Abstract
A series of membrane-spanning bolaamphiphiles (molecules with two hydrophilic end-groups connected by a hydrophobic linker) were prepared by a modular synthetic method and evaluated for their abilities to affect the dynamics of a surrounding bilayer membrane. The goal was to determine if the bolaamphiphiles promote the translocation of phospholipids across vesicle membranes. The bolaamphiphiles were incorporated at low levels (up to 5 mol%) in vesicles composed of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC). Inward translocation assays were performed using fluorescent, NBD-labeled phospholipid probes with phosphocholine (PC) or phosphoglycerol (PG) head-groups. The membrane-spanning bolaamphiphiles promote the translocation of both phospholipid probes in the order PG > PC, while shorter bolaamphiphiles (structures that must adopt a U-shape and keep both end-groups in the same leaflet of the membrane), and regular amphiphiles with one hydrophilic end-group, are inactive. These results are an exception to the rule-of-thumb that membrane-spanning bolaamphiphiles are inherently membrane stabilizing molecules that inhibit all types of membrane transport.
Introduction
Bolaamphiphiles are surface active molecules with two hydrophilic end-groups connected by a hydrophobic spacer that has one, two, or three alkyl chains. Bolaamphiphiles often self-assemble in water to form monolayer lipid membranes that are more tightly packed and less permeable than analogous bilayer membranes.1-3 For example, the membranes of archaebacteria are composed of approximately 90% tetraether isoprenoid based bolaamphiphiles, and as a result they are unusually stable at high temperature and low pH.4,5 Bolaamphiphiles are particularly promising building blocks for the fabrication of highly stable, supported lipid membranes to be used in various membrane mimetic devices.6
The molecular assembly under consideration here is not to a membrane composed predominantly of bolaamphiphiles, but rather a fluid-phase bilayer membrane composed primarily of the common phospholipid, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), with a low concentration (up to 5 mol%) of added bolaamphiphile. The general goal of this work is to ascertain if and how the bolaamphiphile alters the molecular dynamics of the surrounding bilayer. Any effect will, of course, depend on the bolaamphiphile's exact molecular structure. For example, a number of bolaamphiphiles have been designed to act as ion channels or membrane disruptors. These compounds have specific structural elements such as internal hydrophilic residues that promote a significant transport or disruption process.1,7 Much less attention has been paid to the ability of structurally simple bolaamphiles to alter bilayer membrane structure and function.8 We are particularly interested in the dynamic process of phospholipid translocation or flip-flop. In contrast to lateral membrane diffusion, which is rapid, translocation of a labeled-phospholipid across a bilayer membrane is quite slow with a typical half-life in vesicles of many hours or even days (Figure 1).9 The activation barrier is due to the energetic penalty for moving the polar phospholipid head-group through the lipophilic interior of the membrane, although acyl chain structure is also a significant factor.9,10 Recently, we and others, have demonstrated that phospholipid translocation can be accelerated by a number of low molecular weight organic compounds, including molecules that induce membrane pores,11 or form hydrogen bonded complexes with the phospholipid head-group.12-15 Membrane adsorbed polymers can also disrupt packing and induce phospholipid translocation.14
Figure 1.
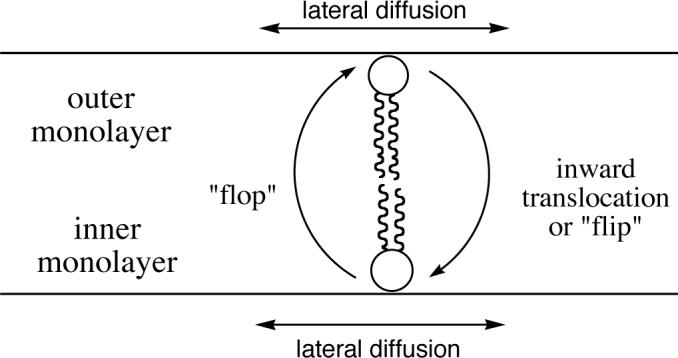
Definitions for phospholipid translocation.
The specific goal of this study was to determine if structurally simple bolaamphiphiles promote the translocation of phospholipids across vesicle membranes. At first glance this idea may seem to be counter-intuitive since, as stated above, bolaamphiphiles are generally considered as inhibitors of membrane transport.1,8,16-18 Nonetheless, our curiosity was raised by a recent study by the group of de Kruijff and co-workers who observed that hydrophobic, single-pass, membrane-spanning helical peptides promote phospholipid flip-flop in vesicle membranes.19 They rationalize their results in terms of a “slip-pop” mechanism (Figure 2) which proposes that the inherent wobbling motion of the peptide causes local perturbations in the head-group region of the membrane, allowing the polar head-groups of the adjacent phospholipids to slip into the hydrophobic center, and subsequently pop out on either side. They also observed that single-pass helices promote phospholipid translocation more effectively than multiple-pass helices.19 Thus, we were interested in seeing if a bolaamphiphile, with a similar structural shape as a single-pass helix,20 could also promote phospholipid translocation. We report the design and synthesis of a series of amphiphiles, 1 − 6, (Scheme 1) that are related by having the same phosphodiester end-groups. The structures of bolaamphiphiles 1 − 4 are sufficiently long (30−32 heavy atoms) to span a vesicle membrane composed of POPC, whereas 5 and 6 are shorter control compounds. We find that the membrane-spanning bolaamphiphiles are indeed able to promote the translocation of fluorescently-labeled phospholipids across surface differentiated vesicle membranes, and the biological implications of our results are discussed.
Figure 2.
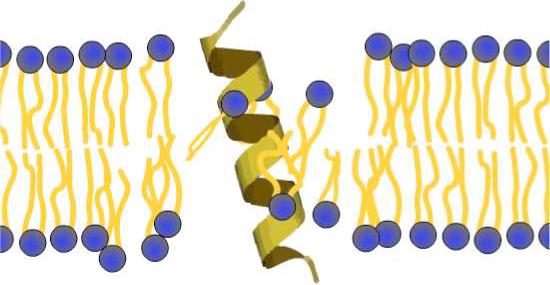
Representation of the “slip-pop” mechanism for phospholipid flipflop. The inherent wobbling motion of a single-pass, membrane-spanning helical peptide causes local defects in the head-group region of the bilayer, which allows neighboring phospholipids to slip into the hydrophobic center of the membrane and subsequently pop outside.19
Scheme 1.
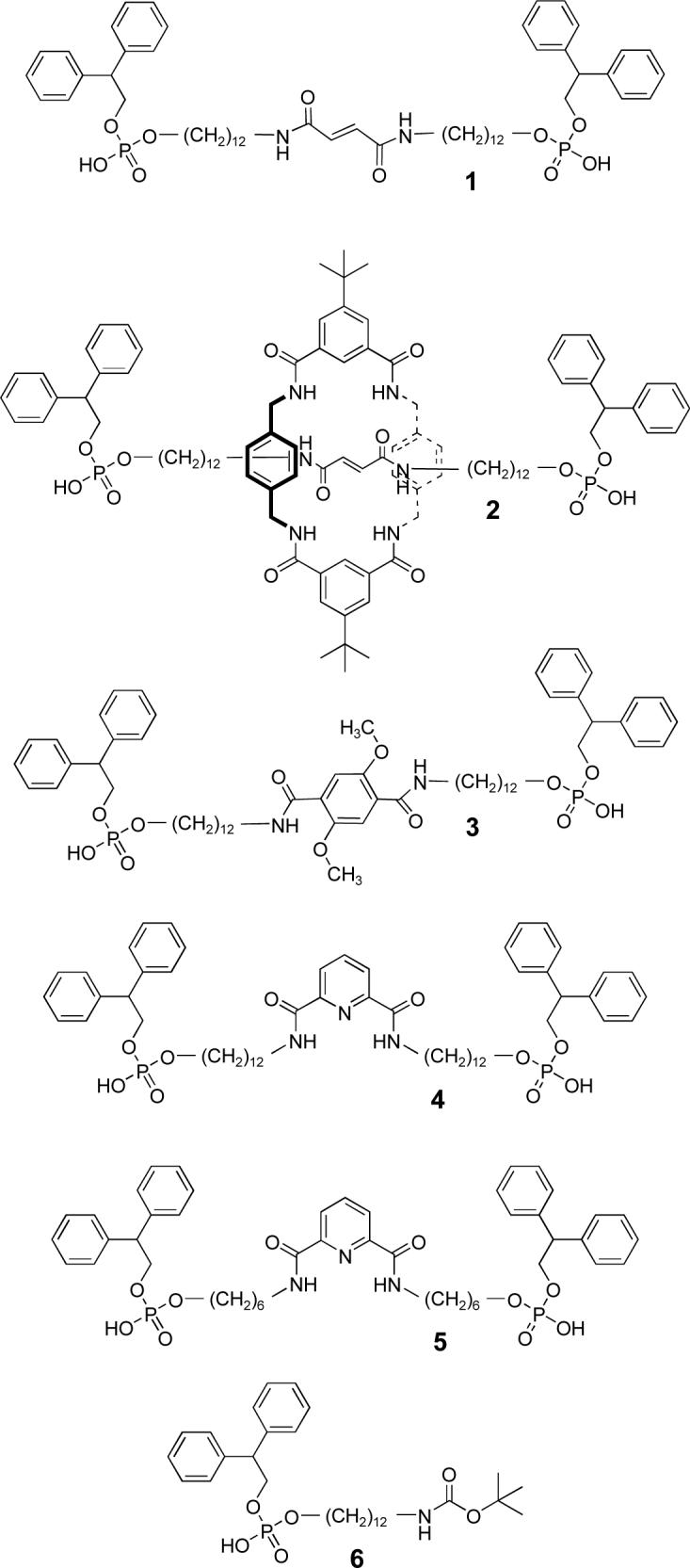
Membrane spanning bolaamphiphiles 1 − 4 and shorter control compounds 5 and 6.
Results
Synthesis
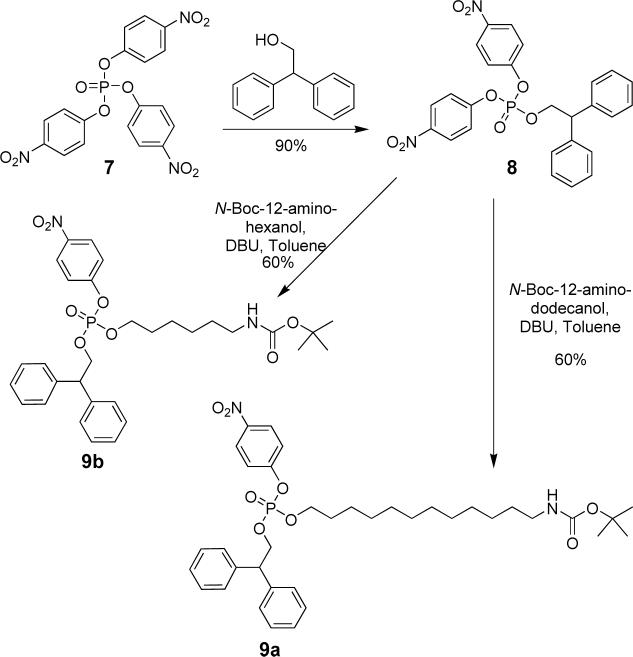
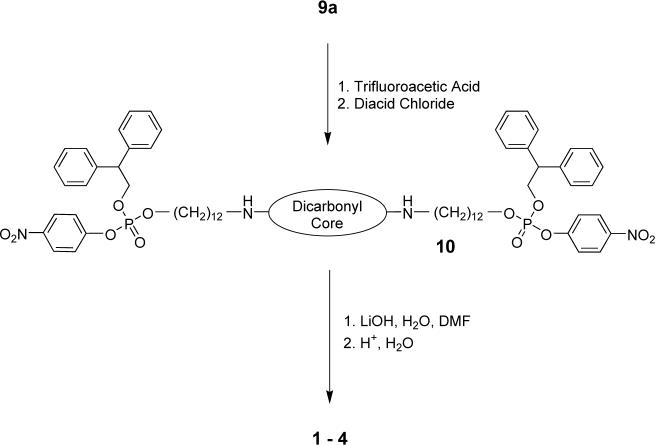
A modular synthetic strategy was employed to prepare the bolaamphiphiles. The six-carbon and twelve-carbon phosphotriester N-Boc-amines 9a and 9b were prepared as shown in Scheme 2. The N-Boc groups were removed by treatment with TFA, but prior to the next synthetic step, the trifluoroacetate counter anions were exchanged for chloride to prevent side reactions. The ammonium chloride salts were treated with the appropriate bis-acid chloride to give the appropriate phosphotriester compounds 1PNP - 4PNP (shown generically as 10 in Scheme 3), and subsequent hydrolysis with LiOH produced the phosphodiesters 1, 3, and 4. The six-carbon control bolaamphiphile 5 was prepared by an analogous pathway. The Leigh-type rotaxane 2 was made by treating the precursor thread compound 1PNP, with 5-tertbutylisophthaloyl chloride, and p-xylylenediamine.21 The rotaxane product, phosphotriester 2PNP, was hydrolyzed to give the rotaxane phosphodiester, 2.
Scheme 2.
Synthesis of the phosphotriester building blocks.
Scheme 3.
General synthetic strategy for compounds 1 − 4
Phospholipid Translocation and Leakage Assays
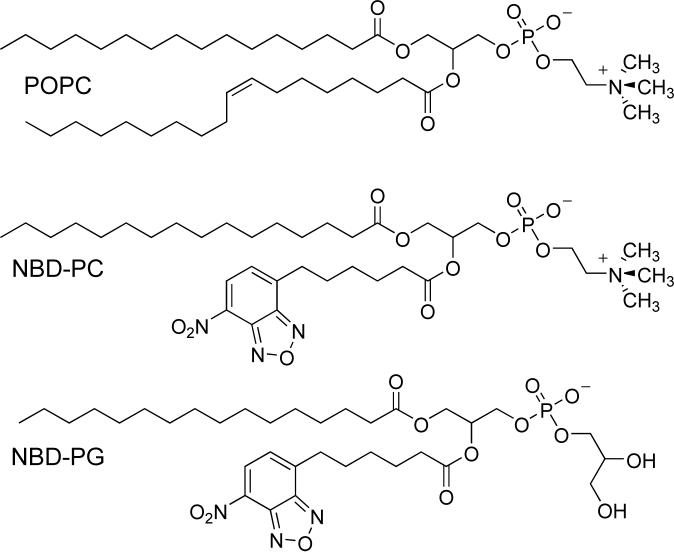
Phospholipid translocation was monitored via the well-established 7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD)/dithionite quenching assay which uses a phospholipid probe containing an NBD group in one of its acyl chains (all phospholipid structures are presented in Scheme 4).14 Exo-labeled vesicles were prepared by addition of a small aliquot of NBD-lipid (0.5 mol% of total phospholipid) in ethanol to a dispersion of vesicles composed of a mixture POPC and bolaamphiphile. The NBD-lipid readily inserts into the outer monolayer of the fluid-phase vesicles. Upon treatment with sodium dithionite (Na2S2O4), the NBD fluorescence is quenched due to reduction of the NBD nitro group. Vesicle membranes are effectively impermeable to dithionite, therefore, only NBD-phospholipid that is located in the outer leaflet is chemically quenched. At any given time, the fraction of probe located in the outer monolayer can be determined from the drop in fluorescence intensity when a portion of the vesicles are subjected to dithionite quenching.
Scheme 4.
Molecular structure of POPC and the fluorescent probes NBD-PG and NBD-PC.
All translocation measurements were conducted in 5 mM TES/100 mM NaCl buffer at pH 7.4 and 25° C with unilamellar vesicles that were prepared by repeated extrusion through polycarbonate filters with 100 nm pores. The inward translocation of NBD-PG or NBD-PC across vesicle membranes composed of POPC and 4 or 5 mol% of amphiphiles 1 − 6 is shown in Figures 3 and 4. The experiments start with an initial value of 100% exo NBD-lipid and progress to an equilibrium value of around 60% exo NBD-lipid.13 The half-lives for these equilibration processes are listed in Table 1, along with the longer half-lives that were observed when the vesicles also contained 30% cholesterol. Inward translocation of NBD-PS was also investigated with the more active amphiphiles but no significant translocation was observed (data not shown). In the case of active bolaamphiphiles 1 and 3, the rate of NBD-PC translocation was measured over the rather narrow range of 2−6 mol% and a linear dependency was observed (uncertainty in the translocation rate measurement increases substantially outside of this concentration range). The fact that the NBD probes become protected from the externally added dithionite is strong evidence that vesicles do not allow passage of hydrophilic ions into the vesicles. This conclusion was independently confirmed by a carboxyfluorescein leakage experiment. The water-soluble fluorescent marker 5(6)-carboxyfluorescein was encapsulated inside vesicles composed of 95% POPC and 5% bolaamphiphile 2 and no significant leakage was observed over several hours.
Figure 3.
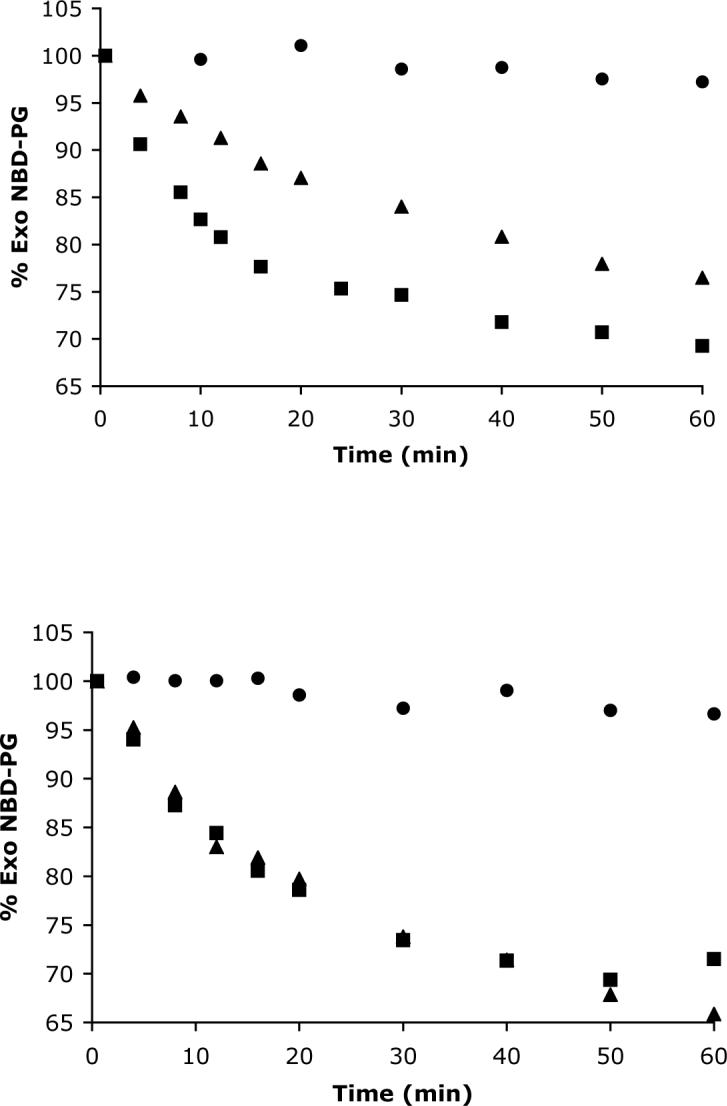
Change in the fraction of NBD-PG in the membrane outer monolayer (% Exo NBDPG). Inward translocation induced at t=0 min by adding 0.125 μM of NBD-PG to POPC vesicles (25 μM) that also contained (Top) 5 mol % of 1(—), 2(π) or 6( ); (Bottom) 4 mol % of 3(—), 4(π) or 5( ).
Figure 4.
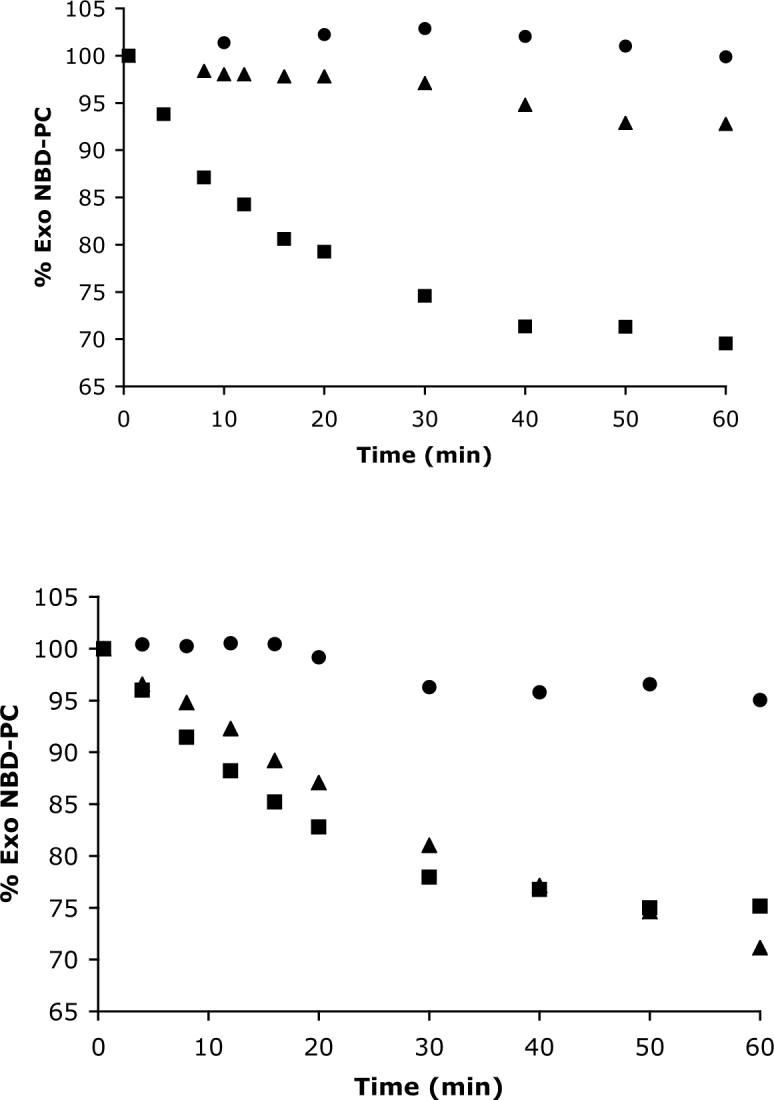
Change in the fraction of NBD-PC in the membrane outer monolayer (% Exo NBDPC). Inward translocation induced at t=0 min by adding 0.125 μM of NBD-PC to POPC vesicles (25 μM) that also contained (Top) 5 mol % of 1(—), 2(π) or 6( ); (Bottom) 4 mol % of 3(—), 4(π) or 5( ).
Table 1.
Half-life (t1/2) for NBD-PG and NBD-PC translocation across vesicles containing amphiphiles 1 − 6.
|
t1/2 (min)a |
||
|---|---|---|
| Vesicle Composition | NBD-PC | NBD-PG |
| POPC:1 (95:5) | 15 | 12 |
| POPC:2 (95:5) | 120 | 40 |
| POPC:3 (96:4) | 25 | 15 |
| POPC:4 (96:4) | 30 | 15 |
| POPC:5 (96:4) | >120 | >120 |
| POPC:6 (95:5) | >120 | >120 |
| POPC:Chol:1 (66:29:5) | >120 | 90 |
| POPC:Chol:2 (66:29:5) | >120 | >120 |
5 mM TES, 100 mM NaCl, pH 7.4, 25 °C; estimated uncertainty is ±33%
Discussion
Factors that were considered when designing the bolaamphiphiles for this study were synthetic accessibility, modular variation of the building blocks, and limited flexibility in the center of linking chain. In the case of 1 − 4 the end-groups are monoanionic phosphodiesters (with terminal diphenylethanes22,23) that are connected by dodecyl chains to a central dicarbonyl core. The two most likely orientations of a bolaamphiphile in a bilayer membrane are illustrated in Figure 5. Depending on the length and flexibility of the linking chain, the bolaamphiphile can extend completely across the membrane or otherwise form a U-shaped conformation in a single membrane leaflet. Previous work by Moss and coworkers has shown that bolaamphiphiles with linking chains that have been stiffened by the inclusion of central aromatic rings strongly prefer extended, membrane-spanning conformations.24 Furthermore, the translocation rate for stiffened bolaamphiphiles is extremely slow. Therefore, bolaamphiphiles 1 − 3 with their rigid central regions are expected to adopt extended, transmembrane orientations.
Figure 5.
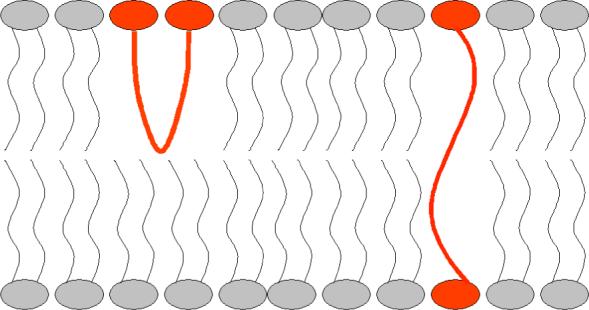
A graphical representation of a bilayer membrane containing a U-shape bolaamphiphile and an extended membrane-spanning bolaamphiphile.
The data in Figures 3 and 4 clearly shows that bolaamphiphiles 1 − 4 can greatly accelerate inward phospholipid translocation across POPC membranes;25 whereas, the control amphiphile 6, with only one phosphodiester end-group, is unable to induce any significant translocation. Why are the bolaamphiphiles so effective at promoting phosphoplipid translocation?26 In the case of bolaamphiphile, 1, we considered the possibility that the central fumaramide core may promote translocation by either forming transient hydrogen bonded complexes with the phospholipid head-groups or self-associating to form phase-separated microdomains. Evidence against the first process is the efficient translocation induced by bolaamphiphiles 2 and 3 which are analogues of 1 except that they are much less likely to form intermolecular hydrogen bonds in the middle of the membrane. The bolaamphiphile 2 is a rotaxane with an amide-containing macrocycle that encapsulates and protects the central fumaramide core. It is well-established that the macrocycle forms strong, internal hydrogen bonds with the fumaramide carbonyl oxygens,21 and this co-conformational preference was confirmed in the case of 2 by inspection of its 1H NMR spectrum in organic solvent (the highly upfield chemical shift for the fumaramide olefin hydrogens indicates that they are located deep inside the macrocycle). Thus, the surrounding macrocycle in bolaamphiphile 2 prevents any hydrogen-bonded self-association of the fumaramide core. A similar situation occurs with bolaamphiphile 3 which contains a 2,5-dimethoxyterephthalamide as the central core. This aromatic group forms strong, internal hydrogen bonds that will stiffen the central region of the bolaamphiphile, and lower the propensity for intermolecular assocation.27 The fact that both 2 and 3 promote the translocation of NBD-PG and NBD-PC almost as well as bolaamphiphile 1, whereas amphiphile 6 is completely ineffective, indicates that intermolecular hydrogen bonding in the middle of the membrane is not a necessary structural feature for accelerated translocation.26 Evidence against the second process, that flip-flop is accelerated by the presence of phase-separated microdomains,28 was gained by measuring the translocation of NBD-PC across vesicle membranes composed of POPC:DPPC (55:45). This membrane composition is known to form a heterogeneous mixture of fluid and gel phase microdomains;29 however, the rate of inward translocation of NBD-PC was as slow as that observed with pure POPC vesicles.
Another translocation pathway that must be considered is a small population of U-shaped orientations that are very effective at disturbing membrane integrity and promoting flip-flop.30,31 To explore the likelihood of this translocation mechanism, bolaamphiphiles 4 and 5 were prepared and evaluated. These two bolaamphiphiles have 2,6-pyridine dicarboxamide units as the central cores. This aromatic group is well-known to form internal hydrogen bonds, with both amide NH residues directed towards the pyridine nitrogen.32 Thus, these bolaamphiphiles are more likely to form a U-shaped conformation, however, an extended membrane-spanning orientation is still possible with version 4 and its two dodecyl chains (Scheme 1). The shorter version, 5, has two hexyl chains and cannot span a bilayer; therefore, it must adopt a U-shaped orientation in one of the membrane leaflets. The data in Figures 3 and 4 show that the longer bolaamphiphile 4 is an efficient translocator, whereas the shorter version 5 is inactive. This latter result is evidence against the hypothesis that a bolaamphiphile in a U-shape orientation is especially active.
The above structure/activity results are consistent with the idea that phospholipid translocaton is strongly promoted by bolaamphiphiles in a transmembrane orientation. Evidence that this occurs by some sort of slip-pop mechanism is the fact that the translocase activities of compounds 1 and 2 are greatly attenuated when the vesicles also include 29 mol% cholesterol (Table 1). The added cholesterol is known to increase order in the acyl chains and raise lateral pressure which is expected to decrease bolaamphiphile wobbling and inhibit the slip-pop pathway.19,31 The generality of this finding needs to be tested with further studies of alternative bolaamphiphile structures, especially compounds with different types of hydrophilic end-groups and numbers of linking chains.33
In summary, membrane-spanning bolaamphiphiles 1 − 4 are able to promote the translocation of monoanionic NBD-PG and to a lesser extent the translocation of zwitterionic NBD-PC across vesicle membranes.25 The data is consistent with the slip-pop model for facilitated phospholipid translocation by membrane-spanning bolaamphiphilic structures.19,31 The membrane disruption effect is subtle and does not create defects that are large enough to allow the passage of small polar molecules. Our results are an exception to the rule of thumb that membrane-spanning bolaamphiphiles are inherently membrane stabilizing molecules that inhibit all types of membrane transport.8,18
Relevance to Flip-Flop in Biogenic Membranes
An intriguing, ongoing question in biomembrane research is the mechanism for accelerated phospholipid flip-flop across biogenic membranes (i.e., membranes that contain the enzymes responsible for phospholipid biosynthesis) such as the bacterial cytoplasmic membrane and the mammalian endoplasmic reticulum.19,34 Since phospholipid biosynthesis occurs only on one face of the membrane, there has to be a process to translocate the newly formed phospholipid and allow the membrane to grow. Indeed, phospholipid translocation across biogenic membranes is known to be rapid, and for some time, researchers have tried to identify the associated translocase protein(s). In the case of the endoplasmic reticulum, there is evidence for an energy-independent, bidirectional translocase that is equally effective with a range of phospholipid head-groups, however, the specific protein(s) has not yet been identified.35 An alterative theory, proposed by de Kruijff and coworkers states that phospholipid translocation across biogenic membranes does not require a dedicated translocase because it can be can be promoted by simple single-pass helical peptides.19 Our results agree with the model that simple, membrane-spanning bolaamphiphilic structures can promote phospholipid translocation. The bolaamphiphiles reported here are about an order of magnitude less efficient than a single-pass helical peptide, but both systems show the same head-group selectivity, that is, the translocation of monoanionic PG is faster than zwitterionic PC.25 Since the fraction of PG in bacterial membranes (15−70%) is much higher than in mammalian membranes (<2%), it appears that the slip-pop mechanism is more likely to be kinetically significant in bacterial membranes.36
Experimental
Phospholipid Translocation Assay
POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine), NBD-PC (1-palmitoyl-2-[6-[(7-nitro-2−1,3-benzoxadiazol-4-yl)amino]hexanoyl]-sn-glycero-3-phosphocholine) and NBD-PG (1-palmitoyl-2-[6-[(7-nitro-2−1,3-benzoxadiazol-4-yl)amino]hexanoyl]-sn-glycero-3-[phospho-rac-(1-glycerol)] (ammonium salt)) were purchased from Avanti Polar Lipids. Cholesterol was purchased from Sigma-Aldrich. An appropriate mixture of POPC (in CHCl3), cholesterol (in CHCl3) and/or bolaamphiphile (in 7:3 CHCl3:CH3OH) was dried in vacuo for 1 h. A stock solution of 10 mM vesicles was made by rehydration at room temperature with TES buffer (5 mM TES, 100 mM NaCl, pH 7.4). Multilamellar vesicles were extruded to form unilamellar vesicles with a Basic LiposoFast device purchased from Avestin, Incorporated. The vesicles were extruded 29 times through a 19-mm polycarbonate Nucleopore filter with 100-nm diameter pores. An aliquot of NBD-phosopholipid (NBD-PC or NBD-PG in 140 μL of 200 proof ethanol, final concentration 0.125 μM) was added to the vesicles (45 mL, 25 μM) in TES buffer. Samples (3.0 mL) were withdrawn over time and assayed in the following way for % exo NBD-phospholipid. At assay time t = 50 s, sodium dithionite (180 _L of 60 mM solution in 1 M Tris, pH ∼10) was added and at t = 180 s, Triton X-100 (20 _L of 20 % v/v) was added. The amount of total NBD-PX which was translocated to the inside of the vesicles was calculated by the following equation: % Exo NBD-phospholipid = ((Fi-Ff)/Fi)*100, where Fi and Ff are the fluorescence intensities (ex: 460 nm, em: 530 nm) just prior to the additions of sodium dithionite and Triton X-100. The half-lives reported are the averages of 2 or 3 independent experiments.
Vesicle Leakage Assay
A solution of 2 (5 mol%) and POPC (100 mol%) in CHCl3:MeOH was evaporated and dried in vacuo for 1 h. A solution of 10 mM vesicles was made by rehydration at room temperature with 5(6)-carboxyfluorescein (CF) buffer (50 mM CF, 5 mM TES, 100 mM NaCl, pH 7.4). The vesicles were frozen and thawed 10 times and then extruded 29 times through a 19-mm polycarbonate Nucleopore filter with 100-nm diameter pores. Unencapsulated dye was removed from the vesicles by separation via a Sephadex-G50 column in TES buffer. The fluorescence intensity of a 3 mL sample of vesicles (25 μM in TES buffer) was monitored for 400 s then Triton X-100 (20 μL of 20 % v:v) was added to lyse the vesicles.
Synthesis37
N-Boc-12-aminododecanoic acid
12-Aminododecanoic acid (1.00 g, 4.65 mmol) was suspended in 15.0 mL of methanol with 0.775 mL (5.58 mmol) of triethylamine and 1.01 g (4.65 mmol) of dissolved di-t-butyl-dicarbonate and heated under a reflux condenser to 60°C overnight. The solvent was evaporated on a rotary evaporator and the residue re-dissolved in ethyl acetate. The mixture was then washed with 0.25 M HCl (2x), dried, filtered and the solvent evaporated to give a white solid. N-Boc-12-aminododecanoic acid (90%) was purified by recrystallization in hexanes. δH(500 MHz; CDCl3; Me4Si) 11.67 (1H, s, COOH), 4.55 (1H, s, Boc NH), 3.10 (2H, quar, J = 6 Hz, Dodec), 2.34 (2H, t, J = 7 Hz, Dodec), 1.62 (2H, quin, J = 7, Dodec), 1.44 (11H, s, Boc + Dodec), 1.27 (16H, s, Dodec). δC(125 MHz; CDCl3; CDCl3) 179.7, 156.2, 79.3, 41.9, 40.8, 34.3, 30.2, 29.63, 29.55, 29.45, 29.38, 29.22, 28.6, 27.0, 24.9.
N-Boc-12-aminododecanol
To a solution of N-Boc-12-aminododecanoic acid (4.22 g, 13.4 mmol) in 100 mL of dry THF, was added Red-Al (12.3 mL, 40.2 mmol-65% weight in toluene) via a syringe over 30 min. Because of the extensive precipitation, a mechanical stirrer was used. The reaction was stirred for 30 min after the precipitate dissolved, then quenched with saturated sodium sulfate and stirred for another 1 h. The solids formed during the quenching process were then filtered, and the solution was concentrated to an oil on a rotary evaporator, re-dissolved in ethyl acetate and washed with water, dried, filtered and evaporated to give the desired product (3.39 g, 84%) as a white solid which was recrystallized in toluene and used without further purification. δH(500 MHz; CDCl3; Me4Si) 4.55 (1H, s, Boc NH), 3.64 (2H, t, J = 7 Hz, Dodec), 3.10 (2H, quar, J = 7 Hz, Dodec), 1.57 (2H, quin, J = 7 Hz, Dodec), 1.44 (11H, s, Boc + Dodec), 1.26 (16H, m, Dodec). δC(125 MHz; CDCl3; CDCl3) 156.2, 79.2, 63.2, 51.3, 40.8, 33.0, 30.2, 29.75, 29.71, 29.60, 29.46, 28.6, 27.0, 25.9.
N-Boc-6-aminohexanol
6-Aminohexanol (1.00 g, 8.50 mmol) was dissolved in 5.00 mL of THF and di-tert-butyl-dicarboxylate (1.86 g, 8.50 mmol) was added. The solution was allowed to stir at room temperature for 3 h, and solvent was removed with a rotary evaporator to give the desired product (99%) as a colorless oil, which crystallized after 2 weeks. The compound was used without further purification. δH(500 MHz; CDCl3; Me4Si) 4.65 (1H, s, Boc NH), 3.62 (2H, t, J = 7 Hz, Hexyl), 3.12 (2H, quar, J = 7 Hz, Hexyl), 2.09 (1H, s, OH), 1.57 (2H, quin, J = 7 Hz, Hexyl), 1.49 (2H, quin, J = 7 Hz, Hexyl), 1.44 (9H, s, Boc), 1.36 (4H, m, Hexyl). δC(125 MHz; CDCl3; CDCl3) 156.3, 79.2, 62.7, 40.5, 32.7, 30.2, 28.6, 26.5, 25.4.
2,5-Dimethoxyterephthaloyl dichloride
This compound (a white solid) was synthesized by a literature method.38 δH(500 MHz; CDCl3; Me4Si) 7.55 (2H, s, Ph), 3.96 (6H, s, Me). δC(125 MHz; CDCl3; CDCl3) 163.8, 152.0, 128.3, 116.6, 57.0.
12-(tert-Butylcarbamoyl)dodecyl-4-nitrophenyl-2,2-diphenylethyl phosphate (9a)
To a stirred solution of tris(p-nitrophenyl) phosphate (200.0 mg; 0.433 mmol) and 2,2-diphenyl ethanol (85.8 mg; 0.433 mmol) in 3 mL of dry chloroform (stabilized with amylenes) was added DBU (68 μL; 0.455 mmol), and the reaction was allowed to stir for 3 h. The reaction was then concentrated to an oil using a rotary evaporator and the residue was re-dissolved in 5.00 mL of dry toluene. N-Boc-12-aminododecanol powder (131 mg; 0.433 mmol) was added to the new solution followed by another 68 μL of DBU and reaction was allowed to stir overnight. The mixture was then diluted with ethyl acetate and washed with 0.25 M HCl. Compound 9a (60%) was purified as a clear viscous oil by column chromatography using chloroform as the eluent. δH(300 MHz; CDCl3; Me4Si) 8.08 (2H, d, J = 9 Hz, PNP), 7.22 (10H, m, DPE), 7.11 (2H, d, J = 9 Hz, PNP), 4.67 (2H, t, J = 7 Hz, DPE), 4.59 (1H, s-br, Boc NH), 4.38 (1H, t, J = 8 Hz, DPE), 3.90 (2H, quar, J = 7 Hz, Dodec), 3.07 (2H, quar, J = 7 Hz, Dodec), 1.58 (2H, quin, J = 7 Hz, Dodec), 1.42 (11H, s, Boc + Dodec), 1.22 (16 Hz, s-br, Dodec). δC(75 MHz; CDCl3; CDCl3) 156.1, 155.4 (d, J = 7 Hz), 144.6, 140.1, 128.8, 128.3, 127.2, 125.6, 120.5, 79.0, 70.7 (d, J = 7 Hz), 69.3 (d, J = 7 Hz), 51.3, 40.7, 30.2, 30.1, 29.59, 29.56, 29.52, 29.36, 29.08, 28.52, 26.9, 25.3
6-(tert-Butylcarbamoyl)hexyl-2,2-diphenylethyl-4-nitrophenyl phosphate (9b)
To a stirred solution of tris(p-nitrophenyl) phosphate (200 mg; 0.433 mmol) and 2,2-diphenyl ethanol (85.8 mg; 0.433 mmol) in 3 mL of dry chloroform (stabilized with amylenes) was added DBU (68 μL; 0.455 mmol), and the reaction was allowed to stir for 3 h. The reaction was then concentrated to an oil using a rotary evaporator and the residue was redissolved in dry toluene. N-Boc-6-aminohexanol (94.0 mg; 0.433 mmol) was added to the new solution followed by another 68 _L of DBU and reaction was allowed to stir overnight. The mixture was then diluted with ethyl acetate and washed with acid. The product (50%) was purified as a clear viscous oil by column chromatography using chloroform as the eluent. δH(500 MHz; CDCl3; Me4Si) 8.08 (2H, d, J = 9 Hz, PNP), 7.25 (10H, m, Ph), 7.11 (2H, d, J = 9 Hz, PNP), 4.95 (1H, t, J = 7 Hz, PNP), 4.68 (2H, t, J = 7 Hz, Boc NH), 4.38 (1H, t, J = 7 Hz, DPE), 3.97 (2H, quar, J = 7 Hz, Hexyl), 3.15 (2H, quar, J = 7 Hz, Hexyl), 1.55 (4H, m, Hexyl) 1.41 (9H, s, Boc), 1.29 (4H, m, Hexyl).
General procedure for the synthesis of compounds 1PNP – 5PNP
The appropriate Boc protected amine was dissolved in the minimal amount of dichloromethane, and then diluted with trifluoroacetic acid to double the volume. The solution was stirred for 20 min after gas evolution stopped and the solvent was removed with a rotary evaporator. The residue was then dissolved in methanol with 0.5 mL of added concentrated HCl and evaporated (achieving ion exchange). The residue was then evaporated twice with toluene and dissolved in dry chloroform. Added to this mixture was the corresponding bis-acid chloride (0.5 molar equivalents). Triethylamine (two molar equivalents with respect to the amine) was first diluted with chloroform and added to the reaction with a dropping funnel over 1 h. The reaction was stirred for 2 h after last addition of base, and the product was purified by chromatography (all 5 compounds required 0−3% methanol in dichloromethane as the eluent).
Bis-N,N-(p-Nitrophenyl-2,2-diphenylethyl-12-aminododecyl phosphate)-fumaramide (1PNP)
The product was a white solid (70%). δH(500 MHz; CDCl3; Me4Si) 8.10 (4H, d, J = 9 Hz, PNP), 7.45 (2H, t, J = 6 Hz, Fu NH), 7.23 (20H, m, Ph), 7.18 (2H, s, Fu CH), 7.12 (4H, d, J = 9 Hz, PNP), 4.69 (4H, t, J = 7 Hz, DPE), 4.40 (2H, t, J = 7 Hz, DPE), 3.99 (4H, quar, J = 7 Hz, Dodec), 3.33 (4H, quar, J = 7 Hz, Dodec), 1.58 (8H, quin, J = 7 Hz, Dodec), 1.23 (32H, m, Dodec). δC(125 MHz; CDCl3; CDCl3) 164.9, 155.4 (d, J = 7 Hz), 144.6, 140.1, 133.3, 128.8, 128.3, 127.2, 125.6, 120.6, 70.7 (d, 7 Hz), 69.3 (d, 7 Hz), 51.4, 40.0, 30.16, 30.11, 29.65, 29.62, 29.55, 29.51, 29.46, 29.1, 27.2, 25.4.
Bis-N,N-(p-Nitrophenyl-2,2-diphenylethyl-12-aminododecyl phosphate)-2,5-dimethoxy–terephthalamide (3PNP)
The product was a white solid (75%). δH(500 MHz; CDCl3; Me4Si) 8.11 (4H, d, J = 9 Hz, PNP), 8.10 (2H, br s, Tera NH), 7.89 (2H, s, Tera Ph), 7.27 (20H, m, Ph), 7.13 (4H, d, J = 9 Hz, PNP), 4.69 (4H, t, J = 7 Hz, DPE), 4.40 (2H, t, J = 7 Hz, DPE), 4.00 (4H, quar, J = 7 Hz, Dodec), 3.99 (6H, s, Tera Me), 3.46 (4H, quar, J = 7 Hz, Dodec), 1.61 (8H, m, Dodec), 1.37 (8H, m, Dodec), 1.24 (24H, m, Dodec). δC(125 MHz; CDCl3; CDCl3) 164.3, 155.5 (d, J = 7 Hz), 151.6, 144.7, 140.1, 128.9, 128.3, 127.3, 125.7, 124.7, 120.6, 115.4, 70.7 (d, J = 7 Hz), 69.3 (d, J = 7 Hz), 56.7, 51.42, 51.37, 40.1, 30.2, 30.2, 29.73, 29.67, 29.63, 29.5, 29.2, 27.3, 25.4.
Bis-N,N-(p-Nitrophenyl-2,2-diphenylethyl-12-aminododecyl phosphate)-2,6-pyridine dicarbamide (4PNP)
The product was a white solid (78%). δH(500 MHz; CDCl3; Me4Si) 8.33 (2H, d, J = 8 Hz, Py), 8.18 (2H, t, J = 6 Hz, Py NH), 8.11 (4H, d, J = 9 Hz, PNP), 7.98 (1H, t, J = 8 Hz, Py), 7.25 (20H, m, Ph), 7.11 (4H, d, J = 9 Hz, PNP), 4.69 (4H, t, J = 7 Hz, DPE), 4.40 (2H, t, J = 7 Hz, DPE), 3.98 (4H, quar, J = 7 Hz, Dodec), 3.41 (4H, quar, J = 7 Hz, Dodec), 1.59 (8H, m, Dodec), 1.24 (32H, m, Dodec).
Bis-N,N-(p-Nitrophenyl-2,2-diphenylethyl-6-aminohexyl phosphate)-2,6-pyridine dicarbamide (5PNP)
The product was a white solid (70%). δH(500 MHz; CDCl3; Me4Si) 8.34 (2H, d, J = 8 Hz, Py NH), 8.18 (2H, t, J = 6 Hz, Py), 8.10 (4H, d, J = 9 Hz, PNP), 8.00 (1H, t, J = 8 Hz, Py), 7.27 (8H, m, Ph), 7.22 (12H, m, Ph), 7.12 (4H, d, J = 9 Hz, PNP), 4.69 (4H, t, J = 7 Hz, DPE), 4.40 (2H, t, J = 7 Hz, DPE), 3.98 (4H, quar, J = 7 Hz, Hexyl), 3.41 (4H, quar, J = 7 Hz, Hexyl), 1.58 (8H, m, Dodec), 1.29 (8H, m, Hexyl). δC(125 MHz; CDCl3; CDCl3) 163.7, 155.3 (d, J = 6 Hz), 149.0, 144.6, 139.9, 139.0, 128.8, 128.2, 127.2, 125.6, 125.0, 120.5, 70.7 (d, J = 6 Hz), 68.9 (d, J = 6 Hz), 51.3, 39.5, 29.9, 29.8, 26.3, 24.9.
Bis-N,N-(2,2-Diphenylethyl-12-aminododecyl phosphate)-fumaramide (1)
Compound 1PNP (200 mg, 0.161 mmol) was dissolved in 10.0 mL of DMSO and 1.0 mL of 1.0 M LiOH solution was added. Reaction was stirred for 1 h and the DMSO solution was added to 50 mL of HCl (0.25 M). The pure product immediately precipitated out of solution as a white solid, was filtered, and washed with water to give a yield of 50%. δH(500 MHz; CDCl3/CD3OD 1:1; Me4Si) 7.26 (20H, m, Ph), 6.81 (2H, s, Fu CH), 4.51 (4H, t, J = 7 Hz, DPE), 4.37 (2H, t, obscured by solvent peak, DPE), 3.75 (4H, quar, J = 7 Hz, Dodec), 3.28 (4H, t, J = 7 Hz, Dodec), 1.54 (4H, m, Dodec), 1.25 (36H, m, Dodec). δC(125 MHz; CDCl3/CD3OD 1:1; CDCl3) 165.3, 140.7, 132.5, 128.5, 128.2, 126.8, 69.0 (d, J = 7 Hz), 67.4 (d, J = 7 Hz), 51.2, 39.8, 30.1, 30.0, 29.44, 29.40, 29.22, 29.04, 28.99, 26.9, 25.3. FAB-MS (NBA matrix): m/z 1004 [M+H]+.
Bis-N,N-(2,2-Diphenylethyl-12-aminododecyl phosphate)-2,5-dimethoxyterephthalamide (3)
Compound 3PNP (200 mg, 0.148 mmol) was dissolved in 10 mL of DMF and 1.0 mL of 1.0 M LiOH was added. The reaction was allowed to stir for 1 h and compound 3 was purified by diluting the reaction mixture with water and washing with chloroform, separating and acidifying the organic layer, and then drying on a high vacuum line (65%). δH(500 MHz; CDCl3; Me4Si) 8.09 (2H, t, J = 6 Hz, Tera NH), 7.88 (2H, s, Tera CH), 7.23 (20H, m, Ph), 4.47 (4H, t, J = 7 Hz, DPE), 4.34 (2H, t, J = 7 Hz, DPE), 3.96 (6H, s, Tera Me), 3.72 (4H, quar, J = 7 Hz, Dodec), 3.47 (4H, quar, J = 7 Hz, Dodec), 1.61 (4H, quin, J = 7 Hz, Dodec), 1.47 (4H, m, Dodec), 1.25 (32H, m, Dodec). δC(125 MHz; CDCl3; CDCl3) 164.4, 151.6, 140.9, 128.7, 128.5, 127.0, 124.7, 115.5, 69.2, 67.7, 56.8, 51.4, 51.3, 40.0, 30.23, 30.17, 29.50, 29.46, 29.28, 29.14, 27.1, 25.4. FAB-MS (NBA matrix): m/z 1114 [M+H]+.
Bis-N,N-(2,2-Diphenylethyl-12-aminododecyl phosphate)-2,6-pyridine dicarboxamide (4)
Compound 4PNP (300 mg, 0.231 mmol) was dissolved in 10 mL of DMF and 1.0 mL of 1.0 M LiOH was added. The reaction was allowed to stir for 1 h, then 1 mL of 2.5 M HCl was added and the resulting solution was concentrated on a rotary evaporator. The residue was washed with water (2x) and then washed with anhydrous diethyl ether (3x) to give 4 (50%). δH(500 MHz; CDCl3/CD3OD 3:1; Me4Si) 8.28 (2H, d, J = 8 Hz, Py), 8.01 (1H, t, J = 8 Hz, Py), 7.22 (20H, m, Ph), 4.51 (4H, t, J = 8 Hz, DPE), 4.37 (2H, t, J = 8 Hz, DPE), 3.75 (4H, quar, J = 7 Hz, Dodec), 3.44 (4H, t, J = 7 Hz, Dodec), 1.66 (4H, quin, J = 8 Hz, Dodec), 1.53 (4H, quin, J = 7 Hz, Dodec), 1.35 (8H, m, Dodec), 1.24 (24H, m, Dodec). δC(125 MHz; CDCl3/CD3OD 3:1; CDCl3) 164.2, 148.8, 140.7, 138.9, 128.4, 128.1, 126.7, 124.6, 69.0 (d, J = 7 Hz), 67.3 (d, J = 7 Hz), 51.2, 39.6, 30.1, 30.0, 29.53, 29.48, 29.42, 29.40, 29.31, 29.0, 27.0, 25.3. FAB-MS (NBA matrix): m/z 1055 [M+H]+, 1077 [M+Na]+.
Bis-N,N-(2,2-Diphenylethyl-6-aminohexyl phosphate)-2,6-pyridine dicarbamide (5)
Compound 5PNP (200 mg, 0.177 mmol) was dissolved in 10 mL of DMF and 1.0 mL of 1.0 M LiOH solution was added. The solution was allowed to stir at room temperature for 1 h and was then concentrated on a rotary evaporator. The residue was dissolved in the minimum amount of water and the bis-lithium phosphate salt of the product was driven out of solution with acetone. The mother liquor was decanted and the remaining oily residue was dissolved in methanol with 3 drops of concentrated HCl solution and the solution evaporated to dryness. The product 5 was selectively extracted into 1:1 methanol/chloroform and regained as an off-white solid after filtration and solvent evaporation (40%). δH(500 MHz; CD3OD; CHD2OD) 8.34 (2H, d, J = 7 Hz, Py), 8.21 (1H, t, J = 7 Hz, Py), 7.25 (20H, m, Ph), 4.45 (4H, t, J = 7 Hz, DPE), 4.33 (2H, t, J = 7 Hz, DPE), 3.75 (4H, quar, J = 6 Hz, Hexy), 3.50 (4H, t, J = 6 Hz, Hexyl), 1.67 (4H, s, Hexyl), 1.55 (4H, s, Hexyl), 1.37 (8H, s, Hexyl). δC(125 MHz; CD3OD; CD3OD) 166.4, 150.3, 142.4, 129.7, 129.5, 128.2, 128.0, 125.7, 70.4, 68.4, 52.8 (d, J = 7 Hz), 40.9, 31.1, 30.4, 27.4, 26.2. FAB-MS (NBA matrix): m/z 925 [M+K]+.
12-(tert-Butylcarbamoyl)dodecyl-2,2-diphenylethyl phosphate (6)
Compound 6PNP was dissolved in 10 mL of DMF and 1.0 mL of 1.0 M LiOH was added. The reaction was allowed to stir for 1 h and was then concentrated on a rotary evaporator. The residue was dissolved in ethyl acetate and washed with 0.25 M NaOH (3x), and then with 0.25 M HCl (2x). The organic layer was dried with magnesium sulfate, filtered, and evaporated to give compound 6 as clear oil (65%). δH(300 MHz; CDCl3; Me4Si) 7.76 (1H, br s, Phos OH), 7.20 (10H, m, Ph), 4.46 (2H, t, J = 7 Hz, DPE), 4.35, (1H, t, J = 7 Hz, DPE), 3.70 (2H, quar, J = 7 Hz, Dodec), 3.09 (2H, t, J = 7 Hz, Dodec), 1.47 (4H, m, Dodec), 1.44 (9H, s, Dodec), 1.22 (16H, m, Dodec). δC(75 MHz; CDCl3; CDCl3) 141.1, 128.7, 128.5, 126.9, 126.2, 69.1, 67.5, 51.4, 51.3, 40.8, 30.4, 30.3, 30.2, 29.7, 29.5, 29.4, 28.6, 27.0, 25.6, 25.1. FAB-MS (NBA matrix): m/z 460 [M-Boc+H]+.
Rotaxane 2PNP
To a solution of 1PNP and 10 equivalents triethylamine in dry chloroform (stabilized with amylenes) was added simultaneously using a dual syringe pump, a chloroform solution of p-xylylene diamine and a chloroform solution of 5-tert-butyl-isophthaloyl dichloride. The resulting precipitate was filtered through celite and the solvent was evaporated using a rotary evaporator. The product was purified by chromatography using 3% methanol in dichloromethane as the mobile phase to give 2PNP as a white solid (15%). δH(300 MHz; CDCl3; Me4Si) 8.45 (2H, s, Wh Iso), 8.21 (4H, s, Wh Iso), 8.06 (4H, d, J = 9 Hz, PNP), 7.69 (4H, t, J = 5 Hz, Wh NH), 7.25 (20H, m, Ph), 7.11 (4H, d, J = 9 Hz, PNP), 7.05 (8H, s, Wh Xy), 6.40 (2H, t, J = 5 Hz, Th NH), 5.72 (2H, s, Th alkene), 4.67 (4H, t, J = 7 Hz, DPE), 4.47, (8H, d, J = 5 Hz, Wh Benz), 4.38 (2H, t, J = 7 Hz, DPE), 3.98 (4H, quar, J = 7 Hz, Dodec), 3.14 (4H, quar, J = 7 Hz, Dodec), 1.51 (8H, m, Dodec), 1.37 (18H, s, t-Bu), 1.22 (32H, m, Dodec).
Rotaxane (2)
Compound 2PNP (100 mg, 0.053 mmol) was dissolved in 10 mL of DMSO and 1.0 mL of 1.0 M LiOH solution was added. The reaction was stirred for 1 h and the DMSO solution was added to 50 mL of HCl (0.25 M). The product, compound 2, precipitated out of solution as a white solid, was filtered, and washed with water three times (50%). Yield = 50% 1H NMR δH(500 MHz, 3:1 CDCl3/CD3OD) 8.60 (s, 2H, Wh Iso), 8.25 (s, 4H, Wh Iso), 7.26 (m, 16H, Ph), 7.20 (m, 4H, Ph), 7.04 (s, 8H, Wh Xy), 5.68 (s, 2H, Th alkene), 4.49 (t, 4H, J = 7 Hz, DPE), 4.45 (s, 8H, Wh CH2), 4.36 (t, 2H, J = 7 Hz, DPE), 3.73 (quar, 4H, J = 7 Hz, Dodec), 3.11 (t, 4H, J = 7 Hz, Dodec), 1.51 (m, 4H, Dodec), 1.43 (s, 18H, t-Bu), 1.26 (m, 36H, Dodec). δC(125 MHz, CDCl3/CD3OD 3:1) δ 167.6, 165.6, 152.8, 140.9, 136.9, 133.4, 129.7, 128.9, 128.5, 128.4, 128.2, 126.7, 122.2, 68.8, 67.1, 51.3, 43.8, 39.8, 35.1, 31.1, 30.2, 30.1, 29.6, 29.5, 29.45, 29.44, 29.28, 29.09, 27.0, 25.4. FAB-MS (NBA matrix): m/z 1647 [M+H]+, 1669 [M+Na]+.
Supplementary Material
Acknowledgments
This work was supported by the NIH, and the University of Notre Dame.
Footnotes
Supporting Information Available: 1H NMR spectra of synthetic amphiphiles 1−6. This material is available free of charge via the Internet at http://pubs.acs.org.
Reference
- 1.Fuhrhop J-H, Wang T. Chem. Rev. 2004;104:2901–2937. doi: 10.1021/cr030602b. and references therein. [DOI] [PubMed] [Google Scholar]
- 2.Boalaamphiphiles self-assemble into a range of morphologies; for a discussion, see: Köhler K, Forster G, Hauser A, Dobner B, Heiser UF, Ziethe F, Richter W, Steiniger F, Drechsler M, Stettin H, Blume A. J. Am. Chem. Soc. 2004;126:16804–16813. doi: 10.1021/ja046537k. and references therein.
- 3.Bolaamphiphiles can even form stable membranes in pure ethanol. Wang X, Liang Y. J. Colloid Interface Sci. 2001;233:364–366. doi: 10.1006/jcis.2000.7245.
- 4.De Rosa M, Gambacorta A, Gliozzi A. Microbiol. Rev. 1986;50:70–80. doi: 10.1128/mr.50.1.70-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan Q, Relini A, Cassinadri D, Gambacorta A, Gliozzi A. Biochim. Biophys. Acta. 1995;1240:83–88. doi: 10.1016/0005-2736(95)00157-x. [DOI] [PubMed] [Google Scholar]
- 6.Kai T, Sun X-L, Faucher KM, Apkarian RP, Chaikof EL. J. Org. Chem. 2005;70:2606–2615. doi: 10.1021/jo048167o. [DOI] [PubMed] [Google Scholar]; Halter M, Nogata Y, Dannenberger O, Sasaki T, Vogel V. Langmuir. 2004;20:2416–2423. doi: 10.1021/la035817v. [DOI] [PubMed] [Google Scholar]
- 7.Examples of bolaamphiphiles as ion channels include: Cameron LM, Fyles TM, Hu C-W. J. Org. Chem. 2002;67:1548–1553. doi: 10.1021/jo0160930. Goto C, Yamamura M, Satake A, Kobuke Y. J. Am. Chem. Soc. 2001;123:12152–12159. doi: 10.1021/ja010761h. Leevy WM, Huettner JE, Pajewski R, Schlesinger PH, Gokel GW. J. Am. Chem. Soc. 2004;126:15747–15753. doi: 10.1021/ja046626x. Gokel GW, Murillo O. Acc. Chem. Res. 1996;29:425–432. For bolaamphiphiles as membrane disruptors, see: Naka K, Sadownik A, Regen SL. J. Am. Chem. Soc. 1993;115:2278–2286.
- 8.Gu Q, Zou A, Yuan C, Guo R. J. Colloid Interface Sci. 2003;266:442–447. doi: 10.1016/s0021-9797(03)00649-0. [DOI] [PubMed] [Google Scholar]
- 9.For recent measurements of translocation rates with unlabeled phospholipids, see: Liu J, Conboy JC. Biophys. J. 2005;89:2522–2532. doi: 10.1529/biophysj.105.065672.
- 10.Phospholipid translocation is disfavored enthalpically but that it can be favored entropically, presumably due to the release of solvating water molecules. Homan R, Pownall H. J. Biochim. Biophys. Acta. 1988;938:155–166. doi: 10.1016/0005-2736(88)90155-1.
- 11.a Fattal E, Nir S, Parente RA, Szoka FC. Biochemistry. 1994;33:6721–6731. doi: 10.1021/bi00187a044. [DOI] [PubMed] [Google Scholar]; b Matsuzaki K, Murase O, Fujii N, Miyajima K. Biochemistry. 1996;35:11361–11368. doi: 10.1021/bi960016v. [DOI] [PubMed] [Google Scholar]
- 12.Smith BD, Lambert TN. Chem. Commun. 2003:2261–2268. doi: 10.1039/b303359g. [DOI] [PubMed] [Google Scholar]
- 13.Boon JM, Lambert TN, Sisson AL, Davis AP, Smith BD. J. Am. Chem. Soc. 2003;125:8195–8201. doi: 10.1021/ja029670q. [DOI] [PubMed] [Google Scholar]
- 14.Boon JM, Smith BD. Med. Res. Rev. 2002;22:251–281. doi: 10.1002/med.10009. [DOI] [PubMed] [Google Scholar]
- 15.Shukla R, Sasaki Y, Krchnak V, Smith BD. J. Comb. Chem. 2004;6:703–709. doi: 10.1021/cc049942g. [DOI] [PubMed] [Google Scholar]
- 16.Kalinowski S, Lotowski Z, Morzycki JW. Cell. Mol. Biol. Lett. 2000;5:107–118. [Google Scholar]
- 17.Wang GJ, Hollingsworth RI. J. Org. Chem. 1999;64:4140–4147. doi: 10.1021/jo9818226. [DOI] [PubMed] [Google Scholar]
- 18.Yan Y, Huang J, Li Z, Ma J, Fu H, Ye J. J. Phys. Chem. B. 2003;107:1479–1482. [Google Scholar]
- 19.Kol MA, de Kroon AIPM, Killian JA, de Kruijff B. Biochemistry. 2004;43:2673–2681. doi: 10.1021/bi036200f. [DOI] [PubMed] [Google Scholar]; Kol MA, van Laak ANC, Rijkers DTS, Killian JA, de Kroon AIPM, de Kruijff B. Biochemistry. 2003;42:231–237. doi: 10.1021/bi0268403. [DOI] [PubMed] [Google Scholar]; Kol MA, de Kroon AIPM, Rijkers DTS, Killian JA, de Kruijff B. Biochemistry. 2001;40:10500–10506. doi: 10.1021/bi010627+. [DOI] [PubMed] [Google Scholar]
- 20.A single pass helical peptide is thicker than the single chain bolaamphiphiles described in this paper but their shapes are similar in that they are both relatively narrow rods that bisect the membrane and wobble.
- 21.Gatti FG, Leigh DA, Nepogodiev SA, Slawin AMZ, Teat SJ, Wong JKY. J. Am. Chem. Soc. 2001;123:5983–5989. doi: 10.1021/ja001697r. [DOI] [PubMed] [Google Scholar]
- 22.A phosphodiester end group with a single phenyl ring is not large enough to prevent unthreading of the macrocycle/thread components in rotaxane 2.
- 23.Polar aromatic groups such as the side chains of tryptophan and tyrosine are known to prefer the interfacial regions of a bilayer membrane, but phenyl groups tend to bury into the membrane interior. Stahelin RV, Cho W. Biochemistry. 2001;40:472–4678. doi: 10.1021/bi0020325. Nonetheless, preorganized hosts with multiple aromatic rings are known to have moderate affinities for the quaternary ammonium cation in the phosphocholine head group. Lambert TN, Smith BD. Coord. Chem. Rev. 2003;240:129–141.. Therefore, it is hard to predict the preferred membrane locations of the diphenylethane end groups in these bolaamphiphiles.
- 24.Moss RA, Li JM. J. Am. Chem. Soc. 1992;114:9227–9229. For additional discussion of the factors controlling translocation of bolamphiphiles, see: Moss RA, Li G, Li J-M. J. Am. Chem. Soc. 1994;116:805–806.
- 25.The half-lives listed in Table 1 indicate that the monoanionic probe NBD-PG is always translocated faster than the zwitterionic NBD-PC, a preference that has been noted in previous studies.13,19 It is not clear if the NBD-PG translocates as an anion or as the neutral acid. Recent work indicates that organic anions may be more permeable than predicted by the pH-partition hypothesis. See: Thomae AV, Wunderli-Allenspach H, Krämer SD. Biophys. J. 2005;89:1802–1811. doi: 10.1529/biophysj.105.060871.
- 26.Previous studies by our group have evaluated small organic molecules that were specifically designed to promote phospholipid flip-flop. These compounds form hydrogen bonded complexes with the polar head group of the phospholipid and the complexes diffuse across the membrane. With these mobile transport carriers the half-lives for NBD-PC and NBD-PG translocation are around 4−30 min and <1 min, respectively. Compared to the bolaamphiphiles reported here, these mobile transport carriers transport NBD-PC equally as well, but they transport NBD-PG almost ten times better.
- 27.a Wu Z-Q, Jiang X-K, Zhu S-Z, Li Z-T. Org. Lett. 2004;6:229–232. doi: 10.1021/ol036108b. [DOI] [PubMed] [Google Scholar]; b Parra RD, Zeng H, Zhu J, Zheng C, Zeng XC, Gong B. Chem.-Eur. J. 2001;7:4352–4357. doi: 10.1002/1521-3765(20011015)7:20<4352::aid-chem4352>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]; c Ernst JT, Becerril J, Park HS, Yin H, Hamilton AD. Angew. Chem. Int. Ed. Engl. 2003;42:535–539. doi: 10.1002/anie.200390154. [DOI] [PubMed] [Google Scholar]; d Yin H, Lee G, Sedey KA, Rodriguez JM, Wang H-G, Sebti SM, Hamilton AD. J. Am. Chem. Soc. 2005;127:5463–5468. doi: 10.1021/ja0446404. [DOI] [PubMed] [Google Scholar]
- 28.The passive permeation of small molecules through a bilayer increases when gel and fluid domains coexist, apparently due to membrane defects at the phase boundaries. Clerc SG, Thompson TE. Biophys. J. 1995;68:2333–2341. doi: 10.1016/S0006-3495(95)80415-7.
- 29.Shoemaker SD, Vanderlick TK. Biophys. J. 2003;84:998–1009. doi: 10.1016/s0006-3495(03)74916-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolaamphiphiles that adopt U-shaped conformations tend to disrupt the membrane so much that salt transport occurs. See, for example: Fyles TM, Zeng B. J. Org. Chem. 1998;63:8337–8345.Fuhrhop JH, Krull M, Schulz A, Möbius D. Langmuir. 1990;6:497–505.
- 31.Another bolaamphiphile motion that may promote phospholipid flip-flop is the interconversion between transmembrane and U-shaped bolaamphiphile orientation (Figure 5). Even if this bolaamphiphile interconversion process is quite slow, it may induce a local defect that lives long enough to permit the translocation of many phospholipids. Since transverse phospholipid diffusion is very rapid, a small but long-lived local defect may be an effective short-circuit point for large amounts of rapid transmembrane flip-flop.
- 32.Affeld A, Hübner GM, Seel C, Schalley CA. Eur. J. Org. Chem. 2001:2877–2890. [Google Scholar]
- 33.Membrane additives that increase the lateral pressure at the membrane surface are known to increase the rate of phospholipid translocation (Bootsveld, A.; Degenhardt, R.; Kamp, D.; Haest, C. W. M. Mol. Membr. Biol. 2004, 21, 315−322). Thus, it is possible that bolaamphiphiles with large hydrophilic end-groups (compared to the cross-sectional area of the linking chain) may lower the barrier for translocation. However, the inactivity of controls 5 and 6 suggests that this is not a dominant factor in the present case.
- 34.Gummadi SN, Kumar KS. Cell. Mol. Biol. Lett. 2005;10:101–121. [PubMed] [Google Scholar]
- 35.Vishwakarma RA, Vehring S, Mehta A, Sinha A, Pomorski T, Herrmann A, Menon AK. Org. Biomol. Chem. 2005;3:1275–1283. doi: 10.1039/b500300h. [DOI] [PubMed] [Google Scholar]
- 36.That is not to say that all translocase proteins in the bacterial membrane are single pass helical structures. For example, the wzxE translocase is a large protein with multiple transmembrane domains. Rick PD, Barr K, Sankaran K, Kajimura J, Rush JS, Waechter CJ. J. Biol. Chem. 2003;278:16534–16542. doi: 10.1074/jbc.M301750200.
- 37.The NMR data includes magnetic field strength, solvent, and chemical shift reference. The 13C NMR signals are quoted to two decimal places only in spectral regions that are crowded.
- 38.Sotzing GA, Reynolds JR, Katritzky AR, Soloducho J, Belyakov S, Musgrave R. Macromolecules. 1996;29:1679–1684. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.