Abstract
Adult metazoans represent the culmination of an intricate developmental process involving the temporally and spatially orchestrated division, migration, differentiation, attachment, polarization and death of individual cells. An elaborate infrastructure connecting the cell cycle and cell attachment machinery is essential for such exquisite integration of developmental processes. Integrin-, cadherin-, Merlin- and planar cell polarity (PCP)-dependent signaling cascades quantitatively and qualitatively program cell division during development. Proteins in this signaling infrastructure may represent an important source of cancer vulnerability in metazoans, as their dysfunction can pleiotropically promote the oncogenic process.
Introduction
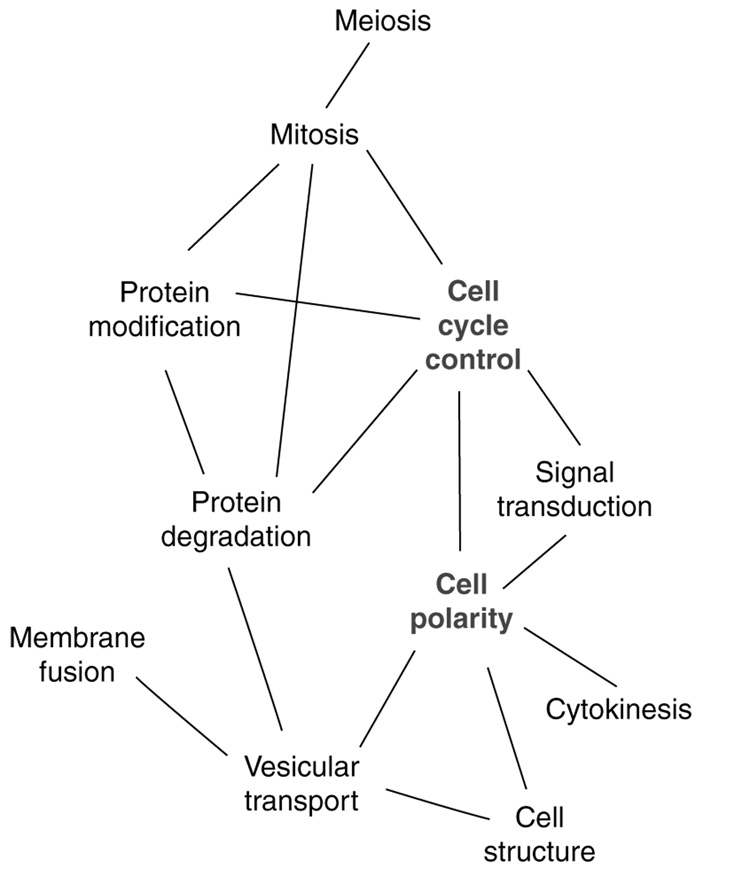
It has long been known that the attachment of cells to basal substrates or to other cells can regulate their progression through specific stages of the cell cycle. Reciprocally, the passage of cells from interphase to mitosis and back is often marked by profound changes in cellular attachment. Mechanistically, the simplest way to achieve such coordination is to use the same signaling proteins to govern both cell cycle and cell attachment. An early suggestion of the extent of interconnection between the cell division and cell polarity/attachment machineries was provided by the first global surveys of yeast protein interactions (e.g. [1]). Meta-analysis of the interaction data to characterize connections between different functional groups (Figure 1) suggested that the cell polarity machinery constituted a central hub connecting cell structure, cytokinesis, signal transduction and cell cycle controls. Recently, this hypothesis has been supported and extended by numerous elegant studies in higher eukaryotes that demonstrate the convergence of these signaling networks, as summarized herein.
Figure 1.
Interdependence of cell polarity and cell cycle pathways. Interaction map of highly connected protein classes (predicted by gene ontology) from a high throughput yeast two-hybrid based analysis of the yeast proteome, modified from [1]. Cell polarity proteins provide an important hub connecting cell architecture, signal transduction and cell cycle progression.
Regulation of the G1-to-S cell cycle transition by cell adhesion
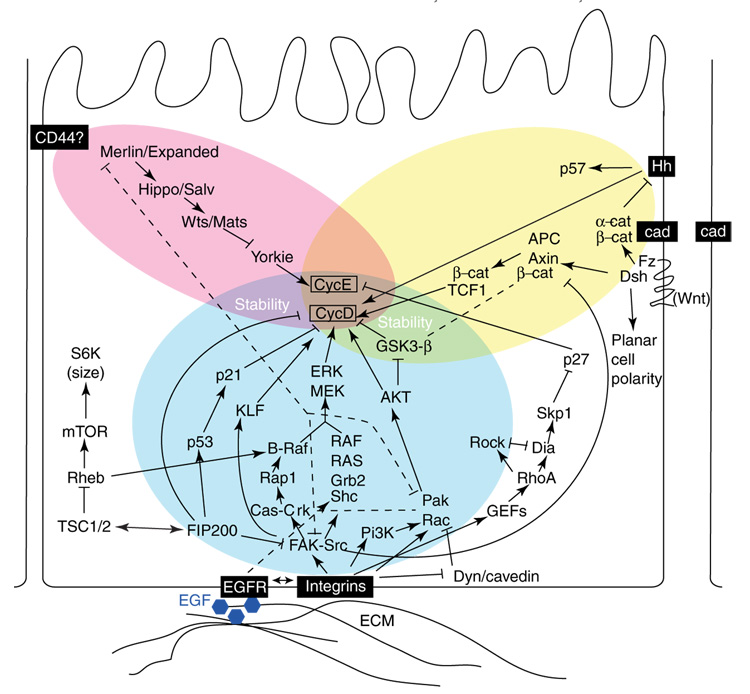
G1 arrest is a major restriction point in the cell cycle. Multiple cytoskeletal and adhesive cues regulate G1-to-S progression, with action in each case culminating in direct control of the expression of cyclins D1 and E. Extracellular cues for adhesion are provided by integrins, cadherins, Merlin, and their associated proteins, as summarized (and simplified) in Figure 2. In non-cancerous adherent cell types, engagement of trans-membrane integrin heterodimers is required for cell progression from G1 to S. Integrin-initiated proliferation signals often synergize with signals provided by growth factor receptors such as EGFR [2], although they can be functionally separated on the basis of requirement for specific downstream effectors (e.g. [3]). In contrast, lateral contact inhibition signals transmitted through cadherins or Merlin commonly block cell proliferation (see [4–8] for extended recent reviews). After cell transformation, transformed cells become anchorage-independent and cease to be contact-inhibited, as a result of altered function of integrins, cadherins, Merlin and their downstream effectors. We note that other transmembrane cell attachment proteins have also been identified and are likely to contribute to this signaling (e.g. RHAMM, CD44, desmosomal proteins), but lack of space prohibits their discussion here.
Figure 2.
Cell adhesion signals regulate proliferation through cyclins D and E. Numerous extracellular cues mediated through transmembrane or membrane-proximal proteins regulate cyclin D and E, controlling progression through G1. Activation of integrin pathways predominantly promotes proliferation, while merlin- and cadherin-dependent signaling predominantly limits proliferation.
Integrin effectors
Integrin engagement by the extracellular matrix (ECM) at focal adhesions activates numerous integrin-proximal signaling proteins, including focal adhesion kinase (FAK), Src, and Cas family members (p130Cas, HEF1/Cas-L/NEDD9, and Efs/Sin). These transmit pro-proliferatory signals downstream through multiple effector pathways [8] that ultimately activate the G1-specific cyclins, cyclins D and E (Figure 2). These mitogenic cascades include one involving Rap1 and B-Raf, another involving phosphoinositol-3-kinase (PI3K) together with Rac, AKT, and PAK, and a third comprising Shc, Grb2, Ras, and Raf. Each of these pathways culminates in activation of MEK and ERK kinases, which in turn activate transcription factors to transcribe cyclin D1. Integrin-activated FAK also directly phosphorylates the transcription factor KLF8, which translocates to the nucleus and activates the cyclin D1 promoter [9]. Additionally, some integrin-dependent signaling pathways stabilize cyclin D1 at the post-translational level (e.g. via AKT inactivation of glycogen synthase kinase 3β [GSK3β] [10]).
Alternatively, some recent studies have indicated that integrins also regulate activation of the mitogenic signaling cascades by a FAK-independent mechanism ([11•] and references therein). In this alternative pathway, integrins inhibit the caveolin- and dynamin-dependent machinery that internalizes cholesterol-enriched membrane microdomains (CEMMs). CEMMs are concentration sites for activated Rac, and help maintain Rac activation. Caveolin expression is lost in many tumor cells; in these cells, activation of Rac, a number of Rac effectors (Pak, ERK), and associated signaling proteins (including PI3K) is no longer anchorage-dependent. In contrast, caveolin expression status has no effect on FAK activation, while caveolin functions normally in FAK−/− cells.
In yet another integrin-dependent pathway regulating G1 progression, integrins also activate GDP–GTP exchange factors (GEFs), which activate the Rho GTPase. Rho signaling can either promote or inhibit G1 progression, depending on the relative degree of activation of the Rho effectors Diaphanous (Dia) and ROCK [12]. A balance favoring Dia activation increases the Skp2-dependent proteolysis of p27kip; as p27kip degrades cyclin E, Dia activation increases cyclin E levels. It is not yet clear what factors influence the Dia/ROCK balance, but their relative abundance may contribute to the observed cell-type-specific influence of Rho on proliferation.
Interestingly, a direct bridge has now been identified between the integrin effector FAK and the tuberous sclerosis complex (TSC) proteins, which may explain how the TSC tumor suppressors counteract integrin-dependent proliferation signals. The recently described protein FIP200 [13,14] directly binds FAK, inhibits FAK signaling and promotes cyclin D1 degradation. FIP200 also binds and modulates activity of the TSC1/TSC2 complex, regulating cell size (and potentially attachment). Further, as one consequence of TSC1/2 signaling is to activate Rheb, changes in TSC1/2 can also influence integrin signaling by inhibiting B-Raf [15]. FIP200 also binds p53 to induce p21, further inhibiting G1 progression. Speculatively, these numerous FIP200 interactions, and the possibility that interaction of FIP200 with one partner may titrate it from interaction with another partner, may contribute to cell-type-specific signaling by TSC and/or FAK in the regulation of cell cycle progression.
Cadherin effectors
Cell–cell contacts are sensed and stabilized by cell-surface members of the cadherin protein family (Figure 2). In stable contacts (adherens junctions or AJs) formed by epithelial cells growing at high density, the transmembrane cadherins form complexes including β-catenin and/or α-catenin, which connect to actin filaments: cells with such contacts are typically contact-inhibited, and do not proliferate. For a long time E-cadherin, α- and β-catenin and actin were assumed to assemble a stable quaternary complex that limited cell growth during contact inhibition. However, this idea has been challenged by recent findings, which show a more dynamic complex, with α- and β-catenin acting separately (e.g. [16••, 17••].).
The absence of stable cell–cell contacts causes displacement of β-catenin from E-cadherin. In the absence (by mutation or downregulation) of E-cadherin, no stable AJ structure recruits β-catenin to the cell periphery. Separately, activation of the Wnt signaling pathway (which includes Wnt and its receptor Frizzled [Frz], the tumor suppressor adenomatous polyposis coli [APC], axin, and GSK3β) causes β-catenin to separate from E-cadherin. In each case, β-catenin translocates to the nucleus and transcribes cyclin D1 and other pro-proliferative proteins. Intriguingly, although regulation of β-catenin has been long thought to be the major means by which cadherin influences cell cycle signaling, a separate role for α-catenin has very recently been demonstrated by Lien et al., whose work suggests that α-catenin at AJs inactivates the cell-cycle-promoting Hedgehog signaling pathway [17••]. This unexpected finding is likely to cause re-examination of cadherin-dependent signaling in the near future.
Merlin effectors
A series of studies in the past year have begun to unravel a third pathway of adhesive/cytoskeletal control of G1. Merlin, encoded by the neurofibromatosis 2 (NF2) tumor suppressor, is a member of the ezrin/radixin/moesin family of proteins associated with cortical actin (Figure 2). Largely on the basis of work in Drosophila, Merlin has been shown to interact with a second protein, Expanded, to control the activation of a signaling cascade proceeding through the Hippo–Salvador kinase complex [18•]. Subsequently, Hippo-Salvador activates the Warts/Mats kinase complex, which inhibits the Yorkie transcriptional factor [19•]. Yorkie is a proximal activator of cyclin E, connecting the Merlin pathway, like the integrin and cadherin pathways, to the core G1 cell cycle machinery.
As multiple components of this novel Merlin-dependent signaling cascade show tumor suppressor activity, this pathway is likely to be increasingly appreciated as an important regulator of adhesion- or cell-density-mediated growth inhibition. Interestingly, Merlin also engages in reciprocal signaling with key effectors of integrin signaling cascades. For example, Merlin downregulates Pak, Rac and FAK, and is in turn inhibited by Pak phosphorylation [20,21]. At present, a key question is the nature of the transmembrane adhesive signal(s) operating upstream of Merlin/Expanded: CD44, cadherin, and EGFR have each been proposed for this function, but the evidence is not clear, as discussed in [6].
Cross-signaling
Although the integrin, cadherin and Merlin signaling pathways are presented separately here, these attachment proteins not only share a number of common downstream effectors, but also influence each other’s action. As a single example, integrin engagement activates Src. Subsequently, Src down-regulates cadherin signaling, by acting through the ubiquitin ligase Hakai and the GTPase Arf6 to stimulate E-cadherin internalization and degradation [22]. Such interconnection can either enforce or antagonize specific signaling cues, allowing fine modulation of cues for cell cycle progression; see also the review by Chen and Gumbiner in this issue.
Mitotic entry and exit
In interphase cells, the main role of attachment proteins may be to act as sentinels for externally originating attachment cues that initiate or inhibit G1 progression. In mitosis, the attachment proteins play a more active role. For many adherent cell types, entry to mitosis involves a reduction in cell contacts accompanied by cell rounding. Subsequently, cytokinesis and re-entry to G1 both require cell attachment, and involve the re-establishment of focal adhesions. Commensurate with such a dynamic attachment profile, many cell attachment proteins are post-translationally modified and/or relocalized during mitosis. In the past several years, exciting research developments have demonstrated that attachment proteins may not only orchestrate mitotic entry and exit, but also affect qualitative aspects of mitosis such as the selection of the cleavage furrow plane, asymmetry of cell division and differentiation of daughter cells. In these studies, a frequent finding is the involvement of the centrosome as a spatial hub that organizes the mitotic cytoskeleton and assembles signaling complexes required for mitotic progression [23].
Early M-phase
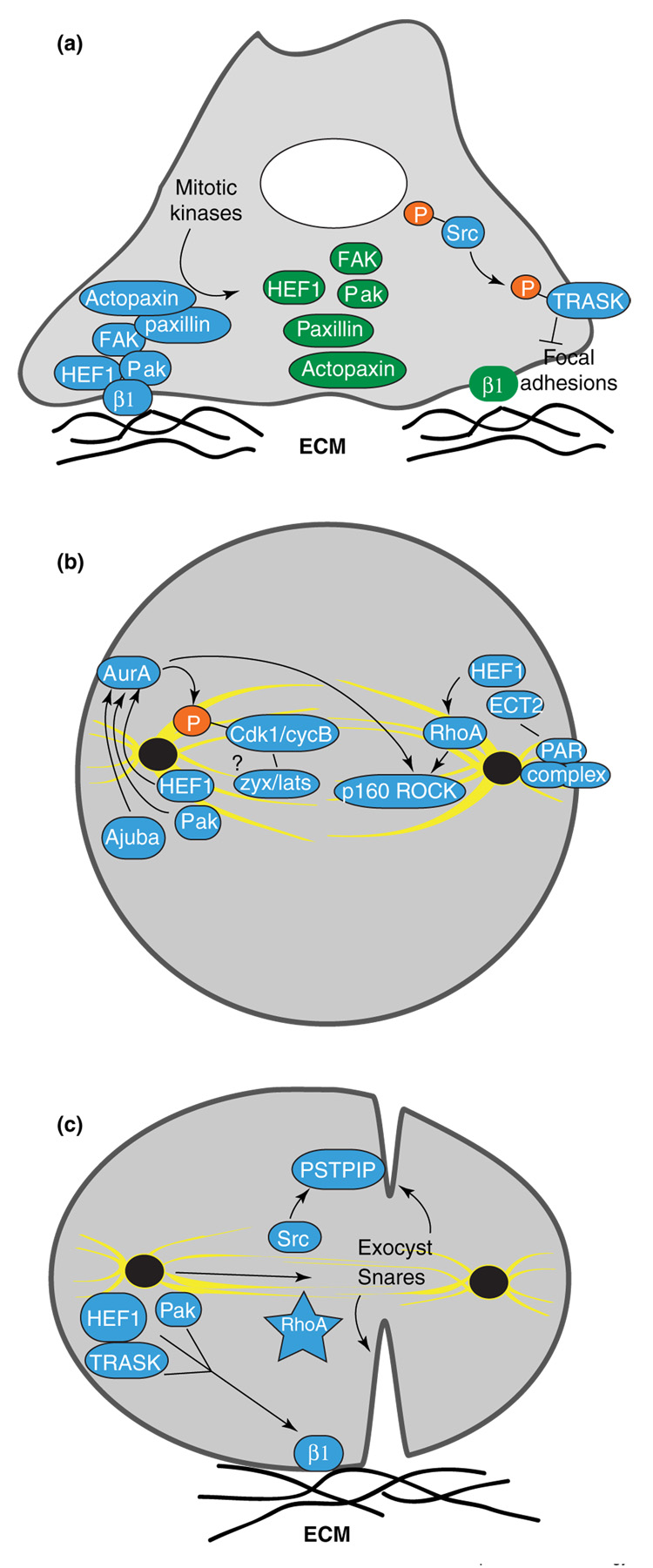
During mitosis, FAK, HEF1, Pak, paxillin, actopaxin and other proteins that target and regulate focal adhesions are phosphorylated at mitosis-specific sites (Figure 3a). These phosphorylations have a number of effects: they limit the ability of these proteins to interact with focal adhesions or with other focal adhesion-associated proteins, contributing to the mitotic disassembly of focal adhesions; they reduce their attachment-related signaling activity [24,25]; and they appear likely to contribute to the relocalization of some of these proteins to the centrosome and mitotic spindle. To date, the nature of the kinase(s) responsible for phosphorylating these proteins during G2–M transition is in most cases unknown.
Figure 3.
Reciprocal regulation of mitotic and adhesion signaling. (a) At G2/M transition, disassembly of focal adhesion complexes (blue) is accompanied by phosphorylation of FAK, HEF1, Pak, paxillin, actopaxin, Src and others by mitotic kinases (green represents phosphorylated/inactive for attachment signaling). Src activation at mitotic entry may disrupt adhesion through phosphorylation of the transmembrane glycoprotein TRASK. (b) During early mitosis, centrosomal HEF1, Pak, and Ajuba activate Aurora A, which activates Cdk1/cyclin B. A Zyxin-Lats complex is also targeted to the mitotic spindle, to regulate Cdk1/cyclinB. Additional interactions — including activation of RhoA by Hef1 and ECT2, Par complex association with ECT2, and Aurora A interaction with the RhoA effector p160ROCK — are indicated. (c) Cell attachment through integrins is required for abscission at cytokinesis. Action of the exocyst complex and SNARE complexes delivers components such as Src to the cleavage furrow to promote abscission. Basal relocalization of proteins such as HEF1, TRASK and Pak may contribute to re-establishment of focal contacts, while RhoA activity contributes to multiple events in cytokinesis (29]. In some systems for asymmetric cell division, abscission cues are provided from only one of the two prospective daughter cells, and may involve transient movement of the centrosome to the midbody.
Speculatively, the transposition of proteins such as HEF1 or Pak from focal adhesions to the mitotic apparatus may provide a ‘readiness’ cue, indicating that cellular detachment from basal surface has successfully concluded, so cell partitioning can proceed. If so, the cue may simply reflect removal of active proteins at focal adhesions, or assumption of new functions for G2-modified proteins at the mitotic apparatus. Indeed, for HEF1, Pak and the Pak-associated protein PIX, recent studies have shown that these proteins are not passive bystanders in the regulation of mitosis, but that their movement to the centrosome plays an important role in allowing mitotic entry [26••,27••,28]. At the centrosome, these proteins are required to activate the Aurora A mitotic kinase (Figure 3b). Activated Aurora A phosphorylates and helps activate the Cdk1/cyclin B complex, which is required for multiple mitotic events, and also associates with TPX2 to drive the formation of the mitotic spindle.
A number of studies support the idea that activation of RhoA may be closely coordinated with Aurora A action in regulating mitotic entry. For example, a second centrosomal kinase, Plk1, also contributes to the activation of Aurora A [29] while additionally activating ECT2, a mitotic GDP–GTP exchange factor for RhoA [30]. The existence of close interactions between Aurora A, HEF1, ECT2 and RhoA is supported by additional observations that overexpressed HEF1 binds ECT2 and activates RhoA [31], while the RhoA effector p160ROCK has been reported to bind and interacts functionally with Aurora A [32]. RhoA is important at all stages of mitosis [33•]: in early M-phase, activation of RhoA promotes mitotic rounding through stimulation of the actin cytoskeleton to enhance cortical rigidity [34]. Intriguingly, overexpression of constitutively activated RhoA causes cultured cells to mis-orient their mitotic spindle axis, and to subsequently undergo cytokinesis at an axis orthogonal to existing epithelial cell sheets [35•]. ECT2 interacts with the cell polarity protein complex (Par3/Par6/Pkcζ) [36]; thus, speculatively, interactions between ECT2, RhoA and the PAR complex may help specify the cellular division plane during development.
Other integrin-dependent effectors also contribute to mitotic entry. For example, phosphorylation by Cdk1 and interaction with RPTPα activates Src specifically at the G2/M transition ([37], Figure 3a). Src family kinases are required for nuclear breakdown and other physical changes associated with mitotic entry. Some mitosis-specific substrates of Src have been defined, including Sam68, an RNA-binding protein, and Trask, a transmembrane glycoprotein [38]. The recently defined Trask is a particularly intriguing Src target, as overexpression of Trask causes detachment from substrate; Src phosphorylation of Trask may thus directly influence mitotic rounding. Of further interest, Trask has also been found to interact physically with cadherins, although the functional significance of this interaction for mitosis remains to be elucidated.
There are hints that other cytoskeletal and attachment signaling proteins are also active participants in M-phase. As one example, the actin regulatory protein zyxin forms a complex with H-warts/LATS that targets the mitotic spindle and regulates Cdk1/cyclin B to control mitotic entry [39]; cells lacking LATS show extensive mitotic defects, including failed cytokinesis [40]. As another example, the LIM-domain protein Ajuba promotes lamellipodial projections and associates with cadherins in interphase, and activates Aurora A at the centrosome at the G2/M boundary [41–43]. It seems inevitable that many further connections will be discovered.
Late M-phase and cytokinesis
A second major point at which attachment influences M-phase occurs during the resolution of cytokinesis (Figure 3c). If normally adherent cells are prevented from attaching to a substrate, they are blocked prior to cytokinesis and frequently become binucleate. Integrin signaling proteins appear to be specifically required for cytokinesis, as impaired signaling by proteins including β1 integrin [44], HEF1 [31] and the focal adhesion regulator PTP-PEST leads to cytokinetic block. Together, these proteins are likely to contribute in at least two ways to abscission. One way is through re-establishment of focal contacts in late M-phase that can be used to generate traction forces that propagate through the cytoskeleton to physically separate the daughter cells at telophase. A second way is through provision of regulatory signals at the region of abscission.
How are focal contacts re-established at the end of mitosis? Although this is currently not well understood, it seems likely that late mitotic phosphorylation of specific attachment proteins by the mitotic kinases with which they associate (e.g. Aurora A, Plk-1 and Cdk1/cyclin B) may license the attachment proteins to reinitiate focal complex assembly. Alternatively, activation of the anaphase promoting complex/cyclosome (APC/C) in late mitosis may degrade proteins targeting HEF1, zyxin and other attachment factors to mitotic structures, causing them instead to migrate to the basal cell surface and contribute to production of focal contacts. Much work remains to be done in this area.
Among the regulatory signals required for resolution of mitosis, changes in the activity, patterns of interaction, and localization of RhoA coordinate multiple aspects of progression through the later stages of mitosis; these extensive RhoA functions have been recently reviewed [33•]. Recent studies have also found that Src is delivered to the cleavage furrow shortly before cytokinesis, becomes activated, and is required to regulate the activity of proteins such as PSTPIP, allowing abscission [45•]. The means of delivery of signals for abscission to the forming midbody is likely to be complex. One study has suggested movement of a centrosome to the midbody region is required to directly transport factors promoting abscission [46], while other work has implicated action of the secretory-vesicle-associated exocyst and SNARE complexes, or targeting of unique membrane domains at the cell surface [45,47•]. Intriguingly, a common factor in several studies is the observation that abscission signals are delivered asymmetrically from only one of the two prospective daughter cells [46,47•]. This first introduces the idea of qualitative regulation of mitosis by cell-autonomous factors and/or extracellular environment.
Qualitative differences in M-phase specified by adhesion cues: regulation of asymmetric cell division and timing of abscission
Recent exciting studies in developmental biology have illuminated the importance of the attachment machinery in controlling the axis of cleavage furrow formation, and symmetry or asymmetry of cell division (Figure 4). In many cases, stem or progenitor cells are characterized by physical connection through cadherins to a ‘hub’ cell. To specify asymmetric divisions away from the hub leading to the spawning of differentiating daughter cells, physical interactions involving APC located both at the cell cortex and the centrosome orient the axis of cell division [48]. Other studies have demonstrated the importance of integrin-mediated attachments in orienting direction of division plane against a natural or synthetic extracellular matrix [49••], while, as noted above, RhoA activation in itself is sufficient to alter the mitotic axis [35•].
Figure 4.
Different classes of extracellular cue regulate spindle orientation. Signals from a neighboring hub cell (via junctional complexes, [50••]), planar cell polarity signaling complexes [48], from extracellular matrix (ECM) [45•], and potentially from directional flow of fluids (sensed by cilia, [49••]) regulates spindle orientation. Adhesion and polarity complexes on the surface of the guided cell sense these cues and transmit them to the mitotic machinery. In some examples, discrete pools of APC at the cell cortex and at the centrosome anchor extended bridges of signaling and cytoskeletal proteins that orient the mitotic spindle [44].
In these qualitative processes of cell division, cross-signaling between the different attachment control systems is critical. Lechler and Fuchs [50••] have described complex signaling between integrins and cadherins that serves to localize polarity complex proteins [51] to the apical surface of dividing skin cells. Two recent exciting studies have for the first time demonstrated that the planar cell polarity (PCP) machinery provides essential cues to orient mitotic cell division and to allow intercalation of post-mitotic cells into cellular sheets during vertebrate development [52••,53••]. Finally, other current findings indicate that asymmetrically generated signals can regulate the timing of cytokinesis by specifying the rate of cleavage furrow progression [54•].
Conclusions
Together, the integrin, cadherin, PCP and other adhesive signals orient the division axis and ultimately specify the differentiation status of post-mitotic cells, promoting developmental processes such as epidermal stratification and neural tube morphogenesis. It is clear that proteins that bridge attachment, mitogenic, mitotic and apoptotic signaling cascades provide a central machinery to generate complex metazoan structures from simple polarized precursors. In the future, exploration of the role of three-dimensional microenvironments in specification of cell division is likely to prove informative for tissue-specific regulation of such signaling, particularly in regulation of M-phase events (see review by Yamada and colleagues in this issue).
It also appears likely that the convergence of cell attachment and cell cycle signaling has important consequences for cancer biology. A long-standing mystery has been why some types of cells are prone to transformation, while others are not. Recent work has focused on the idea of cancer ‘stem cells’ that are uniquely poised to seed tumors. It is clear that polarized epithelial cells (or epithelial progenitor cells, oriented to a hub) express a more elaborate set of attachment proteins to regulate polarity, mechanosensing and cell–cell attachment than do fibroblasts and other non-polarized cells. Altered activity or expression of the proteins involved in these processes may pleiotropically affect proliferation, metastasis and/or genome instability. The tumor suppressor APC exemplifies such a protein (see [5,6]), while HEF1 upregulation has very recently been found to be important for melanoma metastasis [55]. Ultimately, appreciation of the role of highly connected signaling intermediates may yield valuable insights for the design of novel disease therapies.
Acknowledgments
The authors apologize for being unable to cite many additional relevant publications due to journal restrictions. This work was supported by research grant NIH CA63366, the Susan Komen Breast Cancer Foundation, and Tobacco Settlement funding from the State of Pennsylvania (to EAG); and by NIH core grant CA-06927 to Fox Chase Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers cited in this review have been indicated as
• Of special interest
•• Of outstanding interest
- 1.Schwikowski B, Uetz P, Fields S. A network of protein–protein interactions in yeast. Nat Biotechnol. 2000;18:1257–1261. doi: 10.1038/82360. [DOI] [PubMed] [Google Scholar]
- 2.Bill HM, Knudsen B, Moores SL, Muthuswamy SK, Rao VR, Brugge JS, Miranti CK. Epidermal growth factor receptor-dependent regulation of integrin-mediated signaling and cell cycle entry in epithelial cells. Mol Cell Biol. 2004;24:8586–8599. doi: 10.1128/MCB.24.19.8586-8599.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gad A, Thullberg M, Dannenberg JH, te Riele H, Stromblad S. Retinoblastoma susceptibility gene product (pRb) and p107 functionally separate the requirements for serum and anchorage in the cell cycle G1-phase. J Biol Chem. 2004;279:13640–13644. doi: 10.1074/jbc.M314300200. [DOI] [PubMed] [Google Scholar]
- 4.Braga VM, Yap AS. The challenges of abundance: epithelial junctions and small GTPase signalling. Curr Opin Cell Biol. 2005;17:466–474. doi: 10.1016/j.ceb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Besson A, Assoian RK, Roberts JM. Regulation of the cytoskeleton: an oncogenic function for CDK inhibitors? Nat Rev Cancer. 2004;4:948–955. doi: 10.1038/nrc1501. [DOI] [PubMed] [Google Scholar]
- 6.Edgar BA. From cell structure to transcription: Hippo forges a new path. Cell. 2006;124:267–273. doi: 10.1016/j.cell.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Nathke IS. The adenomatous polyposis coli protein: the Achilles heel of the gut epithelium. Annu Rev Cell Dev Biol. 2004;20:337–366. doi: 10.1146/annurev.cellbio.20.012103.094541. [DOI] [PubMed] [Google Scholar]
- 8.Walker JL, Assoian RK. Integrin-dependent signal transduction regulating cyclin D1 expression and G1 phase cell cycle progression. Cancer Metastasis Rev. 2005;24:383–393. doi: 10.1007/s10555-005-5130-7. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Bian ZC, Yee K, Chen BP, Chien S, Guan JL. Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol Cell. 2003;11:1503–1515. doi: 10.1016/s1097-2765(03)00179-5. [DOI] [PubMed] [Google Scholar]
- 10.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •11.del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, Anderson RG, Schwartz MA. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293.This paper, together with earlier studies from the same laboratory, defines an integrin-dependent pathway for activation of mitogenic signaling; this pathway works by inactivating a caveolin–dynamin internalization machinery that regulates proteins associated with lipid rafts/CEMMs. Importantly, this work defines a FAK-independent control pathway for regulating activation of Rac, PI3K, PAK and ERK.
- 12.Mammoto A, Huang S, Moore K, Oh P, Ingber DE. Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2–p27kip1 pathway and the G1/S transition. J Biol Chem. 2004;279:26323–26330. doi: 10.1074/jbc.M402725200. [DOI] [PubMed] [Google Scholar]
- 13.Melkoumian ZK, Peng X, Gan B, Wu X, Guan JL. Mechanism of cell cycle regulation by FIP200 in human breast cancer cells. Cancer Res. 2005;65:6676–6684. doi: 10.1158/0008-5472.CAN-04-4142. [DOI] [PubMed] [Google Scholar]
- 14.Gan B, Melkoumian ZK, Wu X, Guan KL, Guan JL. Identification of FIP200 interaction with the TSC1-TSC2 complex and its role in regulation of cell size control. J Cell Biol. 2005;170:379–389. doi: 10.1083/jcb.200411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karbowniczek M, Cash T, Cheung M, Robertson GP, Astrinidis A, Henske EP. Regulation of B-Raf kinase activity by tuberin and Rheb is mammalian target of rapamycin (mTOR)-independent. J Biol Chem. 2004;279:29930–29937. doi: 10.1074/jbc.M402591200. [DOI] [PubMed] [Google Scholar]
- ••16.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin–catenin–actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020.This paper argues against the long-held view that α-catenin, βcatenin, E-cadherin and actin exist as a quaternary complex. Among other findings, the authors demonstrate that interaction of α-catenin with actin filaments significantly decreases the affinity of α-catenin for the E-cadherin–β-catenin complex. Membrane-associated actin at cell–cell contacts rapidly exchanges with a cytoplasmic actin pool and is much more mobile than α-catenin, β-catenin or E-cadherin, indicating that actin filaments are not stably associated with the cadherin–catenin complex.
- ••17.Lien WH, Klezovitch O, Fernandez TE, Delrow J, Vasioukhin V. αE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science. 2006;311:1609–1612. doi: 10.1126/science.1121449.Central-nervous-system-specific deletion of αE-catenin causes cortical hyperplasia by shortening the cell cycle, increasing the number of mitotic progenitor cells, decreasing apoptosis and inducing dysplasia. αE-catenin−/− ventricular zone cells are dispersed throughout the developing brains, forming invasive tumor-like masses. αE-catenin−/− neural progenitor cells have disrupted apical junction complexes and show loss of cell polarity. Although the general differentiation program is not affected, components and targets of the Hedgehog pathway are significantly up-regulated in αE-catenin−/− brains. Cyclopamine, an inhibitor of this pathway, rescues cell cycle and apoptosis abnormalities but not cortical disorganization.
- •18.Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339.In developing imaginal discs, Merlin (mer) and Expanded (Ex) signal through the recently defined Hippo (Hpo) pathway to inhibit proliferation through control of Cyclin E, and apoptosis through control of Drosophila inhibitor of apoptosis protein-1 (Diap1). Mer, Ex and Hpo regulate the number of cells in epithelial tissues, but, unlike other tumor suppressors such as TSC1, do not affect cell size.
- •19.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007.This study is the first to identify Yorkie (Yki), the Drosophila ortholog of the mammalian transcriptional coactivator Yes-associated protein (YAP), as a downstream target and interactive partner of the Hippo-pathway kinase Warts (Wts). Yki is required for normal tissue growth and Diap1 transcription, and is phosphorylated and inactivated by Wts. Mutant clones of Hpo or Wts, or Yki-overexpressing cells, are characterized by enhanced proliferation: interestingly, each phase of the cell cycle is proportionally accelerated. Yki-overexpressing cells minimize contacts with their neighbors and form round smooth borders, and have a reduced rate of apoptosis.
- 20.Poulikakos PI, Xiao GH, Gallagher R, Jablonski S, Jhanwar SC, Testa JR. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006 doi: 10.1038/sj.onc.1209587. in press. [DOI] [PubMed] [Google Scholar]
- 21.Okada T, Lopez-Lago M, Giancotti FG. Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J Cell Biol. 2005;171:361–371. doi: 10.1083/jcb.200503165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avizienyte E, Frame MC. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol. 2005;17:542–547. doi: 10.1016/j.ceb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Doxsey S, McCollum D, Theurkauf W. Centrosomes in cellular regulation. Annu Rev Cell Dev Biol. 2005;21:411–434. doi: 10.1146/annurev.cellbio.21.122303.120418. [DOI] [PubMed] [Google Scholar]
- 24.Yamakita Y, Totsukawa G, Yamashiro S, Fry D, Zhang X, Hanks SK, Matsumura F. Dissociation of FAK/p130(CAS)/c-Src complex during mitosis: role of mitosis-specific serine phosphorylation of FAK. J Cell Biol. 1999;144:315–324. doi: 10.1083/jcb.144.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke DM, Brown MC, LaLonde DP, Turner CE. Phosphorylation of actopaxin regulates cell spreading and migration. J Cell Biol. 2004;166:901–912. doi: 10.1083/jcb.200404024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••26.Pugacheva EN, Golemis EA. The focal adhesion scaffolding protein HEF1 regulates activation of the Aurora-A and Nek2 kinases at the centrosome. Nat Cell Biol. 2005;7:937–946. doi: 10.1038/ncb1309.The Cas family focal adhesion adaptor protein HEF1 directly regulates centrosome dynamics and mitotic entry through activation of mitotic kinase Aurora A and inhibition of the NUMA-related kinase Nek2. Over-expression of HEF1 causes Aurora A hyperactivation and cytokinetic defects, followed by accumulation of cells with extra centrosomes. Depletion of HEF1 induces premature centriole splitting and activation of Nek2 kinase, and inhibits Aurora A activation.
- ••27.Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell. 2005;20:237–249. doi: 10.1016/j.molcel.2005.08.035.Pak kinase, long appreciated as a mediator of cell migration signals at focal adhesions, is shown to bind GIT at the centrosome. This activates Pak, which in turn promotes Aurora A phosphorylation and progression through mitosis.
- 28.Pugacheva EN, Golemis EA. HEF1-Aurora A interactions: points of dialog between the cell cycle and cell attachment signaling networks. Cell Cycle. 2006;5 doi: 10.4161/cc.5.4.2439. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Luca M, Lavia P, Guarguaglini G. A functional interplay between Aurora-A, Plk1 and TPX2 at spindle poles: Plk1 controls centrosomal localization of Aurora-A and TPX2 spindle association. Cell Cycle. 2006 doi: 10.4161/cc.5.3.2392. in press. [DOI] [PubMed] [Google Scholar]
- 30.Niiya F, Tatsumoto T, Lee KS, Miki T. Phosphorylation of the cytokinesis regulator ECT2 at G2/M phase stimulates association of the mitotic kinase Plk1 and accumulation of GTP-bound RhoA. Oncogene. 2006;25:827–837. doi: 10.1038/sj.onc.1209124. [DOI] [PubMed] [Google Scholar]
- 31.Dadke D, Jarnik M, Pugacheva EN, Singh MK, Golemis EA. Deregulation of HEF1 impairs M-phase progression by disrupting the RhoA activation cycle. Mol Biol Cell. 2006;17:1204–1217. doi: 10.1091/mbc.E05-03-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du J, Hannon GJ. Suppression of p160ROCK bypasses cell cycle arrest after Aurora-A/STK15 depletion. Proc Natl Acad Sci U S A. 2004;101:8975–8980. doi: 10.1073/pnas.0308484101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •33.Narumiya S, Yasuda S. Rho GTPases in animal cell mitosis. Curr Opin Cell Biol. 2006;18:199–205. doi: 10.1016/j.ceb.2006.02.002.A comprehensive recent review of the complex role of Rho signaling during M-phase.
- 34.Maddox AS, Burridge K. RhoA is required for cortical retraction and rigidity during mitotic cell rounding. J Cell Biol. 2003;160:255–265. doi: 10.1083/jcb.200207130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •35.Vasiliev JM, Omelchenko T, Gelfand IM, Feder HH, Bonder EM. Rho overexpression leads to mitosis-associated detachment of cells from epithelial sheets: a link to the mechanism of cancer dissemination. Proc Natl Acad Sci U S A. 2004;101:12526–12530. doi: 10.1073/pnas.0404723101.Colonies of cells transfected with active RhoA are multi-tiered, and have a dramatic decrease in surface area and increased contractile activity, as a result of myosin II being activated. Transfected cells are characterized by a change in the plane of the cleavage furrow from perpendicular to parallel to the substrate. Reorientation occurs as a consequence of misorientation of the mitotic spindle.
- 36.Liu XF, Ishida H, Raziuddin R, Miki T. Nucleotide exchange factor ECT2 interacts with the polarity protein complex Par6/Par3/protein kinase Cζ (PKCζ) and regulates PKCζ activity. Mol Cell Biol. 2004;24:6665–6675. doi: 10.1128/MCB.24.15.6665-6675.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng XM, Shalloway D. Two mechanisms activate PTPα during mitosis. EMBO J. 2001;20:6037–6049. doi: 10.1093/emboj/20.21.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhatt AS, Erdjument-Bromage H, Tempst P, Craik CS, Moasser MM. Adhesion signaling by a novel mitotic substrate of src kinases. Oncogene. 2005;24:5333–5343. doi: 10.1038/sj.onc.1208582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirota T, Morisaki T, Nishiyama Y, Marumoto T, Tada K, Hara T, Masuko N, Inagaki M, Hatakeyama K, Saya H. Zyxin, a regulator of actin filament assembly, targets the mitotic apparatus by interacting with h-warts/LATS1 tumor suppressor. J Cell Biol. 2000;149:1073–1086. doi: 10.1083/jcb.149.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bothos J, Tuttle RL, Ottey M, Luca FC, Halazonetis TD. Human LATS1 is a mitotic exit network kinase. Cancer Res. 2005;65:6568–6575. doi: 10.1158/0008-5472.CAN-05-0862. [DOI] [PubMed] [Google Scholar]
- 41.Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, Hatakeyama K, Saya H. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114:585–598. doi: 10.1016/s0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- 42.Marie H, Pratt SJ, Betson M, Epple H, Kittler JT, Meek L, Moss SJ, Troyanovsky S, Attwell D, Longmore GD, et al. The LIM protein Ajuba is recruited to cadherin-dependent cell junctions through an association with α-catenin. J Biol Chem. 2003;278:1220–1228. doi: 10.1074/jbc.M205391200. [DOI] [PubMed] [Google Scholar]
- 43.Pratt SJ, Epple H, Ward M, Feng Y, Braga VM, Longmore GD. The LIM protein Ajuba influences p130Cas localization and Rac1 activity during cell migration. J Cell Biol. 2005;168:813–824. doi: 10.1083/jcb.200406083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aszodi A, Hunziker EB, Brakebusch C, Fassler R. Beta1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev. 2003;17:2465–2479. doi: 10.1101/gad.277003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •45.Ng MM, Chang F, Burgess DR. Movement of membrane domains and requirement of membrane signaling molecules for cytokinesis. Dev Cell. 2005;9:781–790. doi: 10.1016/j.devcel.2005.11.002.This study shows formation of an equatorial membrane domainproxiaml to the contractile ring and enriched in ganglioside GM1 and cholesterol, occurs prior to furrow formation and remains as a distinct band in the cleavage furrow until late cytokinesis. Src and PLCγ are important components of this domain; these proteins are specifically phosphorylated at cytokinesis, and their activity is required for cleavage furrow progression.
- 46.Piel M, Nordberg J, Euteneuer U, Bornens M. Centrosome-dependent exit of cytokinesis in animal cells. Science. 2001;291:1550–1553. doi: 10.1126/science.1057330. [DOI] [PubMed] [Google Scholar]
- •47.Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027.The authors demonstrate that centriolin interacts with snapin (sec15), sec3 and sec8, proteins associated with vesicle-targeting exocyst complexes and vesicle-fusion SNARE complexes. Vesicles move to the midbody ring asymmetrically from one prospective daughter cell, promoting membrane fusion and abscission. Centriolin is required for midbody localization of the exocyst; disruption of exocyst of SNARE complexes inhibits abscission.
- 48.Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- ••49.Thery M, Racine V, Pepin A, Piel M, Chen Y, Sibarita JB, Bornens M. The extracellular matrix guides the orientation of the cell division axis. Nat Cell Biol. 2005;7:947–953. doi: 10.1038/ncb1307.Using a micro-contact printing technique to control the spatial distribution of an ECM substrate, the authors investigate the role of the ECM in determining the orientation of the division axis of HeLa cells. In round mitotic cells, the cortex is not homogeneous and contains cortical cues that modulate spindle orientation. These cues are associated with retraction fibers, and originate from the cortical heterogeneity of the pre-mitotic cell. The localization of and requirement for intracellular actin regulatory proteins were investigated, with cortactin and ezrin being implicated in this signaling.
- ••50.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922.Polarization cues program basal epidermal cells to divide asymmetrically, generating a committed suprabasal cell and a proliferative basal cell. This work demonstrates that both integrins and cadherins are essential for the apical localization of atypical protein kinase C, the Par3–LGN–Inscuteable complex and NuMA–dynactin. Together, these complexes align the spindle, contributing to the stratification of squamous epithelium.
- 51.Munro EM. PAR proteins and the cytoskeleton: a marriage of equals. Curr Opin Cell Biol. 2006;18:86–94. doi: 10.1016/j.ceb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- ••52.Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signaling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–224. doi: 10.1038/nature04375.This study is one of the first to demonstrate the role of PCP signaling in vertebrate development. Genetic analysis of the interactions of Wnt, Van Gogh-like 2 (Vangl2/Strabismustrilobite) and Prickle shows the Wnt/PCP pathway polarizes neural progenitors along the anteroposterior axis. While this polarity is transiently lost during cell division, anchoring of dividing cells through these signals allows re-intercalation of daughter cells into the neuroepithelium. Defects in this pathway result in accumulation of ectopic neural progenitor cells and neural tube defects.
- ••53.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–23. doi: 10.1038/ng1701.This study demonstrates that lengthening of renal tubules is associated with mitotic orientation of cells along the tubule axis, requiring intrinsic planar cell polarization. The authors also find that mitotic orientations are significantly distorted in rodent polycystic kidney models, providing insights into the etiology of this disease. In this study, the non-motile renal cell cilia are suggested to act as mechanosensors for directional flow of fluids, organizing the PCP response.
- •54.Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436.Drosophila germline cystocytes generated either in second instar larval ovaries or in adults over-producing the BMP4-like stem cell signal Decapentaplegic efficiently convert into single stem-like cells. These de-differentiated cells can develop into functional germline stem cells and support normal fertility. As part of this study, the authors demonstrate that polarized Dpp cues emanating from a germline stem cell can regulate the timing of ring canal closure, a cytokinetic process previously shown to be under the control of Src.
- 55.Kim M, Gans JD, Nogueira C, Wang A, Paik JH, Feng B, Brennan C, Hahn WC, Cordon-Cardo C, Wagner SN, et al. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell. 2006;125:1269–1281. doi: 10.1016/j.cell.2006.06.008. [DOI] [PubMed] [Google Scholar]