Abstract
Antegrade cerebral perfusion (ACP) is a cardiopulmonary bypass technique that uses special cannulation procedures to perfuse only the brain during neonatal and infant aortic arch reconstruction. It is used in lieu of deep hypothermic circulatory arrest (DHCA), and thus has the theoretical advantage of protecting the brain from hypoxic ischemic injury. Despite this, recent comparative studies have demonstrated no difference in neurodevelopmental outcomes with ACP vs. DHCA for neonatal arch repair. This article presents animal and human data demonstrating that ACP flows less than 30 ml/kg/min are inadequate for many patients, and may be the explanation for lack of outcome difference vs. DHCA. A technique for ACP, its physiologic basis, and a neuromonitoring strategy are presented, and then the results of an outcome study are reviewed demonstrating that with ACP technique at higher flows of 50–80 ml/kg/min guided by neuromonitoring, periventricular leukomalacia (PVL) is eliminated on postoperative brain MRI after neonatal cardiac surgery.
Introduction
Antegrade cerebral perfusion (ACP), also known as regional low-flow cerebral perfusion (RLFP), selective cerebral perfusion (SCP), low-flow cerebral perfusion (LFCP), continuous cerebral perfusion (CCP) or regional cerebral perfusion (RCP) was devised as a perfusion method that could minimize or eliminate deep hypothermic circulatory arrest (DHCA) during aortic arch reconstruction in neonates. Although earlier descriptions were published (1), the paper by Pigula et al in 1999 (2) described the technique as it is used in most centers. This group also demonstrated that in addition to providing cerebral oxygenation, ACP provided blood flow to sub diaphragmatic organs as well (3). Potential benefits of this technique became more apparent after publication of the 8 year Boston Circulatory Arrest Study follow-up data, demonstrating that a “cut point” of 41 minutes of DHCA existed beyond which neurodevelopmental abnormalities became significantly more prevalent (4). Because the median of DHCA times for the Norwood Stage I palliation for hypoplastic left heart syndrome (HLHS) in papers from 2000-05 was 50 minutes (5–7), an additional theoretical benefit of ACP is to provide the surgeon adequate time to create an adequate aortic reconstruction. This premise is validated by the reported ACP times of 40–70 minutes in subsequent publications (8,9).
Contemporary techniques for ACP have varied significantly. As noted elsewhere in this monograph, there are a variety of cannulation techniques (see Chapter 8: Hanley FL. Strategy for Achieving Antegrade Cerebral Perfusion –Cannulation Technique). ACP bypass flow rates in published papers have varied from 20–94 ml/kg/min (8–12). Monitoring techniques during ACP have varied from none (11), to arterial pressure only (12), to near infrared spectroscopy (NIRS) for cerebral oxygenation (3), and also transcranial Doppler ultrasound (TCD)(8,9).
Despite the theoretical advantage of ACP in providing cerebral blood flow and oxygenation during aortic arch reconstruction at a time when DHCA would traditionally be utilized, the recent outcome studies comparing neurodevelopmental outcomes at 1 year with DHCA vs. ACP have revealed no differences (11,12). Lack of standardization in cannulation, ACP flow rates, and cerebral physiological monitoring have, in our view, prevented meaningful comparisons of these techniques. In this paper we hypothesize that most of the previously published ACP studies, including the outcome studies, have used ACP flow rates that are inadequate for many patients, and indeed may be worse than conventional DHCA. To argue for this hypothesis we review our own, and other groups’ cerebral physiologic monitoring data using both NIRS and TCD. We then review the available animal model data. Finally, we detail our own outcome study, utilizing brain MRI changes before and after neonatal aortic arch reconstruction, to demonstrate improved early brain outcomes when adequate ACP flow rates are utilized.
Details of ACP Technique
The original description of ACP by Pigula et al (2) is illustrated in Figure 1. A 3.5 mm polytetrafluoroethylene (PTFE) graft is sewn to the right innominate artery with fine prolene sutures, the brachiocephalic vessels and descending thoracic aorta are snared, and flow is provided to the brain via the right innominate and right vertebral arteries (arising from the base of the right subclavian artery, which is above the snare) only. This group was the first to prove that the brain was oxygenated during this technique, and also demonstrated both blood pressure and flow to the subdiaphragmatic viscera during this technique via the extensive system of collateral arterial supply in neonates. This flow and oxygenation to the lower body is lower than normal bypass flows (13), but nonetheless demonstrates another potential advantage of ACP over DHCA. As detailed elsewhere in this monograph, cannulation technique is critical, because other techniques of ACP cannulation involving direct cannulation of the right innominate artery, with snaring that excludes the subclavian-vertebral artery, could theoretically result in less perfusion to the brain. Our cannulation and ACP flow technique is a modification of the Pigula description, details noted in Table 1.
Figure 1.
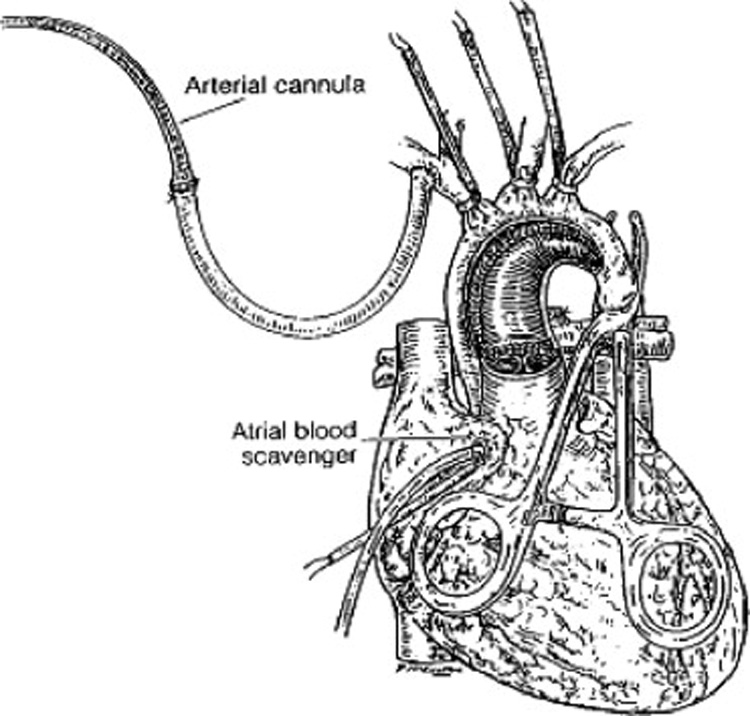
Depiction of the operative field during aortic arch reconstruction with antegrade cerebral perfusion (ACP). Arterial inflow is through the cannulated 3.5 mm polytetrafluoroethylene (PTFE) graft after the anastomosis to the innominate artery. Exposure is maintained by the brachiocephalic snares and a snare or clamp on the descending aorta, and the right atrial cannula. The PTFE graft will admit an 8 or 10 french aortic cannula. Reproduced with permission from Pigula et al. (3)
Table 1.
Texas Children’s Hospital Antegrade Cerebral Perfusion Technique
| 1. | 3.5 mm PTFE graft to right innominate artery, after 100 units/kg heparin, with 8–0 prolene sutures |
| 2. | 10 fr standard aortic cannula to distal end of graft |
| 3. | Single atrial or bicaval cannulation |
| 4. | Use bilateral bifrontal NIRS and TCD through anterior fontanelle or temporal window for cerebral physiological monitoring. |
| 5. | Establish baseline mean cerebral blood flow velocity using TCD, and rSO2 using NIRS, at 18–22° C, at full flow CPB: 150 ml/kg/min; MAP 30–35 mm Hg; utilize α-receptor blockade with phentolamine or phenoxybenzamine if necessary. (rSO2normally 90–95% bilaterally, mean CBFV normally 18–25 cm/sec). |
| 6. | Use pH stat management, hct 30–35, all phases of CPB. |
| 7. | ACP initiated after brief DHCA for atrial septectomy for Norwood: all brachiocephalic vessels and descending thoracic aorta snared; temperature always 18° C: begin at 37.5 ml/kg/min. |
| 8. | Adjust ACP flow using TCD to achieve CBFV within ±10% of baseline at full CPB flow. |
| 9. | rSO2 should be within ±10% of baseline, or 90–95% bilaterally; if left rSO2 falls to more than 10% below right, increase ACP flow. |
Abbreviations: PTFE, polytetrafluroethylene; NIRS, near-infrared spectroscopy; TCD, transcranial Doppler; rSO2, regional brain oxygen saturation; CPB; cardiopulmonary bypass; MAP, mean arterial pressure; CBFV; cerebral blood flow velocity; ACP, antegrade cerebral perfusion; DHCA, deep hypothermic circulatory arrest
The critical differences in our technique relate to cerebral physiologic monitoring and ACP flow rates. We utilize bifrontal NIRS because both we and others have demonstrated that during ACP, the left side of the brain may develop significantly lower regional oxygen saturation (rSO2) than the right side, presumably because all flow to the left side during ACP must occur via the Circle of Willis, and because of known anatomic variations, obstruction to left sided venous drainage, or positioning of the patient. (8,9,14). This occurs in approximately 50% of patients, and rSO2 differences as large as 30% have been described.(14). Thus, without left sided monitoring, undetected left sided cerebral desaturation may occur. In addition, the TCD is used to guide ACP flow rates, to match cerebral blood flow velocity (CBFV) at full flow hypothermic baseline, before ACP. This is also a critical point, as the maximal rSO2 scale value is 95%, and the TCD monitoring should prevent excessive flow. Another important point is that we have demonstrated that when NIRS and TCD are utilized to guide ACP flow, left or right radial artery pressure do not correlate with the flow required; in other words simply increasing flow to a predetermined radial artery pressure will not suffice for many patients. We also noted that during ACP performed in this manner, the mean pressure in those patients with right radial artery catheters was approximately 10 mm Hg higher than those with left radial artery catheters.(8,9,14). Using the above noted techniques, ACP flow rates in our institution vary from 24–94 ml/kg/min, with a median value of 63 and 64 ml/kg/min in our published studies. As noted in the review below, this median value is significantly higher than other reports.
Disadvantages of the Texas Children's Hospital monitoring system include the fact that we monitor a small frontal cerebral sample volume with NIRS, meaning other portions of the brain may not be receiving adequate flow and are unmonitored. The addition of TCD for the anterior and middle cerebral artery territory should ensure monitoring of larger territory of the brain, however. In practice the TCD window may be difficult to maintain, and TCD may not be available at some centers. In lieu of TCD, we advocate bilateral NIRS monitoring, with a goal to keep both sides at rSO2 of 90–95%. If the left side decreases to >10% below the right, or <80–85%, we advocate maneuvers to increase left sided rSO2, including higher flows and hematocrits. One reason for the higher required flows in our institution may be the routine use of α-receptor blockade using phenoxybenzamine or phentolamine, increasing the volume of non-cerebral tissue beds perfused during this technique.
Review of ACP Publications
In the following review of ACP publications (Table 2), the technique of cannulation for ACP is identical to the description by Pigula et al (2) unless otherwise noted. In the Pigula et al series of 6 Norwood patients, ACP flow was initiated at 5 ml/kg/min, and increased gradually to 20 ml/kg/min. They utilized cerebral NIRS and found that rSO2 increased back to baseline of 90% at this level of flow. They further utilized a NIRS research parameter, cerebral blood volume index, which purports to equate cerebral blood volume with total hemoglobin signal, to determine that this level of flow was adequate.
Table 2.
Antegrade Cerebral Perfusion Clinical Reports
| Study (reference) | No of patients | ACP Flow Rate (ml/kg/min) | Radial Arterial Pressure Mm Hg | NIRS rSO2% | TCD cm/sec mean velocit y | Outcomes |
|---|---|---|---|---|---|---|
| Pigula (3) | 6 | 20 | L = 22 mm Hg | 90 | NA | No early deaths or gross neurological deficits |
| Andropoul os (8) | 34 | 63 ±19 (24–94) | 29 mm Hg | 88 | 22 | No gross neurological deficits in 32 survivors at 1–11 months |
| Andropoul os (9) | 20 | 64 ± 15 | R = 33, L=23 | R = 95%, L = 87% | R = 19, L = 20 | No in-hospital mortality, no new neurological deficits or seizures |
| Tchervenk ov (16) | 18 | 44 (18–76) | R = 24 mm Hg | NA | NA | 1 in hospital death; no acute neurologic complications in survivors |
| McQuillen (10) | 10 | 30 | NA | NA | NA | New brain ischemic MRI lesions in 8 of 10 |
| Dent (15) | 15 | 30 | NA | NA | NA | New or worsened ischemic brain MRI lesions in 11 of 15 patients |
| Goldberg (12) | 40 | 20 | NA | NA | NA | No difference at 1 year in neurodevelopmenta 1 outcomes |
| Visconti (11) | 29 | 30–40 | L = 20–25 mm Hg | NA | NA | No difference at 1 year in neurodevelopmenta l outcomes |
| Tweddell (17) | 21 | 30–40 | NA | NA | NA | 100% survival to stage 2; no brain imaging or long term follow-up |
Abbreviations: ACP, antegrade cerebral perfusion; NIRS, near-infrared spectroscopy; rSO2, regional brain oxygen saturation; TCD, transcranial Doppler ultrasound; L, left; R, right
Dent et al (15) studied 22 neonates undergoing ACP for Norwood Stage I palliation, using pre- and postoperative brain MRI to assess incidence of new brain injury in the perioperative period. ACP flow was 30 ml/kg/min and was not varied. NIRS monitoring was utilized but apparently not to determine ACP flow rates. Of the 15 patients able to have a postoperative brain MRI, new or worsened ischemic lesions were seen in 11 patients, including 7 with periventricular leukomalacia (a white matter injury associated with significant ischemia), and focal ischemic or ischemic-hemorrhagic lesions in 8.
McQuillen et al (10) reported pre-and postoperative brain MRI results on 53 neonates undergoing cardiac surgery. ACP flow was 30 ml/kg/min in those who had this technique, and no monitoring method is described. Of the 10 who had ACP, 8 had new postoperative brain injury, vs. 8 of 34 who had full flow CPB, and 3 of 9 who had DHCA as primary strategy. There was a significant association of ACP with new brain injury, vs. the other techniques.
Tchervenkov et al (16) described 18 patients with varying degrees of aortic arch obstruction, and 3 different methods of cannulation to provide ACP. Median ACP flow rate was 44 ml/kg/min, with a range from 18 to 76 ml/kg/min. Methods to determine necessary flow rates were not described.
Tweddell et al (17), in their important paper reporting improved outcomes in the Norwood Stage I palliation, described 21 patients who received ACP, noting the flow rate to be 30–40 ml/kg/min, but not further details as to monitoring or decision-making to determine flow rates. The use of ACP was significantly associated with survival to second stage operation in this series.
Goldberg et al (11) prospectively randomized 77 neonates undergoing Norwood Stage I palliation for HLHS to DHCA or ACP (39 ACP, 38 DHCA). ACP was initiated at 5 ml/kg/min, and increased to 20 ml/kg/min. Again, cerebral NIRS was used, but no adjustments to ACP flow rates made on this basis. Both ACP and DHCA times were a mean of 41 minutes, but the ACP group required a mean of 5.7 minutes of DHCA.
Because of technical and anatomic considerations, 3 of 39 ACP patients required significant periods of DHCA, but were kept in the ACP group for analysis. Of the 57 survivors at one year, 27 were in the ACP group, and 30 in the DHCA group. At one year the Bayley Scales of Infant Development II scores were not different between groups, with Mental Development Index (MDI) 94 for DHCA (28 patients) vs. 89 for ACP (22 patients), and Physical Development Index (PDI) 80 for DHCA 74 for ACP vs. 74 for ACP.
Visconti et al (12) retrospectively reviewed 29 patients undergoing the Norwood Stage I Palliation, 9 of whom received ACP, and 20 DHCA, in a non-randomized assignment at the surgeon’s discretion. ACP flow rates were 30–40 ml/kg/min, and monitored with left radial artery pressure kept at 20–25 mm Hg. ACP patients still required 23.5 minutes of DHCA, although this was less than the DHCA patients, at 44.5 minutes. At one year, there was no difference between techniques on the Bayley Scales of Infant Development II (MDI 88 for both, PDI 76 for ACP vs. 75 for DHCA).
It is our position that, with the exception of the published studies from our group, other ACP studies have a significant number of patients with ACP flows that are inadequate to support cerebral oxygenation and perfusion. Hofer et al (18) studied 10 Norwood stage I palliation neonates receiving ACP, beginning at 30 ml/kg/min for 2 minutes, decreasing to 20 ml/kg/min for 2 minutes, and finally to 10 ml/kg/min for 2 minutes, at 20 ° C. Bilateral NIRS, and left middle cerebral artery (MCA) blood flow velocity were measured, the latter with pulsed wave Doppler ultrasound through the temporal window.
At 30 ml/kg/min 1 of 10 patients had no detectable left MCA blood flow, at 20 ml/kg/min 2 of 10 had no left MCA flow, and at 10 ml/kg/min, 3 of 10 had no detectable left MCA flow. Cerebral rSO2 and jugular venous bulb oxygen saturation declined from baseline at 20 and 10 ml/kg/min, but measurements were made after 2 minutes at each flow level, because investigators did not allow the cerebral saturation to decrease further. This data is direct evidence that ACP flows of 30 ml/kg/min or less, which are frequently used in the studies cited above, are inadequate for a number of patients. Inadequate flow ACP may actually be worse than DHCA, as the ACP times in the studies cited are 40–70 minutes, longer than reported DHCA times.
Several animal model studies address the question of what ACP flow is adequate to support the brain. Schears et al (19) compared ACP delivered through a cannula advanced into the carotid artery in a neonatal piglet model, with a sham-operated group, with regard to cerebral cortical oxygen tensions measured by oxygen-dependent quenching of phosphorescence. ACP flow was 20 ml/kg/min for 90 minutes, and average cortical oxygen tension fell from 49 mm Hg before CPB, to 8 mm Hg at the end of 90 minutes of ACP. These investigators calculated that by the end of 90 minutes of ACP, 14% of the brain tissue had oxygen tension below 5 mm Hg, the value assumed to represent serious tissue oxygen deprivation. Thus, although the production of extracellular striatal dopamine and oxygen free radicals with ACP in this model were similar to sham operated animals, the oxygen tension data suggest 20 ml/kg/min is not adequate ACP flow for portions of the brain in this model. A similar study from the same group of investigators demonstrated that cortical oxygen tension decreased from 55 mm Hg before bypass, to 9 mm Hg after 90 minutes of ACP at 20 ml/kg/min, a value similar to a low-flow cardiopulmonary bypass group with flows of 20 ml/kg/min, but higher than the DHCA group, with a cortical oxygen tension of 1.1 mm Hg after 90 minutes DHCA. (20).
Chock et al (21) studied 18 neonatal piglets receiving ACP vs. DHCA for 45 minutes, with ACP at 40 ml/kg/min via direct right carotid artery cannulation. This flow rate maintained brain capillary oxygen saturation at 50–70%, and reduced apoptotic cell death seven fold compared to DHCA, although only to a level slightly above control sham operated animals. de Campli et al (22) studied neonatal piglets with an ACP model at 20 vs. 40 ml/kg/min, and determined that the higher ACP flow rates maintained cortical oxygen tension at pre-CPB levels, whereas the lower flow rate did not.
Amir et al (23) used a neonatal piglet model, and visual light spectroscopy to measure superficial and deep brain oxygen saturation during standard CPB, and ACP at 10–40 ml/kg/min before and after a period of DHCA. They concluded that even ACP flows of 40 ml/kg/min failed to maintain both superficial and deep brain oxygen saturation above 40% after a period of DHCA. (Figure 2).
Figure 2.
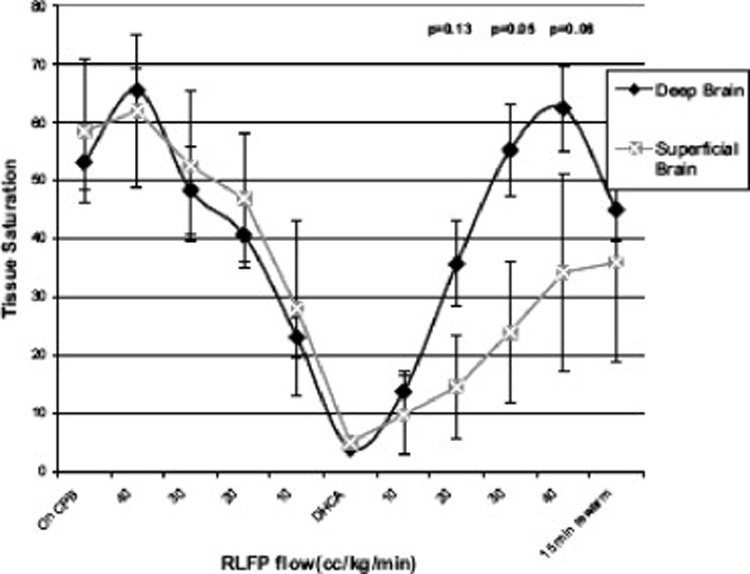
Flow related changes in superficial and deep brain saturations during regional low flow perfusion (RLFP). Values are plotted as means ± standard error of the mean. A significant difference was found between the deep and superficial brain during RLFP after a period of deep hypothermic circulatory arrest. RLFP is synonymous with antegrade cerebral perfusion. Reproduced with permission from Amir et al. (23)
All of this data suggests that ACP flow rates of less than 40 ml/kg/min may well be inadequate for many patients, to maintain adequate cerebral blood flow and oxygen delivery. Furthermore, the variability in cannulation techniques, anatomy, pH management, hematocrits, and temperatures argues for an individual strategy for each patient, which employs the readily available cerebral physiologic monitoring to adjust ACP flow on a minute by minute basis, to meet the needs of the patient, with the goal to provide adequate brain oxygenation to improve outcomes. In the next section we will describe the results of a pilot study designed to demonstrate early brain outcomes with the optimized ACP strategy described above.
Data from Texas Children’s Neuromonitoring Pilot Study
Understanding that more detailed outcome data is necessary in the study of ACP and other CPB techniques, we have undertaken a longitudinal study of neonates undergoing complex repairs, utilizing change in brain MRI from before, and 7 days postoperative studies. (24,25) Entry criteria include HLHS or variant undergoing Norwood stage I palliation, aortic arch hypoplasia with other intracardiac defects undergoing aortic arch advancement with full 2 ventricle repair, and D-transposition of the great arteries (DTGA) undergoing arterial switch operation (ASO). CPB techniques follow our standard practices and include ACP for Norwood or aortic arch advancement utilizing the monitoring scheme detailed above. DHCA is avoided, and the ASO patients receive full flow CPB at 150 ml/kg/min. pH stat management, and hematocrit of 30–35% is used during cooling and hypothermia. Brain MRI is obtained immediately before entering the operating room for surgery, and again at 7 days postoperatively, or when the patient is stable enough to travel to the MRI suite. MRI techniques include T1 and T2 weighted imaging, MR spectroscopy, and diffusion weighted imaging to assess for intraparenchymal or intraventricular hemorrhage, infarction, and significant white matter injury (periventricular leukomalacia—PVL) (26). Cerebral NIRS monitoring is used for 12 hours before surgery, and during and for 72 hours postoperatively. Continuous video electroencephalogram (EEG) is used for a 6 hour baseline study the day before surgery, and for 72 hours after surgery. A formal neurological examination by a pediatric neurologist is done before surgery, and before hospital discharge. Longer term follow-up includes a third brain MRI at age 3–6 months at the time of the bidirectional cavopulmonary connection for single ventricle patients, or as an outpatient for others. Bayley Scales of Infant Development III are performed at 1 year and 3 years of age, with a final battery of developmental testing at age 5 years. The primary outcome variable is new MRI change on the 7 day postoperative scan.
Because we have used ACP since 2000 as our primary method of CPB support during aortic reconstruction, we did not have equipoise to randomize to DHCA vs. ACP. (27,28) Therefore, we have compared cerebral outcomes in the ACP patients, vs. those receiving full flow CPB for ASO and other 2 ventricle repairs. Of 45 patients entering the study, 1 patient with unbalanced atrioventricular canal and aortic arch hypoplasia underwent attempted complete repair, which was revised to a Damus-Kaye-Stansel operation, and could not be weaned from ECMO and died 8 days postoperatively. The other 44survived and underwent a second MRI. 24 patients (20 Stage I Norwood palliation, one palliative ASO with aortic arch advancement, and 3 aortic arch advancement with VSD) received ACP and 20 patients (17 ASO, 3 truncus arteriosus) full flow CPB. The operative data is reported in Table 3. (25). Of the ACP patients 8 had new lesions on postoperative MRI: 7 with very small hemorrhages and 2 with small infarctions—1 subcortical and 1 basal ganglia injury (One patient had both hemorrhage and infarction). There was no periventricular leukomalacia. The MRI findings are reported in Table 4. Of note is that there was no evidence of cerebral edema, or larger hemorrhage. Of those 20 patients receiving full flow CPB, the number of patients with new MRI findings were not different from ACP patients, and included 3 with new findings: 2 with very small hemorrhages and 1 small gray matter thalamic infarction. Again there was no evidence of cerebral edema, or large hemorrhage. In 44 patients, with over 2500 hours of continuous EEG monitoring, we observed only two patients with EEG seizures: the first patient was an HLHS neonate who underwent ACP without problems. This neonate had a single 30 second seizure, in had experienced prolonged cerebral desaturation postoperatively. A second patient had 2 brief seizures after uncomplicated ACP, but had abnormal preoperative EEG, and evidence of ischemia on preoperative MRI, and also had prolonged low rSO2 postoperatively (26). This low seizure burden represents a significant decrease from previous studies of neonatal EEG seizures after cardiac surgery, and again demonstrates that ACP with adequate flows may be superior to DHCA or low-flow cardiopulmonary bypass to protect the brain. (29,30)
Table 3.
Patient Data
| Parameter | ACP, n=24 | No ACP, n=20 |
|---|---|---|
| Age (days) | 8 (5–11) | 8 (6–14) |
| Weight (kg) | 3.0 ± 0.3 | 3.4 ± 0.5* |
| Preoperative mechanical ventilation (no., %) | 6 (25) | 3 (15) |
| CPB time (min) | 217 ± 65 | 220 ± 40 |
| AoXcl time (min) | 101 (88–109) | 114 (106–147)* |
| Lowest temperature (°C) | 17.5 (17.4–17.8) | 24.8 (24.1–27.3)* |
| Hematocrit during hypothermia (%) | 30 ± 2 | 31 ± 1 |
| DHCA time (min) | 12 ± 5 (22 pts) | 8 (1 pt) |
| ACP time (min) | 76 ± 24 | NA |
| ACP flow rates (ml/kg/min) | 57 ± 11 | NA |
Data expressed as means ± SD or median (25th–75th percentile interquartile range);
p<0.05 by T-test or Mann Whitney Rank Sum Test. CPB, cardiopulmonary bypass; AoXcl, aortic crossclamp time; DHCA, deep hypothermic circulatory arrest; ACP, antegrade cerebral perfusion.
Table 4.
MRI Data
| Parameter | ACP, n=24 | No ACP, n=20 | Total |
|---|---|---|---|
| MRI postop day | 8 (7–10) | 7 (7–8)* | 7 (7–9) |
| Preop hemorrhage, no., (%) | 1 (4) | 2 (10) | 3 (7) |
| Postop hemorrhage, no., (%) | 6 (25) | 3 (15) | 9 (20) |
| Preop infarction, no., (%) | 3 (13) | 1 (4) | 4 (9) |
| Postop infarction, no., (%) | 4 (17) | 3 (15) | 7 (16) |
| Preop PVL, no., (%) | 0 | 0 | 0 |
| Postop PVL, no., (%) | 0 | 0 | 0 |
| Any preop finding, no., (%) | 4 (17) | 3 (15) | 7 (16) |
| Any pre/postop finding, no., (%) | 8 (33) | 4 (20) | 12 (27) |
| New postop lesions, no., (%) | 8 (33) | 3 (15) | 11 (25) |
Data expressed as numbers of patients and percentages for MRI findings, and median days (25th–75th percentile interquartile range) for MRI days postoperatively. No significant changes detected by Fisher Exact Test, for between groups at each time, or within groups for before/after MRI.
Significant difference, p<0.05, between groups for postop MRI day by MannWhitney Rank Sum Test. PVL, periventicular leukomalacia.
The question of whether flow rates in our system are excessive are addressed in two ways: 1. the transcranial doppler cerebral blood flow velocity is continually monitored during ACP, and adjusted to maintain it within ± 10% of the baseline established at cold, full flow CPB. Although only velocity is measured, an argument can be made that deep hypothermia, with cerebral pressure flow autoregulaton lost, and cerebral CO2 blood flow reactivity blunted (31), that CBFV should be proportional to cerebral blood flow under these conditions. Thus, although rSO2 is often 95% the maximum value on the scale, flow is limited using the TCD. 2. On postoperative cerebral MRI, we would expect to see evidence of injury from excessive flow or pressure, especially significant intracerebral or intraventricular hemorrhage, or evidence of cerebral edema. Aside from the few very small hemorrhages in 7 patients, there is no other damage seen on the postoperative MRI in our series. Thus there is no evidence that our ACP technique produces excessive flows.
This data is preliminary evidence that ACP with higher flows, guided by physiologic cerebral monitoring, provides adequate but not excessive blood flow to the brain during neonatal aortic arch reconstruction. The very low level of new brain injury after ACP is not different from injury after standard full flow CPB. This absence of new periventricular leukomalacia after surgery is a significant improvement over previous MRI studies of neonatal cardiac surgery, where new PVL was seen in 50–70% of patients whose bypass strategy was DHCA or inadequate ACP (15, 32). Data from the 3rd MRI to assess permanent change, myelination, brain growth, and other parameters are critical to determine if early favorable outcomes are sustained. Longer term neurodevelopmental outcomes will be assessed out to at least 5 years, to determine the relationship of ACP to these outcomes. In a second phase of this study, the neuroprotective effects of high dose erythropoeitin are being studied in a prospective, randomized, blinded, placebo-controlled study design, to see if the incidence of MRI change, and long term adverse neurodevelopemtnal outcomes can be improved with this strategy. (33,34).
Discussion
ACP is an intuitively attractive CPB technique, that was devised and applied to patients with little data to validate how much flow is necessary and sufficient, and to determine if it is superior to older techniques, i.e. DHCA. ACP in its multiple variations was widely adopted by many centers in lieu of animal models, or human data with careful monitoring techniques to establish the safety of this technique. As detailed above, cannulation techniques, ACP flow rates, and monitoring techniques vary widely with little uniformity in the published reports and in clinical practice. In addition, the natural biological variability and unique aspects of each patient require, in our opinion, an individualized strategy based on cerebral physiologic monitoring values, particularly bilateral NIRS. (28) In the absence of such monitoring, we believe the individual patient is at risk for cerebral hypoperfusion and hypoxemia.
The animal and human data reviewed above suggest that ACP flows, with the technique described by Pigula et al (2), should be at least 40 ml/kg/min or more for most patients, again guided by neuromonitoring (18,19,21,23). Flows less than this are consistently associated with the development of low cerebral oxygen tensions, placing the patient at risk, which may be silent in the absence of monitoring. In addition, ACP at inadequate flows may be worse than DHCA, if the surgical team is lulled into a false sense of security and has an ACP time that is substantially longer than would be used for DHCA. Finally, if ACP is used at temperatures above deep hypothermia, this could further increase risk for cerebral hypoxemia if inadequate flows are utilized.
To ensure a fair and full evaluation of ACP, additional animal studies should be done measuring brain tissue oxygenation in multiple regions of the brain under varying conditions of flow, pressure, temperature, pH management, hematocrit, and with different cannulation strategies. Additional measures of cell damage and death such as immunohistochemical staining for apoptotic cell death should be evaluated, as well as functional neurological testing in the recovered intact animal.
Conclusion
ACP is a promising CPB technique with increasing experimental and clinical data supporting its use in lieu of DHCA for aortic arch reconstruction in the neonate. If adequate ACP flows of 40 ml/kg/min or above for most patients are used, guided by neuromonitoring, the brain should be well protected against hypoxic insults. However, more randomized and longitudinal outcome data in both animal models and humans is needed, with study designs that ensure adequate ACP flows. Outcome studies with inadequate ACP flows have not answered the question of superiority or equivalence of ACP vs. DHCA.
Figure 3.
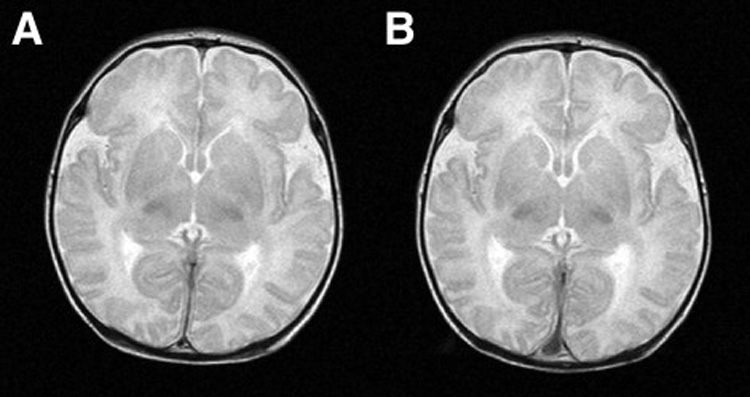
Figure 3A (left): Normal preoperative T2 weighted sagittal MRI scan in patient with hypoplastic left heart syndrome. Figure 3B (right): The same patient with normal postoperative scan after Norwood Stage I palliation using antegrade cerebral perfusion.
Figure 4.
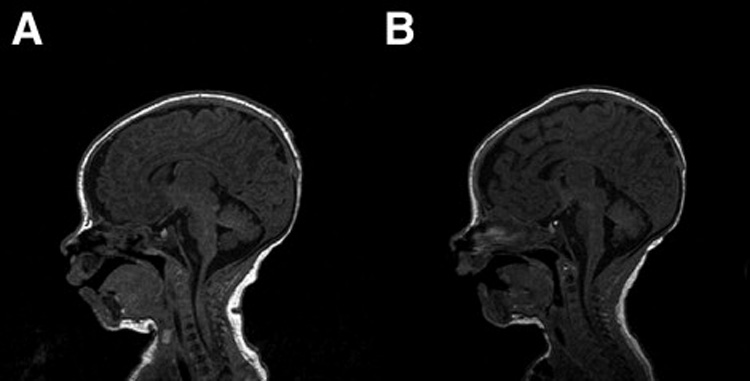
Figure 4A (left): Normal preoperative T1 weighted sagittal MRI scan in patient with interuupted aortic arch, ventricular septal defect. Figure 4B (right) : Same patient with normal postoperative scan after complete repair using antegrade cerebral perfusion.
Acknowledgments
Dr. Andropoulos is supported in part by NIH NICHD grant 1R21 HD055501-01, Baylor College of Medicine General Clinical Research Center Grant #0942, funded by NIH M01 RR00188, and Dana Foundation Brain Imaging Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Fraser has no disclosures.
Dr. Andropoulos financial disclosure: Single honorarium from Somanetics, Inc.
Contributor Information
Charles D. Fraser, Jr, Chief, Congenital Heart Surgery, Texas Children's Hospital, Professor of Surgery and Pediatrics, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas
Dean B. Andropoulos, Chief of Anesthesiology, Texas Children's Hospital, Professor of Anesthesiology and Pediatrics, Baylor College of Medicine, Houston, Texas.
References
- 1.Asou T, Kado H, Imoto Y, et al. Selective cerebral perfusion technique during aortic arch repair in neonates. Ann Thorac Surg. 1996;61:1546–1548. doi: 10.1016/0003-4975(96)80002-S. [DOI] [PubMed] [Google Scholar]
- 2.Pigula FA, Nemoto EM, Siewers RD, Griffith BP. Regional perfusion of the brain during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg. 2000;117:1023–1024. doi: 10.1016/S0022-5223(99)70387-9. [DOI] [PubMed] [Google Scholar]
- 3.Pigula FA, Nemoto EM, Griffith BP, et al. Regional low-flow perfusion provides cerebral circulatory support during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg. 2000;119:331–339. doi: 10.1016/S0022-5223(00)70189-9. [DOI] [PubMed] [Google Scholar]
- 4.Wypij D, Newburger JW, Rappaport LA, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397–1403. doi: 10.1016/s0022-5223(03)00940-1. [DOI] [PubMed] [Google Scholar]
- 5.Mahle WT, Cuadrado AR, Tam VK. Early experience with a modified Norwood procedure using right ventricle to pulmonary artery conduit. Ann Thorac Surg. 2003;76:1084–1088. doi: 10.1016/s0003-4975(03)00343-6. [DOI] [PubMed] [Google Scholar]
- 6.Poirier NC, Drummond-Webb JJ, Hisamochi K, et al. Modified Norwood procedure with a high-flow cardiopulmonary bypass strategy results in low mortality without late arch obstruction. J Thorac Cardiovasc Surg. 2000;120:875–878. doi: 10.1067/mtc.2000.109540. [DOI] [PubMed] [Google Scholar]
- 7.Pizarro C, Malec E, Maher KO, et al. Right ventricle to pulmonary artery conduit improves outcome after stage I Norwood for hypoplastic left heart syndrome. Circulation. 2003 Sep 9;108 Suppl 1:II155–11160. doi: 10.1161/01.cir.0000087390.94142.1d. [DOI] [PubMed] [Google Scholar]
- 8.Andropoulos DB, Stayer SA, McKenzie ED, Fraser CD., Jr Novel cerebral physiologic monitoring to guide low-flow cerebral perfusion during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg. 2003;125:491–499. doi: 10.1067/mtc.2003.159. [DOI] [PubMed] [Google Scholar]
- 9.Andropoulos DB, Stayer SA, McKenzie ED, Fraser CD., Jr Regional low-flow perfusion provides comparable blood flow and oxygenation to both cerebral hemispheres during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg. 2003;126:1712–1717. doi: 10.1016/s0022-5223(03)01027-4. [DOI] [PubMed] [Google Scholar]
- 10.McQuillen PS, Barkovich AJ, Hamrick SE, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007 Feb;38(2 Suppl):736–741. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg CS, Bove EL, Devaney EJ, et al. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest: outcomes for infants with functional single ventricle. J Thorac Cardiovasc Surg. 2007;133:880–887. doi: 10.1016/j.jtcvs.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 12.Visconti KJ, Rimmer D, Gauvreau K, et al. Regional low-flow perfusion versus circulatory arrest in neonates: one-year neurodevelopmental outcome. Ann Thorac Surg. 2006;82:2207–2211. doi: 10.1016/j.athoracsur.2006.06.069. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman GM, Stuth EA, Jaquiss RD, et al. Changes in cerebral and somatic oxygenation during stage 1 palliation of hypoplastic left heart syndrome using continuous regional cerebral perfusion. J Thorac Cardiovasc Surg. 2004;127:223–233. doi: 10.1016/j.jtcvs.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Andropoulos DB, Diaz LK, Fraser CD, Jr, et al. Is bilateral monitoring of cerebral oxygen saturation necessary during neonatal aortic arch reconstruction? Anesth Analg. 2004;98:1267–1272. doi: 10.1213/01.ane.0000111114.48702.59. [DOI] [PubMed] [Google Scholar]
- 15.Dent CL, Spaeth JP, Jones BV, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2006;131:190–197. doi: 10.1016/j.jtcvs.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Tchervenkov CI, Korkola SJ, Shum-Tim D, et al. Neonatal aortic arch reconstruction avoiding circulatory arrest and direct arch vessel cannulation. Ann Thorac Surg. 2001;72:1615–1620. doi: 10.1016/s0003-4975(01)03063-6. [DOI] [PubMed] [Google Scholar]
- 17.Tweddell JS, Hoffman GM, Mussatto KA, et al. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients.Circulation. Circulation. 2002 Sep 24;106 12 Suppl 1:I82–I89. [PubMed] [Google Scholar]
- 18.Hofer A, Haizinger B, Geiselseder G, et al. Monitoring of selective antegrade cerebral perfusion using near infrared spectroscopy in neonatal aortic arch surgery. Eur J Anaesthesiol. 2005;22:293–298. doi: 10.1017/s0265021505000499. [DOI] [PubMed] [Google Scholar]
- 19.Schears G, Zaitseva T, Schultz S, et al. Brain oxygenation and metabolism during selective cerebral perfusion in neonates. Eur J Cardiothorac Surg. 2006 Feb;29(2):168–174. doi: 10.1016/j.ejcts.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaitseva T, Schultz S, Schears G, et al. Regulation of brain cell death and survival after cardiopulmonary bypass. Ann Thorac Surg. 2006;82:2247–2253. doi: 10.1016/j.athoracsur.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Chock VY, Amir G, Davis CR, et al. Antegrade cerebral perfusion reduces apoptotic neuronal injury in a neonatal piglet model of cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2006;131:659–665. doi: 10.1016/j.jtcvs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 22.DeCampli WM, Schears G, Myung R, et al. Tissue oxygen tension during regional low-flow perfusion in neonates. J Thorac Cardiovasc Surg. 2003;125:472–480. doi: 10.1067/mtc.2003.13. [DOI] [PubMed] [Google Scholar]
- 23.Amir G, Ramamoorthy C, Riemer RK, et al. Visual light spectroscopy reflects flow-related changes in brain oxygenation during regional low-flow perfusion and deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2006;132:1307–1313. doi: 10.1016/j.jtcvs.2006.04.056. [DOI] [PubMed] [Google Scholar]
- 24.Andropoulos DB, Mizrahi EM, Hrachovy RA, Nelson DP, Fraser CD. Optimized bypass strategy virtually eliminates EEG seizures following neonatal cardiac surgery. Anesthesiology. 2007;107:A218. [Google Scholar]
- 25.Andropoulos DB, Stayer SA, Nelson DP, Hunter JV, Fraser CD. Optimized bypass strategy eliminates periventricular leukomalacia following neonatal cardiac surgery. Anesthesiology. 2007;107:A209. [Google Scholar]
- 26.Ment LR, Bada HS, Barnes P, et al. Practice parameter: Neuroimaging of the neonate: Report of the Quality Standards Subcommittee of the America Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58:1726–1738. doi: 10.1212/wnl.58.12.1726. [DOI] [PubMed] [Google Scholar]
- 27.Fraser CD, Andropoulos DB. Neurologic monitoring for special cardiopulmonary bypass techniques. Pediatric Cardiac Surgery Annual of the Seminars in Thoracic and Cardiovascular Surgery. 2004;7:125–132. doi: 10.1053/j.pcsu.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Andropoulos DB, Stayer SA, Diaz LK, et al. Neurological monitoring for congenital heart surgery. Anesth Analg. 2004;99:1365–1375. doi: 10.1213/01.ANE.0000134808.52676.4D. [DOI] [PubMed] [Google Scholar]
- 29.Clancy RR, Sharif U, Ichord R, et al. Electrographic neonatal seizures after infant heart surgery. Epilepsia. 2005;46:84–90. doi: 10.1111/j.0013-9580.2005.22504.x. [DOI] [PubMed] [Google Scholar]
- 30.Rappaport LA, Wypij D, Bellinger DC, et al. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Boston Circulatory Arrest Study Group. Circulation. 1998;97:773–779. doi: 10.1161/01.cir.97.8.773. [DOI] [PubMed] [Google Scholar]
- 31.Taylor RH, Burrows FA, Bissonnette B. Cerebral pressure-flow velocity relationship during hypothermic cardiopulmonary bypass in neonates and infants. Anesth Analg. 1992;74:636–642. doi: 10.1213/00000539-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Mahle WT, Tavani F, Zimmerman RA, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002 Sep 24;106(12 Suppl 1):I109–I114. [PubMed] [Google Scholar]
- 33.Sola A, Wen T, Hamrick SE, et al. Potential for protection and repair following injury to the developing brain: a role for erythropoetin? Pediatr Res. 2005;57:110R–117R. doi: 10.1203/01.PDR.0000159571.50758.39. [DOI] [PubMed] [Google Scholar]
- 34.Maise K, Li F, Chiong ZZ. New avenues of exploration for erythropoetin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]


