg TP: n=9; oil: n=6) during 40-80 days of gestation, days of blood sample collection in dams, fetuses and infants, timing of caesarean-section delivery, and fetal and infant GnRH tests.
Fetal GnRH test
To assess pituitary gonadotropin responsiveness to exogenous GnRH in fetal PA female monkeys, an intravenous (IV) bolus injection of 5 μg GnRH (∼15-20 μg/kg) was administered in 0.1ml sterile saline at ∼0700h at 120 days of gestation via the fetal circulation (Figure 1). Fetal blood samples were collected by ultrasound-guided cardiocentesis immediately before (0 min) and at 10, 20, 30 and 40 min following the GnRH injection. The ∼15-20 μg/kg dose of GnRH was chosen in an attempt to consistently stimulate LH release, as reported in earlier studies of fetal and infant rhesus monkeys (41: 80μg (∼240-320 μg/kg); 42: 10-50μg (∼30-200 μg/kg), 43: 500μg (∼625 μg/kg)). Inconsistent LH release occurs when lower doses (44: 50 ng/kg) of exogenous GnRH are employed (LH release occurred in only 3 out of 7 female infant controls and none of the 6 female infant PA monkeys; 44).
Infant GnRH tests
A similar assessment of pituitary gonadotropin responsiveness to exogenous GnRH was performed in infant PA female monkeys. GnRH was injected via a peripheral vessel, then blood samples were collected in hand-held, unanesthetized animals from a femoral vein. For infants, GnRH tests were administered during both morning (∼0700h) and evening (∼2100h) hours on different days to detect any diurnal changes in pituitary gonadotropin responsiveness (Figure 1). The morning GnRH test was administered on postnatal day 30, when an IV GnRH bolus (20 μg, ∼25 μg/kg) was given in 0.4ml sterile saline. Blood samples were obtained post-injection before (0 min) and at 10, 20, 30 and 40 min following the GnRH injection. The evening GnRH test was administered on postnatal day 37, using an identical injection and blood sample collection protocol.
Steroid hormone assay procedures
All hormones were assayed in the Assay Services Laboratories of the Wisconsin National Primate Research Center (WNPRC; 12, 45, 46). All steroid hormones used as reference preparations in standard curves were obtained from Steraloids (Newport, RI). Serum steroid hormone concentrations in all samples, except those for conjugated estrogen determinations, were analyzed using an Agilent 1100 liquid chromatography/mass spectrometer (LCMS) equipped with an electrospray ionization source and Chemstation software version A 10.02. The LCMS methods were validated using Federal Drug Administration protocols (May, 2001), and adapted from those previously described for humans (47).
Using positive ion identification for testosterone (m/z 289.2) and androstenedione (m/z 286.8), the LCMS standard curve for testosterone was 0-80 ng/ml and that for androstenedione ranged from 0 to 2 ng/ml. The calibration plots over 3 days showed slopes of 0.91 and 1.67, intercepts of 0.0008 and 0.003, and regression coefficients of 0.999 and 0.991, respectively. Relevant characteristics for LCMS determination of both testosterone and androstenedione values are presented in Table 1. For sample preparation, 100 μl aliquots from individual serum samples or quality control rhesus monkey serum pools were placed into 13×100 mm borosilicate glass culture tubes, and to each of these 500 μl of 18.2 Mohm-cm filtered (distilled and UV-purified) water was added as well as 20 μl of both d5-testosterone (m/z 294.2; CDN Isotopes, Quebec, Canada) and d7-androstenedione solutions (m/z 293.8), at 100 pg/μl of each (in ethanol, Sigma, St. Louis, MO). The deuterated androgens were added as internal standards to monitor recovery. Each standard, pool, or individual sample was then extracted with 2 ml diethyl ether (Fisher, Hampton, NH), the separated solvent fraction evaporated, and re-constituted in 20 μl of water:acetonitrile (EMD Chemicals, Norwood, OH; 1:1, v/v), from which 4 μl (20%) was injected onto a Phenomenex Synergi Max-RP 150×1.0mm column with 4 μm particles at 80A. All solvents used were HPLC grade. Mobile phase A comprised water:acetonitrile (95/5%) with 0.01% formic acid, while mobile phase B comprised acetonitrile:water (95/5%) with 0.01% formic acid. Prior to injecting each sample, the LCMS column was equilibrated for 10 min at 150 μl/min with 60% mobile phase B and 40% mobile phase A, and the column was maintained at 35°C. Serial dilutions of rhesus monkey serum (100-400 μl) yielded testosterone and androstenedione values parallel to LCMS standards and regression analyses favorably compared LCMS determinations to those made by our previously validated methods (enzymeimmunoassay (EIA) for testosterone and radioimmunoassay (RIA) for androstenedione; 45; Table 1). The only major difference in LCMS compared to EIA or RIA determined serum androgen values was reflected in the consistent, but reliable, lower serum values generated by LCMS (Table 1).
Table 1.
Characteristics of the LCMS methods for determination of serum androgen and estrogen levels in control and testosterone propionate treated female rhesus monkeys and their comparison to previously validated ‘in house’ RIA and EIAs.
| LCMS hormone parameter | LLOQ | Accuracy (%) | Recovery (%) | Within day CV (%) | Between day CV (%) | ‘In house’ RIA/EIAa | Regression of LCMS vs RIA/EIA |
|---|---|---|---|---|---|---|---|
| Testosterone | 0.03 ng/ml | 103.7 | 72.4 | 4.4 | 10.8 | EIA | y=1.71x-0.2b |
| r2=0.98, p<0.001 | |||||||
| Androstenedione | 0.03 ng/ml | 110.0 | 75,3 | 6.9 | 14.0 | RIA | y=0.83x+0.3c |
| r2=0.70, p<0.001 | |||||||
| Estradiol | 12.0 pg/ml | 102.0 | 84.8 | 11.0 | 14.0 | RIA | y=1.21x+60.4 d |
| r2=0.92, p<0.001 | |||||||
| Estrone | 12.0 pg/ml | 95.7 | 78.8 | 9.8 | 15.2 | RIA | y=0.61x+0.2 e |
| r2=0.96, p<0.001 |
LLOQ: lower limit of quantitation (Food and Drug Administration protocols, May 2001)
p-values indicating whether the slopes of the regression lines for LCMS vs RIA/EIA values differ from that of 1, and whether the y-axis intercept of the lines differ from 0:
testosterone: slope 0.005, intercept NS
androstenedione: slope NS, intercept 0.04
estradiol: slope 0.01, intercept 0.02
estrone: slope 0.005, intercept NS
For estrogen determinations by LCMS, we derivatized estradiol and estrone with dansyl chloride to improve ionization efficiency and LCMS sensitivity, as previously described (48). Standards, pools and individual samples were prepared similarly to those for androgens, except that 20 μl of ethanol solutions of both d5-estradiol dansyl (m/z 511.4; 2 pg/μl) and d4-estrone dansyl (m/z 504.4; 4 pg/μl), were added as internal standards to monitor recovery. We then extracted each standard, pool, or individual samples as for androgens, but re-constituted in 100 μl ethanol, which was vortexed briefly before adding 500 μl of 18.2Mohm-cm filtered water with 2 ml dichloromethane, and vortexing again for 1 min. The organic layer was removed, evaporated to dryness and re-constituted with 20 μl of 100 mM sodium bicarbonate (adjusted to pH 10.5 with 1M sodium hydroxide). The mixture was vortexed for 1 min before the addition of 20 μl dansyl chloride (1 mg/ml in acetone) and incubation at 33°C for 2.5 min. Twenty microlitres (50%) of the derivatized estrogen solution was then injected on to the LCMS column.
Using positive ion identification for estradiol dansyl (m/z 506.4) and estrone dansyl (m/z 504.4), the standard curves for both estrogens were 0-40 pg/ml. The calibration plots over 3 days showed slopes of 0.63 and 1.16, intercepts of 0.009 and 0.008, and regression coefficients of 0.926 and 0.927, respectively. Relevant characteristics for LCMS determination of both estradiol and estrone are presented in Table 1. Similar equilibration procedures and mobile phase dynamics were employed to those used for LCMS androgen determinations. Serial dilutions of rhesus monkey serum (50-400 μl) yielded estradiol and estrone values parallel to LCMS standards and regression analyses favorably compared LCMS determinations to those made using our previously validated methods (RIA; 12, 45; Table 1). While LCMS determined values were highly correlated with those from our established assays, they were consistently, but reliably, either lower than those produced by our previously established methods (estradiol RIA) or higher than those produced by previous methods (estrone RIA; Table 1).
Estrogen conjugate determinations
As primates exhibit extensive conjugation of estrogens in the liver, placenta and other reproductive organs during the process of bio-inactivation and excretion of these steroids (49, 50), we employed sequential enzyme hydrolysis and acid solvolysis to maternal serum samples to quantify total conjugate concentrations for both estradiol and estrone (51) that better reflect total estrogen in the circulation. Serum estriol concentrations were not determined as this estrogen is present only at very low levels in rhesus monkey pregnancies (52). There were insufficient remaining sample volumes from fetuses and infants to assess estrogen conjugate levels in their serum. All fractions of de-conjugated estrogens were re-suspended in celite chromatography solvents, and the estrogens were separated through celite column chromatography and assayed by RIA, as previously described (45). Intra- assay CVs for QC values for the single RIAs performed were, respectively, estradiol, 5.8% and estrone, 7.3%.
Gonadotropin assay procedures
Serum bioLH and immunoactive FSH were determined by mouse Leydig cell bioassay and RIA, using the reference preparations of recombinant (r-) cyno.LH (AFP-6936A) and r-cyno.FSH (AFP-6940A), respectively, obtained from Dr. Parlow at NHPP (12). Intra- and inter-assay CVs for QC values were: bioLH, 10.8% and 17.7%; FSH, 4.6% and 10.9%, respectively. While the mouse Leydig cell bioassay cannot distinguish between bioactive LH and bioactive chorionic gonadotropin (CG), rhesus placentae secrete little or no CG by 40 days of gestation (52-54). Thus, there is little likelihood of placental CG contributing to fetal bioLH levels at 80-120 days of gestation (Figures 6 and 7).
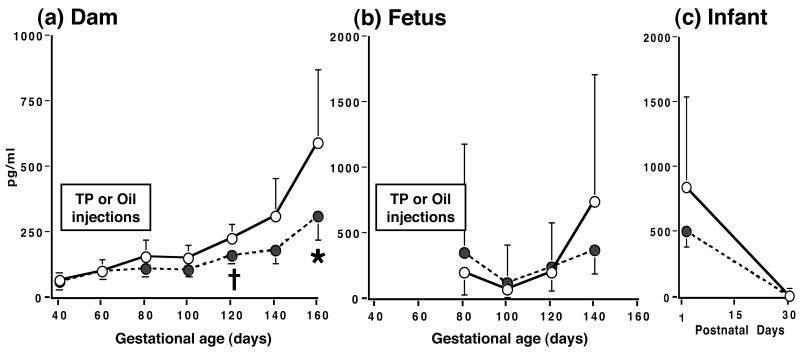
Figure 6.
Serum bioLH levels (backtransformed means and individual values) in prenatally androgenized (filled bars and symbols) and control (open bars and symbols) female (a) fetuses on the last day of TP or oil subcutaneous injections, and 20 and 40 days later, and (b) newborn and 30-day old infants. * p<0.001, # p<0.025 vs controls on the same day of gestation, † p<0.015 vs controls on postnatal days 1 and 30 combined.
Figure 7.
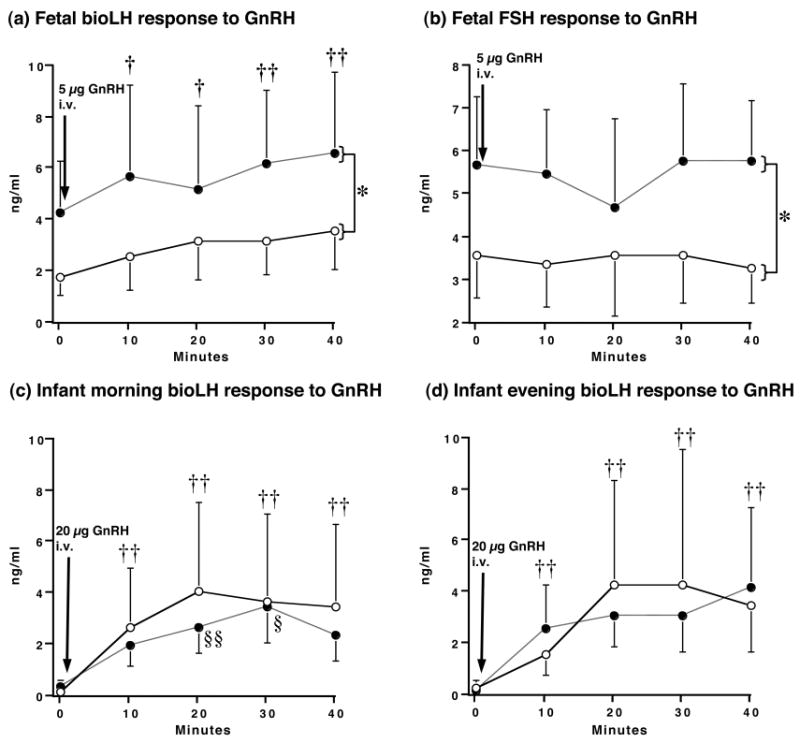
Serum gonadotropin levels following an intravenous injection of exogenous GnRH in prenatally androgenized (PA; filled circles) and control (open circles) female monkeys: fetal responses to 5μg GnRH at 120 days of gestation (a) bioLH and (b) FSH, and infant responses to 20μg GnRH at (c) 30 days postpartum (bioLH; ∼0700h), and (d) 37 days postpartum (bioLH; ∼2100h). * p<0.05 vs controls, all times combined, † p<0.05, vs. 0 min, all females combined, †† p<0.002, vs. 0 min, all females combined, §§ p<0.015, vs 10 min, PA females only, § p<0.03, vs 20 min, PA females only.
Statistical analysis
All hormonal data were tested for normality using a Lilliefors test (two-sided) and were log transformed to achieve homogeneity of variance and to increase linearity (55). All hormonal variables were analyzed using separate two-way ANOVAs (with repeated measures designs as appropriate) for dams, fetuses and infants (the latter two sets of ANOVAs reflecting distinctly different developmental stages and hormone parameter values within the same individuals), with fetal androgen exposure and time as independent variables. When significant (P<0.05) statistical interactions were identified by ANOVA, posthoc univariate analyses were performed on the variables (Systat, Version 5.2, 1992; Macintosh, Evanston, IL). The Extreme Studentized Deviate (ESD) outlier test (56; one-sided) identified one control female fetus as an outlier (p<0.01) for bioLH values when compared to all other study fetuses, combined. The data from this control female were thus excluded from analyses for bioLH and FSH. All log transformed parameters are expressed as the antilog of the transformed mean [95% confidence limit], except for fetal and infant bioLH data when individual values are shown instead of the 95% confidence limit (Figure 6). All other data are shown as mean±SEM.
Results
Newborn weight and appearance of PA infants
Immediately following Cesarean-section at term, the newborn weights of PA female infants (0.44±0.16 kg) did not differ from those of female controls (0.46±0.20 kg). The external genitalia of PA female infants, however, were virilized, exhibiting an empty scrotum and a preputial opening housing an immature phallus, a typical phenotypic marker of early gestational androgen excess indicating sufficient testosterone treatment provided at an appropriate gestational age.
Experimentally-induced changes in circulating androgen concentrations
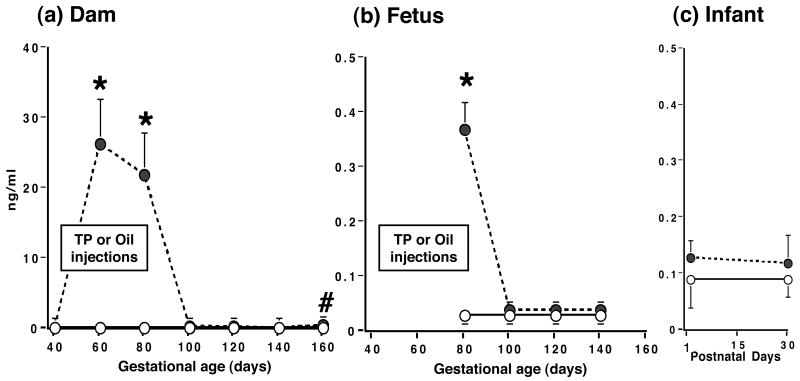
TP injections induced a ∼110-fold increase (p<0.001) in maternal serum testosterone levels, with a considerably smaller increase (∼11-fold; p<0.001) in fetal levels (Figure 2). The increases in maternal and fetal testosterone levels were restricted to the duration of daily TP injections. Circulating testosterone levels were otherwise similar in both treatment groups of dams, fetuses and infants, except for a ∼112% increase (p<0.006) in previously TP-injected dams at term (TP-injected: 0.53 [0.39, 0.70] ng/ml vs. control: 0.25 [0.17, 0.35] ng/ml; Figure 2). There were no other changes in circulating testosterone levels in dams and fetuses across gestation or during early infancy.
Figure 2.
Serum testosterone levels (backtransformed means [95% confidence limits]) in (a) gravid female rhesus monkeys receiving 15 mg TP (filled circles, dashed line) or oil (open circles, solid line) injections during 40-80 days of gestation, and their fetuses (b) and infants (c). * p <0.001, # p<0.006 vs controls on the same day of gestation.
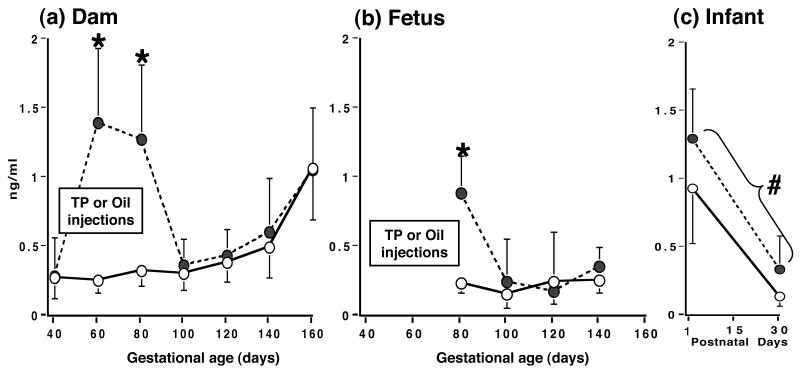
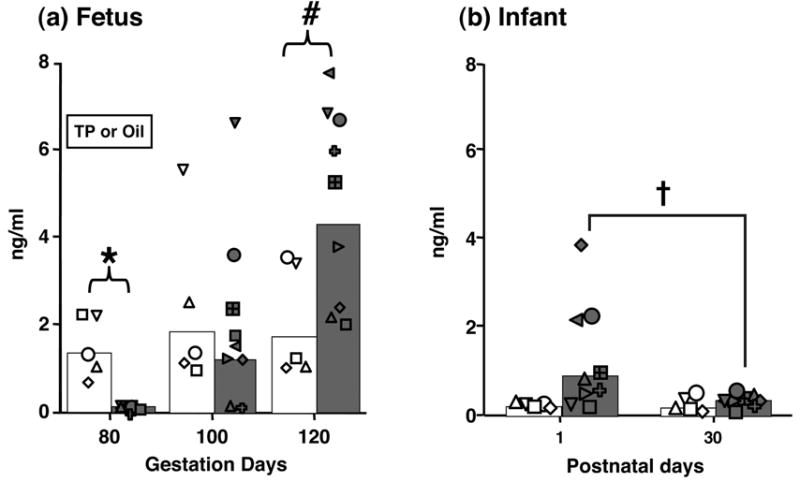
Serum androstenedione changes following TP injection mostly paralleled those of serum testosterone, except that the magnitude of the serum androstenedione increase was less than that of serum testosterone, being comparable in both dams (∼4-5-fold; p<0.001 vs controls) and fetuses (∼3-fold, p<0.001 vs controls; Figure 3). In contrast to serum testosterone concentrations, serum androstenedione levels in young PA infants were elevated (p<0.019) about 50-100% above those values normally found in infant controls between postnatal days 1 and 30. Serum androstenedione levels also exhibited temporal changes across gestation and early infancy. In both female groups, maternal serum androstenedione levels rose progressively (p<0.001) as gestation progressed, while serum androstenedione levels declined (p<0.03) in early infancy (Figure 3).
Figure 3.
Serum androstenedione levels (backtransformed means [95% confidence limits]) in (a) gravid female rhesus monkeys receiving 15 mg TP (filled circles, dashed line) or oil (open circles, solid line) injections during 40-80 days of gestation, and their fetuses (b) and infants (c). * p<0.001 vs controls on the same day of gestation, # p<0.03 vs controls on postnatal days 1 and 30 combined.
Experimentally-induced changes in circulating estrogen and progesterone concentrations
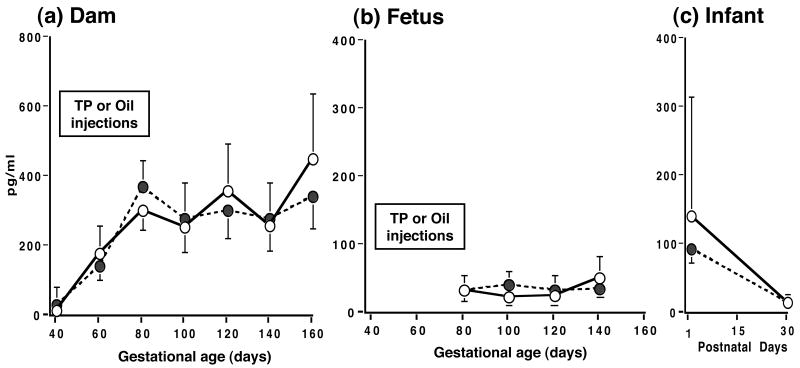
Unlike circulating androgen levels, TP injections did not elevate serum estradiol or estrone levels in dams, fetuses or infants (Figures 4 and 5). Nevertheless, maternal circulating levels of total conjugated estrogen were elevated (p<0.03) by TP injections on day 80 of gestation (TP-injected: 3.1[2.1, 4.7] ng/ml vs controls: 1.3[0.8, 2.1] ng/ml). In TP-injected dams, serum estrone levels were lower (p<0.05) during late gestation (Figure 5). There were no between-group differences, however, in temporal changes in fetuses across gestation and in early infancy for serum estradiol and estrone levels. Maternal estrone levels rose steadily (p<0.001) during gestation (Figure 5), while maternal estradiol levels reached a plateau by mid-gestation (Figure 4). During infancy, circulating levels of both estrogens declined precipitously (p<0.01) between postnatal days 1 and 30.
Figure 4.
Serum estradiol levels (backtransformed means [95% confidence limits]) in (a) gravid female rhesus monkeys receiving 15 mg TP (filled circles, dashed line) or oil (open circles, solid line) injections during 40-80 days of gestation, and their fetuses (b) and infants (c).
Figure 5.
Serum estrone levels (backtransformed means [95% confidence limits]) in (a) gravid female rhesus monkeys receiving 15 mg TP (filled circles, dashed line) or oil (open circles, solid line) injections during 40-80 days of gestation, and their fetuses (b) and infants (c). * p<0.03, † p<0.05 vs controls on the same day of gestation.
Maternal serum progesterone levels did not differ between TP-injected and control females across gestation (40-160 days gestational age), averaging 3.8 [3.4, 4.2] and 3.3 [2.3, 4.3] ng/ml, respectively.
Experimentally-induced changes in circulating bioLH and FSH levels in fetuses and infants
As expected for rhesus monkey dams, the majority of bioLH serum values in both female groups were equal or less than the bioassay sensitivity limit of 0.06 ng/ml from 40-160 days of gestation (data not shown).
In 80-day gestation (second trimester) PA fetuses, maternal TP injections reduced (p<0.001) fetal circulating bioLH levels to ∼10% of those found in oil-injected controls (Figure 6). Following cessation of TP injections, bioLH levels in PA female fetuses were comparable to controls within 20 days (day 100 of gestation), but exceeded control values within 40 days (day 120 of gestation, Figure 6). In contrast, serum bioLH levels in controls remained constant between the same gestational ages. During the fetal GnRH test administered on day 120 of gestation, serum bioLH levels were elevated in PA compared to control females before and after GnRH injection (Figure 7), resulting in increased (p<0.048) area-under-the-curve bioLH values in PA females (PA: 2.4 [1.6, 3.4] ng/ml*min vs. control: 1.2 [0.7, 2.0] ng/ml*min). The absolute increase in the GnRH-induced increase in serum bioLH levels, however, paralleled that in controls. Additionally, serum FSH levels were also elevated in PA fetuses before and after GnRH injection, while fetal FSH levels were unresponsive to the exogenous GnRH stimulus (Figure 7).
In newborn PA infants, serum bioLH values remained elevated for the first 30 postnatal days (p<0.019; Figure 6). Thereafter, there was a decline (p<0.014) in bioLH levels in PA infants between postnatal day 1 (0.9 [0.7, 1.2] ng/ml) and 30 (0.4 [0.2, 0.5] ng/ml), while values in control females remained unchanged during this time period (day 1, 0.2 [0.1, 0.5] vs. day 30, 0.2 [0.1, 0.4] ng/ml). Morning and evening infant GnRH tests elicited no female group differences in bioLH responsiveness to GnRH (Figure 7). During the morning GnRH test, serum bioLH levels in PA infants, however, progessively increased until 30 min following GnRH injection, while bioLH levels in controls failed to do so after 10 min (type of female × time interaction, p<0.008), but area-under-the-curve bioLH values were similar in PA (1.0 [0.6, 1.6] ng/ml*min) and control (1.3 [0.7, 2.3] ng/ml*min) infants. Insufficient sample volumes remained to obtain meaningful infant serum FSH values.
Discussion
Elevated circulating LH levels and rapid, pulsatile LH release are hypothalamo-pituitary hallmarks of adult PA female rhesus monkeys, women exposed to androgen excess during early gestation (1, 19, 25) and PCOS women (19, 23). It has been proposed that pre- or perinatal androgen excess (2, 4, 24-27), peripubertal hyperandrogenism (23, 24), or excessive and prolonged adult ovarian production of estrogen from androstenedione (57), may contribute to the persistently rapid GnRH pulse frequency in women with PCOS via impaired hypothalamic feedback inhibition. Such LH excess in PCOS women contributes to ovarian hyperandrogenism in adulthood (23, 58, but see 59). The present evidence of fetal and infant LH excess in a nonhuman primate model of PCOS is consistent with early life programming of neuroendocrine dysfunction that will manifest as postpubertal LH hypersecretion (1, 2) in a manner resembling PCOS in women.
Hypothalamic-pituitary changes seen in fetal PA female rhesus monkeys resemble those normally observed in fetal males (60), suggesting some degree of masculinized hypothalamic-pituitary function in PA females. Mid-gestational circulating testosterone levels in normal fetal male monkeys mostly exceed those of normal fetal females (61), while LH levels in such fetal males are usually lower than those of respective fetal females (62). This sexually dimorphic LH differential probably represents testicular testosterone-mediated negative feedback at the hypothalamic level in fetal males (63). Our present findings in PA females emulate this LH differential: specifically, fetal PA females, exposed to testosterone levels typical of those seen in the lower range of normal fetal males, have low circulating bioLH levels versus normal female controls at 80 days of gestation (early second trimester). In fetal males and PA females alike, such LH suppression may represent testosterone-mediated changes in hypothalamic regulation of LH secretion (63), altered either directly via androgen receptors (64, 65), or indirectly via estrogen receptors, through target tissue aromatization of exogenous testosterone (66, 67).
Such an explanation is further supported by increases in circulating LH levels observed in fetal male, but not fetal female monkeys, following gonadectomy at 100 days of gestation (60), which are prevented by testosterone replacement at the time of gonadectomy (68). Corresponding with fetal androgen programming of the hypothalamic-pituitary unit, reduced LH responses of PA female fetuses at 80 days of gestation were followed by a rise of serum bioLH levels to those of control females by 100 days of gestation (when circulating testosterone levels in PA females decreased to control female values), after which they exceeded control values by 120 days of gestation. This serum LH response of PA females to diminished testosterone exposure closely resembles that exhibited by fetal male rhesus monkeys (at the same gestational age) following gonadectomy at 100 days of gestation (61), although it remains to be determined whether circulating testosterone levels are equivalent between ovary-intact female and gonadectomized male monkeys at these gestational ages.
Persistence of elevated serum LH levels in newborn, ovary-intact PA female infants confirms an earlier finding of LH excess in infant PA female monkeys exposed to a much lower dose of testosterone during early gestation (weekly IM injections of 20 mg testosterone enanthate given to gravid monkeys; 44). Male infant rhesus monkeys also exhibit elevated circulating LH levels compared to normal infant females (69, 70). Since the lower dose of androgen in the previous study (44) also masculinized components of infant female monkey behavior (71) without virilizing external genitalia (44), elevations of fetal female testosterone insufficient to induce genital masculinization are nevertheless sufficient to re-program sexually-dimorphic aspects of hypothalamo-pituitary-gonadal function and behavior. Short-term loss of bioLH hypersecretion as PA female infants age in both the present and previous (44) studies probably represents the onset of pre-pubertal quiescence of hypothalamic GnRH release found in immature, gonadally intact or gonadectomized male and female rhesus monkeys (70, 72, 73). Elevated circulating androstenedione levels in PA female infants probably do not contribute a sufficient additional negative feedback component to LH suppression (70, 72, 74).
In concert with LH hypersecretion of female fetal PA monkeys, circulating FSH levels were also elevated in the same monkeys at 120 days of gestation. Concomitant LH and FSH excess likely represents enhanced hypothalamic GnRH stimulation of pituitary function, together with an apparent absence of ovarian hormone negative feedback regulation. Absence of ovarian feedback may represent an immature stage of ovarian development at this gestational age, since such rhesus monkey ovaries have not yet developed gonadotropin responsiveness or the capacity to secrete estradiol (75). It is interesting to speculate that subsequent LH excess found in adult PA female monkeys, in the absence of excessive levels of FSH (12, 45), may represent differential negative feedback on FSH from ovarian hormones, including inhibins (76).
In addition to gonadotropin excess, hyperandrogenism was also apparent in infant female PA monkeys. It is possible that elevated androstenedione levels in these young infants represent ovarian responses to LH excess, since congenital LH excess can induce pre-pubertal ovarian hyperandrogenism in humans (71), that may be conditional on sufficient FSH stimulation of the ovary (78, 79). Alternatively, adult PA female monkeys exhibit adrenal androgen excess (46), and adrenarche occurs around the time of birth in rhesus monkeys coincident with fetal zone regression (80) well before the conventional prepubertal timing found in Great Apes and humans (80, 81). Therefore, the elevated androstenedione levels in PA female infants may indicate an adrenal and/or ovarian endocrine antecedent of adult hyperandrogenism, particularly since the adrenal gland normally contributes to elevated circulating androgen levels in infant male rhesus monkeys (74).
Apart from transient increases in circulating androgen and conjugated estrogen levels during TP injections, there was little impact on maternal reproductive hormonal profiles during the affected pregnancies. Circulating levels of progesterone and estradiol were normal in TP-injected dams and comparable to values previously reported across gestation in rhesus monkeys (52, 82). Not surprisingly, bioLH or CG levels were low to undetectable in the circulation of all pregnant females in the current study, since CGβ mRNA expression is undetectable in rhesus placentae and cultured rhesus syncytiotrophoblast cells by 28 days of gestation (83), and rhesus placentae secrete little or no CG following this stage of gestation (52-54). The increased levels of conjugated estrogens in the maternal circulation during the time interval of TP injections, however, may indicate androgen excess-induced alterations in estrogen bio-inactivation and excretion (49, 50). The biological relevance of diminished elevation in circulating estrone levels in late gestation TP-injected dams after TP injections is unclear since estradiol is the predominant, more bioactive, placental estrogen in rhesus monkeys (52). It is unlikely that diminished estrone levels alone in TP-injected mothers reflect diminished placental function (84), since the body weights of PA infants on the day of delivery were similar to those of controls. Furthermore, there was no obvious fetal growth restriction in PA monkey infants, confirming previous reports (44, 85), unlike non-primate PA females that have low birthweight indicative of fetal growth restriction (rats: 86; sheep: 87).
Taken together, our experimental findings provide evidence for the developmental progression of bioLH hypersecretion with endogenous hyperandrogenism in female monkey fetuses or infants exposed to exogenous androgen excess during early gestation, which resembles that found in adult female monkeys, similarly-exposed hyperandrogenic women (1, 28) and in many PCOS women (6). They further suggest that genetically- or environmentally-determined differentiation of ovarian function during fetal life may contribute to ovarian hyperandrogenism during mid- to late gestation (4) since ovarian steroidogenesis (rhesus monkeys: 75; humans: 88) and steroid receptors exist at this time (humans: 89) in which the external genital are less responsive to androgen action (rhesus monkeys: 90; humans: 92). Such ovarian hyperandrogenism may, in turn, reprogram the fetal hypothalamo-pituitary-gonadal axis to promote exaggerated pulsatile LH, and presumably GnRH, release with puberty in a manner that resembles that of PCOS and other hyperandrogenic conditions.
Acknowledgments
We thank J Turk, S Muller, J Lange and Assay Services of WNPRC for assistance with endocrine determinations; Ravinder J. Singh, Ph.D. of the Endocrine Laboratory, Mayo Foundation and Clinic, Rochester, MN for assistance with LCMS development and confirmation of LCMS values at WNPRC; and members of the animal care staff at the CNPRC for expert technical assistance.
This work was supported by NIH grants P50 HD044405, U01 HD044650, P51 RR000167 (WNPRC base operating grant) and RR00169 (CNPRC base operating grant), and was partly conducted at a facility (WNPRC) constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01.
References
- 1.Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- 2.Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW. Insights into the development of PCOS from studies of prenatally androgenized female rhesus monkeys. Trends in Endocrinology and Metabolism. 1998;9:62–67. doi: 10.1016/s1043-2760(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 3.Eisner JR, Barnett MA, Dumesic DA, Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril. 2002;77:167–172. doi: 10.1016/s0015-0282(01)02947-8. [DOI] [PubMed] [Google Scholar]
- 4.Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome - a hypothesis. J Endocrinol. 2002;174:1–5. doi: 10.1677/joe.0.1740001. [DOI] [PubMed] [Google Scholar]
- 5.Zawadzki JA, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic Ovary Syndrome. Boston: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 6.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 7.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF. Position statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 8.Franks S. Diagnosis of polycystic ovarian syndrome: in defense of the Rotterdam criteria. J Clin Endocrinol Metab. 2006;91:786–789. doi: 10.1210/jc.2005-2501. [DOI] [PubMed] [Google Scholar]
- 9.Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN PCOS/Troglitazone Study Group. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:48–53. doi: 10.1210/jc.2005-1329. [DOI] [PubMed] [Google Scholar]
- 10.Goodarzi MO, Azziz R. Diagnosis, epidemiology, and genetics of the polycystic ovary syndrome. Best Pract Res Clin Endocrinol Metab. 2006;20:193–205. doi: 10.1016/j.beem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Dunaif A. Insulin resistance in women with polycystic ovary syndrome. Fertil Steril. 2006;86 1:S13–4. doi: 10.1016/j.fertnstert.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Dumesic DA, Abbott DH, Eisner JR, Goy RW. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril. 1997;67:155–163. doi: 10.1016/s0015-0282(97)81873-0. [DOI] [PubMed] [Google Scholar]
- 13.Barnes RB, Rosenfield RL, Burstein S, Ehrmann DA. Pituitary-ovarian responses to nafarelin testing in the polycystic ovary syndrome. N Engl J Med. 1989;320:559–565. doi: 10.1056/NEJM198903023200904. [DOI] [PubMed] [Google Scholar]
- 14.Christman GM, Randolph JF, Kelch RP, Marshall JC. Reduction of gonadotropin-releasing hormone pulse frequency is associated with subsequent selective follicle-stimulating hormone secretion in women with polycystic ovarian disease. J Clin Endocrinol Metab. 1991;72:1278–1285. doi: 10.1210/jcem-72-6-1278. [DOI] [PubMed] [Google Scholar]
- 15.Steiner RA, Clifton DK, Spies HG, Resko JA. Sexual differentiation and feedback control of luteinizing hormone secretion in the rhesus monkey. Biol Reprod. 1976;15:206–212. doi: 10.1095/biolreprod15.2.206. [DOI] [PubMed] [Google Scholar]
- 16.Levine JE, Terasawa E, Hoffmann SM, Dobbert MJW, Foecking EM, Abbott DH. Luteinizing hormone (LH) hypersecretion and diminished LH responses to RU486 in a nonhuman primate model for polycystic ovary syndrome (PCOS). Abstract P1-85 presented at the 87th Annual Meeting of the Endocrine Society; San Diego, CA. 2005, June 4-7. [Google Scholar]
- 17.Daniels TL, Berga SL. Resistance of gonadotropin releasing hormone drive to sex steroid-induced suppression in hyperandrogenic anovulation. J Clin Endocrinol Metab. 1997;82:4179–4183. doi: 10.1210/jcem.82.12.4402. [DOI] [PubMed] [Google Scholar]
- 18.Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85:4047–4052. doi: 10.1210/jcem.85.11.6992. [DOI] [PubMed] [Google Scholar]
- 19.McCartney CR, Eagleson CA, Marshall JC. Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin Reprod Med. 2002;20:317–326. doi: 10.1055/s-2002-36706. [DOI] [PubMed] [Google Scholar]
- 20.JE Levine, Hoffmann SM, Terasawa E, Horton T, Abbott DH. Unpublished results
- 21.Abbott DH, Dumesic DA, Eisner JW, Kemnitz JW, Goy RW. The prenatally androgenized female rhesus monkey as a model for polycystic ovarian syndrome. In: Azziz R, Nestler JE, Dewailly D, editors. Androgen Excess Disorders in Women. 1997. pp. 369–382. [Google Scholar]
- 22.Franks S, Mason H, Willis D. Follicular dynamics in the polycystic ovary syndrome. Mol Cell Endocrinol. 2000;163:49–52. doi: 10.1016/s0303-7207(99)00239-7. [DOI] [PubMed] [Google Scholar]
- 23.Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update. 2006;12:351–361. doi: 10.1093/humupd/dml017. [DOI] [PubMed] [Google Scholar]
- 24.Escobar ME, Ropelato MG, Ballerini MG, Gryngarten MG, Garcia Rudaz MC, Veldhuis JD, Barontini M. Acceleration of Luteinizing Hormone Pulse Frequency in Adolescent Girls with a History of Central Precocious Puberty with versus without Hyperandrogenism. Horm Res. 2007;68:278–285. doi: 10.1159/000104177. [DOI] [PubMed] [Google Scholar]
- 25.Barnes RB, Rosenfield RL, Ehrmann DA, Cara JF, Cuttler L, Levitsky LL, Rosenthal IM. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79:1328–1333. doi: 10.1210/jcem.79.5.7962325. [DOI] [PubMed] [Google Scholar]
- 26.Xita N, Tsatsoulis A. Review: fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies. J Clin Endocrinol Metab. 2006;91:1660–1666. doi: 10.1210/jc.2005-2757. [DOI] [PubMed] [Google Scholar]
- 27.Sarma HN, Manikkam M, Herkimer C, Dell'Orco J, Welch KB, Foster DL, Padmanabhan V. Fetal programming: excess prenatal testosterone reduces postnatal luteinizing hormone, but not follicle-stimulating hormone responsiveness, to estradiol negative feedback in the female. Endocrinology. 2005;146:4281–4291. doi: 10.1210/en.2005-0322. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfield RL. Clinical review: Identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:787–796. doi: 10.1210/jc.2006-2012. [DOI] [PubMed] [Google Scholar]
- 29.Zhou R, Bruns CM, Bird IM, Kemnitz JW, Goodfriend TL, Dumesic DA, Abbott DH. Pioglitazone improves insulin action and normalizes menstrual cycles in a majority of prenatally androgenized female rhesus monkeys. Reprod Toxicol. 2007;23:438–434. doi: 10.1016/j.reprotox.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarantal AF. Ultrasound imaging in rhesus (Macaca mulatta) and long-tailed (Macaca fascicularis) macaques: Reproductive and research applications. In: Wolfe-Coote S, editor. The Laboratory Primate. Elsevier Academic Press; San Diego, CA: 2005. pp. 317–352. [Google Scholar]
- 31.Tarantal AF, Gargosky SE. Characterization of the insulin-like growth factor (IGF) axis in the serum of maternal and fetal macaques (Macaca mulatta and Macaca fascicularis) Growth Regul. 1995;5:190–198. [PubMed] [Google Scholar]
- 32.Jimenez DF, Tarantal AF. Quantitative analysis of male fetal DNA in maternal serum of gravid rhesus monkeys (Macaca mulatta) Pediatr Res. 2003;53:18–23. doi: 10.1203/00006450-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Resko JA, Buhl AE, Phoenix CH. Treatment of pregnant rhesus macaques with testosterone propionate: observations on its fate in the fetus. Biol Reprod. 1987;37:1185–1191. doi: 10.1095/biolreprod37.5.1185. [DOI] [PubMed] [Google Scholar]
- 34.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, Zapanti ED, Bartzis MI. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–4011. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 35.Asunción M, Calvo RM, San Millán JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 36.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 37.Beck-Peccoz P, Padmanabhan V, Baggiani AM, Cortelazzi D, Buscaglia M, Medri G, Marconi AM, Pardi G, Beitins IZ. Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: circulating levels of gonadotropins, their common alpha-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-stimulating hormone. J Clin Endocrinol Metab. 1991;73:525–532. doi: 10.1210/jcem-73-3-525. [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi T. The problem of species comparison of developmental toxicity: can we extrapolate human developmental toxicity induced by environmental chemicals from the data on rodents? Yakugaku Zasshi. 2007;127:491–500. doi: 10.1248/yakushi.127.491. [DOI] [PubMed] [Google Scholar]
- 39.France JT, Mason JI, Magness RR, Murry BA, Rosenfeld CR. Ovine placental aromatase: studies of activity levels, kinetic characteristics and effects of aromatase inhibitors. J Steroid Biochem. 1987;28:155–160. doi: 10.1016/0022-4731(87)90371-2. [DOI] [PubMed] [Google Scholar]
- 40.Tarantal AF. Interventional ultrasound in pregnant macaques: embryonic/fetal applications. J Med Primatol. 1990;19:47–58. [PubMed] [Google Scholar]
- 41.Norman RL, Spies HG. Effect of luteinizing hormone-releasing hormone on the pituitary-gonadal axis in fetal and infant rhesus monkeys. Endocrinology. 1979;10:655–659. doi: 10.1210/endo-105-3-655. [DOI] [PubMed] [Google Scholar]
- 42.Huhtaniemi IT, Korenbrot CC, Seron-Ferre M, Foster DB, Parer JT, Jaffe RB. Stimulation of testosterone production in vivo and in vitro in the male rhesus monkey fetus in late gestation. Endocrinology. 1977;100:839–844. doi: 10.1210/endo-100-3-839. [DOI] [PubMed] [Google Scholar]
- 43.Monroe SE, Yamamoto M, Jaffe RB. Changes in gonadotrope responsivity to gonadotropin releasing hormone during development of the rhesus monkey. Biol Reprod. 1983;29:422–431. doi: 10.1095/biolreprod29.2.422. [DOI] [PubMed] [Google Scholar]
- 44.Herman RA, Jones B, Mann DR, Wallen K. Timing of prenatal androgen exposure: anatomical and endocrine effects on juvenile male and female rhesus monkeys. Horm Behav. 2000;38:52–66. doi: 10.1006/hbeh.2000.1608. [DOI] [PubMed] [Google Scholar]
- 45.Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:1111–1119. doi: 10.1210/jcem.87.3.8287. [DOI] [PubMed] [Google Scholar]
- 46.Zhou R, Bird IM, Dumesic DA, Abbott DH. Adrenal hyperandrogenism is induced by fetal androgen excess in a rhesus monkey model of polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:6630–6637. doi: 10.1210/jc.2005-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89:534–543. doi: 10.1210/jc.2003-031287. [DOI] [PubMed] [Google Scholar]
- 48.Nelson RE, Grebe SK, OKane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–384. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 49.Barbier O, Belanger A. The cynomolgus monkey (Macaca fascicularis) is the best animal model for the study of steroid glucuronidation. J Steroid Biochem Mol Biol. 2003;85:235–245. doi: 10.1016/s0960-0760(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 50.Leung KC, Lee YT, Fung KP, Yu WN, Yu CC. beta-Glucuronidase and oestrogens in hydatidiform mole. Clin Chim Act. 1976;72:295–300. doi: 10.1016/0009-8981(76)90191-1. [DOI] [PubMed] [Google Scholar]
- 51.Ziegler TE, Scheffler G, Wittwer DJ, Schultz-Darken N, Snowdon CT, Abbott DH. Metabolism of reproductive steroids during the ovarian cycle in two species of callitrichids, Saguinus oedipus and Callithrix jacchus, and estimation of the ovulatory period from fecal steroids. Biol Reprod. 1996;54:91–99. doi: 10.1095/biolreprod54.1.91. [DOI] [PubMed] [Google Scholar]
- 52.Atkinson LE, Hotchkiss J, Fritz GR, Surve AH, Neill JD, Knobil E. Circulating levels of steroids and chorionic gonadotropin during pregnancy in the rhesus monkey, with special attention to the rescue of the corpus luteum in early pregnancy. Biol Reprod. 1975;12:335–345. doi: 10.1095/biolreprod12.3.335. [DOI] [PubMed] [Google Scholar]
- 53.Hodgen GD. Primate chorionic gonadotropins: their comparative biological, immunologic and chemical properties. In: Nathanielsz PW, editor. Fetal Endocrinology. New York: Academic Press; 1981. pp. 95–110. [Google Scholar]
- 54.Hobson BM, Wide L. The similarity of chorionic gonadotrophin and its subunits in term placentae from man, apes, old and New World monkeys and a prosimian. Folia Primatol (Basel) 1981;35:51–64. doi: 10.1159/000155965. [DOI] [PubMed] [Google Scholar]
- 55.Sokal RR, Rohlf FJ. Biometry. 3. New York: W. H. Freeman and Co.; 1995. the principles and practice of statistics in biological research; pp. 413–422. [Google Scholar]
- 56.Iglewicz B, Hoaglin DC. How to detect and handle outliers. ASQC basic references in quality control. v 16. Milwaukee: ASQC Quality Press; 1993. [Google Scholar]
- 57.Baird DT, Corker CS, Davidson DW, Hunter WM, Michie EA, Van Look PF. Pituitary-ovarian relationships in polycystic ovary syndrome. J Clin Endocrinol Metab. 1977;45:798–801. doi: 10.1210/jcem-45-4-798. [DOI] [PubMed] [Google Scholar]
- 58.Rosenfield RL, Barnes RB, Ehrmann DA. Studies of the nature of 17-hydroxyprogesterone hyperresonsiveness to gonadotropin-releasing hormone agonist challenge in functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1994;79:1686–1692. doi: 10.1210/jcem.79.6.7989476. [DOI] [PubMed] [Google Scholar]
- 59.Gilling-Smith C, Story H, Rogers V, Franks S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin Endocrinol (Oxf) 1997;47:93–99. doi: 10.1046/j.1365-2265.1997.2321049.x. [DOI] [PubMed] [Google Scholar]
- 60.Ellinwood WE, Baughman WL, Resko JA. The effects of gonadectomy and testosterone treatment on luteinizing hormone secretion in fetal rhesus monkeys. Endocrinology. 1982;110:183–189. doi: 10.1210/endo-110-1-183. [DOI] [PubMed] [Google Scholar]
- 61.Resko JA, Ellinwood WE. Sexual differentiation of the brain of primates. In: Serio M, Motta M, Zanisi M, Martini L, editors. Sexual Differentiation: Basic and Clinical Aspects. Raven Press; New York, NY: 1984. pp. 169–181. [Google Scholar]
- 62.Ellinwood WE, Resko JA. Sex differences in biologically active and immunoreactive gonadotropins in the fetal circulation of rhesus monkeys. Endocrinology. 1980;107:902–907. doi: 10.1210/endo-107-4-902. [DOI] [PubMed] [Google Scholar]
- 63.Plant TM, Dubey AK. Evidence from the rhesus monkey (Macaca mulatta) for the view that negative feedback control of luteinizing hormone secretion by the testis is mediated by a deceleration of hypothalamic gonadotropin-releasing hormone pulse frequency. Endocrinology. 1984;115:2145–2153. doi: 10.1210/endo-115-6-2145. [DOI] [PubMed] [Google Scholar]
- 64.Abbott DH, Batty KA, Dubey AK, Herbert J, Shiers HM. The passage of 5 alpha-dihydrotestosterone from serum into cerebrospinal fluid and LH negative feedback in castrated rhesus monkeys. J Endocrinol. 1985;104:325–330. doi: 10.1677/joe.0.1040325. [DOI] [PubMed] [Google Scholar]
- 65.Scott CJ, Clarke IJ, Rao A, Tilbrook AJ. Sex differences in the distribution and abundance of androgen receptor mRNA-containing cells in the preoptic area and hypothalamus of the ram and ewe. J Neuroendocrinol. 2004;16:956–963. doi: 10.1111/j.1365-2826.2005.01261.x. [DOI] [PubMed] [Google Scholar]
- 66.Resko JA, Roselli CE. Prenatal hormones organize sex differences of the neuroendocrine reproductive system: observations on guinea pigs and nonhuman primates. Cell Mol Neurobiol. 1997;17:627–648. doi: 10.1023/a:1022534019718. [DOI] [PubMed] [Google Scholar]
- 67.Resko JA, Pereyra-Martinez AC, Stadelman HL, Roselli CE. Cellular observations and hormonal correlates of feedback control of luteinizing hormone secretion by testosterone in long-term castrated male rhesus monkeys. Biol Reprod. 2000;63:872–878. doi: 10.1095/biolreprod63.3.872. [DOI] [PubMed] [Google Scholar]
- 68.Resko JA, Ellinwood WE. Negative feedback regulation of gonadotropin secretion by androgens in fetal rhesus macaques. Biol Reprod. 1985;33:346–352. doi: 10.1095/biolreprod33.2.346. [DOI] [PubMed] [Google Scholar]
- 69.Mann DR, Davis-DaSilva M, Wallen K, Coan P, Evans DE, Collins DC. Blockade of neonatal activation of the pituitary-testicular axis with continuous administration of a gonadotropin-releasing hormone agonist in male rhesus monkeys. J Clin Endocrinol Metab. 1984;59:207–211. doi: 10.1210/jcem-59-2-207. [DOI] [PubMed] [Google Scholar]
- 70.Plant TM. The effects of neonatal orchidectomy on the developmental pattern of gonadotropin secretion in the male rhesus monkey (Macaca mulatta) Endocrinology. 1980;106:1451–1454. doi: 10.1210/endo-106-5-1451. [DOI] [PubMed] [Google Scholar]
- 71.Tomaszycki ML, Davis JE, Gouzoules H, Wallen K. Sex differences in infant rhesus macaque separation-rejection vocalizations and effects of prenatal androgens. Horm Behav. 2001;39:267–276. doi: 10.1006/hbeh.2001.1659. [DOI] [PubMed] [Google Scholar]
- 72.Plant TM. A striking sex difference in the gonadotropin response to gonadectomy during infantile development in the rhesus monkey (Macaca mulatta) Endocrinology. 1986;119:539–545. doi: 10.1210/endo-119-2-539. [DOI] [PubMed] [Google Scholar]
- 73.Terasawa E, Bridson WE, Nass TE, Noonan JJ, Dierschke DJ. Developmental changes in the luteinizing hormone secretory pattern in peripubertal female rhesus monkeys: comparisons between gonadally intact and ovariectomized animals. Endocrinology. 1984;115:2233–2240. doi: 10.1210/endo-115-6-2233. [DOI] [PubMed] [Google Scholar]
- 74.Plant TM, Zorub DS. A study of the role of the adrenal glands in the initiation of the hiatus in gonadotropin secretion during prepubertal development in the male rhesus monkey (Macaca mulatta) Endocrinology. 1984;114:560–565. doi: 10.1210/endo-114-2-560. [DOI] [PubMed] [Google Scholar]
- 75.Ellinwood WE, McClellan MC, Brenner RM, Resko JA. Estradiol synthesis by fetal monkey ovaries correlates with antral follicle formation. Biol Reprod. 1983;28:505–516. doi: 10.1095/biolreprod28.2.505. [DOI] [PubMed] [Google Scholar]
- 76.Abbott DH, Eisner JR, Groome NP, Dumesic DA, McNeilly AS. Decreased serum levels of inhibin A and inhibin B in anovulatory prenatally androgenized female rhesus monkeys. J Endocrinol. 1997;152 Suppl:P258. [Google Scholar]
- 77.Rosenfeld RG, Reitz RE, King AB, Hintz RL. Familial precocious puberty associated with isolated elevation of luteinizing hormone. N Engl J Med. 1980;303:859–862. doi: 10.1056/NEJM198010093031506. [DOI] [PubMed] [Google Scholar]
- 78.Barnes RB, Namnoum AB, Rosenfield RL, Layman LC. The role of LH and FSH in ovarian androgen secretion and ovarian follicular development: clinical studies in a patient with isolated FSH deficiency and multicystic ovaries. Hum Reprod. 2002;17:88–91. doi: 10.1093/humrep/17.1.88. [DOI] [PubMed] [Google Scholar]
- 79.Latronico AC, Lins TS, Brito VN, Arnhold IJ, Mendonca BB. The effect of distinct activating mutations of the luteinizing hormone receptor gene on the pituitary-gonadal axis in both sexes. Clin Endocrinol (Oxf) 2000;53:609–613. doi: 10.1046/j.1365-2265.2000.01135.x. [DOI] [PubMed] [Google Scholar]
- 80.Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med. 2004;22:311–326. doi: 10.1055/s-2004-861548. [DOI] [PubMed] [Google Scholar]
- 81.Cutler GB, Jr, Glenn M, Bush M, Hodgen GD, Graham CE, Loriaux DL. Adrenarche: a survey of rodents, domestic animals, and primates. Endocrinology. 1978;103:2112–2118. doi: 10.1210/endo-103-6-2112. [DOI] [PubMed] [Google Scholar]
- 82.Stanczyk FZ, Hess DL, Namkung PC, Senner JW, Petra PH, Novy MJ. Alterations in sex steroid-binding protein (SBP), corticosteroid-binding globulin (CBG), and steroid hormone concentrations during pregnancy in rhesus macaques. Biol Reprod. 1986;35:126–132. doi: 10.1095/biolreprod35.1.126. [DOI] [PubMed] [Google Scholar]
- 83.Golos TG, Handrow RR, Durning M, Fisher JM, Rilling JK. Regulation of chorionic gonadotropin-alpha and chorionic somatomammotropin messenger ribonucleic acid expression by 8-bromo-adenosine 3′,5′-monophosphate and dexamethasone in cultured rhesus monkey syncytiotrophoblasts. Endocrinology. 1992;131:89–100. doi: 10.1210/endo.131.1.1612035. [DOI] [PubMed] [Google Scholar]
- 84.Albrecht ED, Pepe GJ. Central integrative role of oestrogen in modulating the communication between the placenta and fetus that results in primate fecal-placental development. Placenta. 1999;20:129–139. doi: 10.1053/plac.1998.0359. [DOI] [PubMed] [Google Scholar]
- 85.Abbott DH, Bruns CM, Barnett DK, Dumesic DA. Fetal programming of polycystic ovary syndrome. In: Kovacs WG, Norman RL, editors. Polycystic Ovary Syndrome. 2nd. Cambridge: Cambridge University Press; 2006. pp. 262–287. [Google Scholar]
- 86.Slob AK, den Hamer R, Woutersen PJ, van der Werff ten Bosch JJ. Prenatal testosterone propionate and postnatal ovarian activity in the rat. Acta Endocrinol (Copenh) 1983;103:420–427. doi: 10.1530/acta.0.1030420. [DOI] [PubMed] [Google Scholar]
- 87.Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- 88.Cole B, Hensinger K, Maciel GA, Chang RJ, Erickson GF. Human fetal ovary development involves the spatiotemporal expression of P450c17 protein. J Clin Endocrinol Metab. 2006;91:3654–3661. doi: 10.1210/jc.2006-0641. [DOI] [PubMed] [Google Scholar]
- 89.Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol. 1996;120:51–57. doi: 10.1016/0303-7207(96)03819-1. [DOI] [PubMed] [Google Scholar]
- 90.Goy RW, Bercovitch FB, McBrair MC. Behavioral masculinization is independent of genital masculinization in prenatally androgenized female rhesus macaques. Horm Behav. 1988;22:552–571. doi: 10.1016/0018-506x(88)90058-x. [DOI] [PubMed] [Google Scholar]
- 91.Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev. 1995;16:271–321. doi: 10.1210/edrv-16-3-271. [DOI] [PubMed] [Google Scholar]