Rationale for Enzyme Induction as a Chemopreventive Strategy
Numerous epidemiological studies from many parts of the world report strikingly lower cancer risks among individuals who consume large quantities of fruit and vegetables (reviewed in (1, 2)). As a consequence, a great variety of foods and supplements have been implicated as being sources of protective phytochemical factors. These factors can be used as discrete chemicals, dietary supplements, or as functional foods. Others have contrasted the fact that dietary supplements are generally considered to be time-tested, but in large part scientifically unproven, whereas functional foods are components of the normal diet that are increasingly shown to have inherent value for maintaining human health (3). The popular literature repeatedly highlights some of these phytochemicals more than others (e.g., capsaicin from peppers, coumarins from citrus and tomatoes, epigallocatechin-3-gallate (EGCG) from green tea, genistein from soybeans, indoles from broccoli and cabbage, isothiocyanates from cruciferous vegetables, lycopene from tomatoes and red grapefruit, allicin from garlic and onions, triterpenoids from licorice root and citrus, pectin from grapefruit, resveratrol from grapes, carotenoids from red grapefruit, quercetin from onions and broccoli, and flavonoids from a variety of fruits and vegetables). The mechanisms responsible for the possible protective effects derived from the consumption of these foods are multiple, probably involve complex interactions and are incompletely understood. Nonetheless, there is extensive literature detailing the actions of specific phytochemicals as well as the intact plants themselves towards altering the expression of genes that reflect an adaptive stress response which enhances normal cell survival, and may also promote apoptosis or cell cycle arrest in tumor cells. In the face of electrophilic and oxidative insults, these inducible gene products have been shown to facilitate the conjugation of xenobiotics, the nucleophilic trapping of activated electrophilic xenobiotics, as well as to increase overall antioxidative capacity in cells, animals and in some cases humans (4–8). Moreover, substantive experimental evidence in animals has been developed to support the view that the coordinated induction of these cytoprotective enzymes is a critical and sufficient mechanism to engender protection against the toxic and carcinogenic actions of reactive intermediates (reviewed in (6, 9)). As a consequence, monitoring for inducers of this stress response can be an informative means to identify plants and constituent phytochemicals of potential chemopreventive utility.
Chemical Classes of Inducers
A great deal of effort has gone into the identification of structural moieties that induce chemoprotective enzymes and this structure-inducer potency data has been reviewed recently (10). Some of these protective-enzyme inducing phytochemicals, for example the glucosinolate derivative isothiocyanates and indoles, are found exclusively in broccoli and its close relatives. Others, such as the carotenoids, flavonoids, and chlorophyll, are of almost universal provenance in green plants, with antioxidant, anti-inflammatory, anti-allergic, anticarcinogenic, and even antiviral activities having been ascribed to them (11, 12). The major structural classes of naturally occurring inducers are summarized in Table 1. Most, but not all of them are phytochemicals. In our work, we have given special attention to the glucosinolates and isothiocyanates from Brassica vegetables such as cauliflower, Brussels sprouts, broccoli, and cabbage (13), to chlorophyllin (14–16) which can be derived from extracts of virtually any green plant, and to certain flavonoids (17) and triterpenoids (18). To identify and isolate inducers of chemoprotective enzymes, we and others have utilized the Prochaska microtiter plate NQO1 assay (which measures the induction of quinone reductase, one of the quintessential detoxification enzymes in the adaptive stress response (19, 20). Guided by this approach, sulforaphane was isolated from broccoli in 1992 (21). The use of this bioassay has also guided studies of structure-activity relationships, the synthesis of analogues, elucidation of mechanisms, and assessment of potencies (22).
Table 1.
General structural classes of natural compounds containing significant potency inducing chemoprotective enzymes.
| General Class | Recent Review |
|---|---|
| diphenols, phenylenediamines and quinones | (10) |
| Michael reaction acceptors | (10) |
| Glucosinolate-derived isothiocyanates (e.g., sulforaphane), dithiocarbamates (e.g., brassinin), and indoles (e.g., indole-3-carbinol [I3C] and di-indoly-methane [DIM]) | (10) |
| aliphatic sulfides (including 1,2-dithiole-3-thiones & oxathiolene oxides) | (10) |
| carotenoids and other conjugated polyenes such as chlorophyll | (10) |
| terpenoids (including triterpenoids) | (10, 44) |
| ceramides | (44) |
| withanolides | (44) |
| flavonoids (including flavones, flavanones, flavonols, isoflavones, chalcones) | (10, 44) |
| alkaloids | (44) |
| diaryl heptanoids | (44) |
| phenylpropenoids | (45) |
Mode of Action
Multiple defense systems have evolved in all multicellular organisms in order to ensure protection against the toxic effects of the plethora of endogenous and exogenous oxidants and electrophiles to which they are exposed. DNA damage resulting from these reactive intermediates is an integral component of carcinogenesis, and strategies to reduce the burden of damage to the genome have been clearly shown to prevent cancer development in animals (6, 23). There exist several signaling pathways that evoke an adaptive response to the stress elicited by oxidants and electrophiles. A key pathway is that of Keap1-Nrf2-ARE signaling. In mice, disruption of this pathway dramatically alters the response of animals to chemical carcinogens, oxidants, inflammatory states, and other toxins targeting a variety of organs (7). Induction of this cytoprotective response system requires at least three essential components: (i) Antioxidant Response Elements (AREs) (24), upstream regulatory sequences present on each responsive gene either in single or multiple copies; (ii) Nrf2, the principal transcription factor that heterodimerizes with members of the small Maf family of transcription factors, binds to the ARE, and recruits the general transcriptional machinery for expression of ARE-regulated genes (25); (iii) Keap1, a cytosolic repressor protein that binds to Nrf2, retains it in the cytoplasm, and promotes its proteasomal degradation (26). Inducers react with thiols in Keap1 at rates that are closely related to their potencies, leading to disruption of the Nrf2-Keap1 complex and nuclear accumulation of Nrf2 (27). The molecular features of this pathway have been recently reviewed in Chemical Research in Toxicology (28) and elsewhere (29), but it is already clear that phytochemicals such as sulforaphane directly interact with Keap1 to trigger a gene expression response (30).
Sources and Delivery of Cytoprotective Agents
Extracts of plants and isolated natural products from those plants exhibit a bewildering array of activities in bioassays and in animal models, and we are beginning to see such products subjected to rigorous scrutiny in human volunteers. The induction of the cytoprotective enzymes has now been widely studied in cell culture and in animal models, and clinical investigations are beginning to unfold (31). A wealth of information exists on cell-based assays that have good predictive value, at least to the stage of animal models of carcinogenesis. An interesting conclusion that can be drawn from this body of literature: There are a number of potent, naturally occurring inducers of protective enzymes that also have low cytotoxicity, (e.g., sulforaphane, resveratrol, pinostrobin, and EGCG). By our calculations, consumption of a serving or two of foods like broccoli sprouts (sulforaphane glucosinolate) or Thai ginger (pinostrobin) can be expected to be effective in inducing the chemoprotective enzyme response (17, 32). For example, we recently conducted a clinical study in China using standardized doses of broccoli sprout derived sulforaphane glucosinolate, and observed a dose-dependant reduction in markers of DNA damage (33). Levels of sulforaphane were calibrated to be achievable by dietary means. However, the consumption of quite high levels of the plants in which some of these compounds are found would be necessary to achieve what can be reasonably expected to be a protective, but not toxic level of active phytochemical. When calculations are made of the quantity of certain other potentially protective phytochemicals (e.g., from wasabi or mustard), achieving an efficacious dose does not appear to be possible (or advisable) by dietary means.
This presents a conundrum. Should persons at increased risk for particular cancers be encouraged to take supplements or food products enriched in potentially protective compounds? There is tremendous variation in the inducer potency of these compounds (Figure 1). The consumption of a varied diet rich in potentially protective foods (Table 2) has clear attraction from the perspective of safety (low risk of overdosing or toxicity). On the other hand, the evolution of modern humans’ diet has been phenomenally rapid over the past few generations (Figure 2) and an argument can be made that dietary supplementation may be called for if we are to achieve a more uniformly enhanced longevity across populations – an incompletely understood phenomenon which is now enjoyed by a fraction of individuals in Western and other populations. Thus, we have also approached the issue of substances which have a very high safety index (e.g., one can consume a lot of it without adverse effects) but relatively low inducer potency, in an alternative fashion. We have examined compounds such as chlorophyllin and chlorophyll (14–16) and foods such as honey (17) which have very long and clear records of safe consumption, but do not have particularly high potency as inducers of chemoprotective enzymes. Dogmatic approaches to protection frequently assume that one would need to attain high levels of protection in order for there to be value in this strategy. Whereas this may be the only paradigm which permits us to measure risk reduction using currently available tools, it also embodies the flawed logic that the only good risk reduction is a large risk reduction. Consider the following examples.
Figure 1.
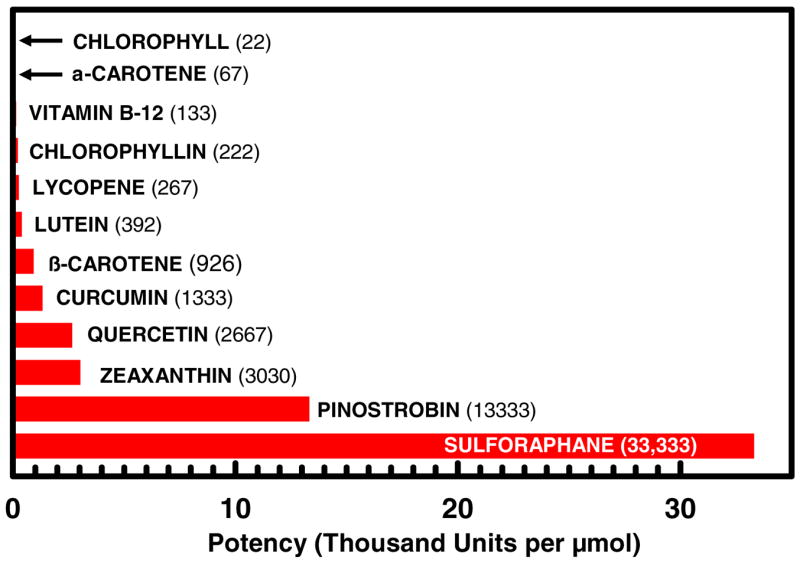
Induction of the chemoprotective enzyme NQO1 by phytochemicals in cell culture. Bioassay data adapted from references (16, 17, 46).
Table 2.
Common Edible Plants and Plant Products that Induce Chemoprotective Enzymes.
| Common Name | Scientific Name | Predominant Enzyme-Inducing Phytochemical(s) |
|---|---|---|
| Crucifer family | ||
| Arugula | Eruca sativa | isothiocyanates (e.g., erucin) |
| Broccoli | Brassica oleracea | isothiocyanates (e.g., sulforaphane) |
| Brussels sprouts | Brassica oleracea | indole glucosinolate metabolites (I3C, DIM) |
| Cabbage | Brassica oleracea | isothiocyanates (e.g., allyl-) |
| Horseradish | Armoracia rusticana | isothiocyanates (e.g., allyl-) |
| Mustard | Sinapis spp. | isothiocyanates (e.g., allyl-) |
| Radish | Raphanus sativus | isothiocyanates (e.g., sulforaphene) |
| Watercress | Nasturtium nasturtium-aquaticum | isothiocyanates (e.g., phenylethyl-) |
| Wasabi | Wasabia japonica | isothiocyanates (e.g., allyl-) |
| Onion (Lilly) family | ||
| Onion | Allium cepa | quercetin glycoside(s) + allyl sulfide |
| Garlic | Allium sativum | allyl sulfide |
| Other | ||
| Banana | Musa spp. | bicyclic diarylheptanoid |
| Green Tea | Camillia sinensis | polyphenols (e.g., EGCG) |
| Ginger | Zingiber officinale | flavonoids |
| Tephrosia | Tephrosia spp. | flavonoids |
| Thai ginger (fingerroot) | Boesenbergia pandurata | flavonoids (e.g., pinostrobin) |
| Tomatillos | Physalis philadelphica | withanolides |
| Turmeric | Curcuma longa | curcumin |
Figure 2.
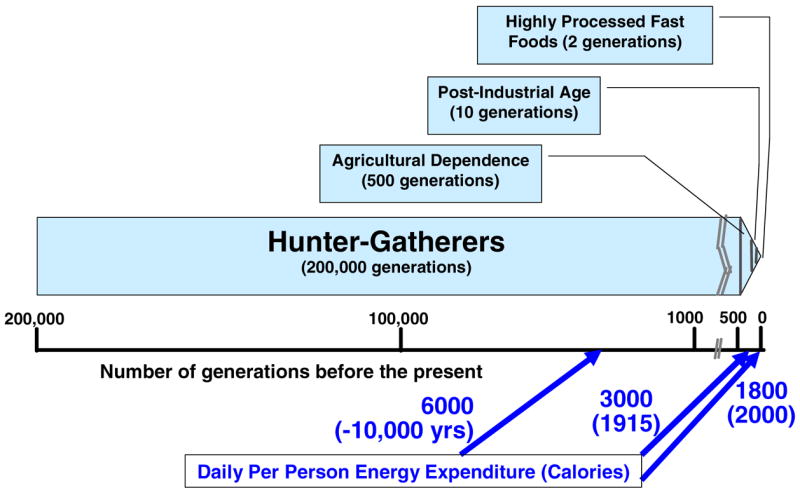
The human dietary experience.
In the context of the amount of sugar consumed in economically advantaged countries (194 g/d of sugar is eaten by Americans) (34), we have determined that replacing a small fraction of this quantity with honey, might well yield small but significant cancer protective results in addition to all of the other obvious benefits of replacing white sugar or high fructose corn syrup with honey (17). A similar argument can be made for chlorophyll and some of the accessory plant pigments (e.g., carotenoids and xanthophylls) which are present in essentially all higher plants (fruits and vegetables), yet are only moderately to slightly potent inducers in the assay systems that have been employed (16). Thus, the additive effect of a multitude of weak to moderately potent inducers for people eating 5 or more servings a day of fruits and vegetables may be highly significant. Reducing the risk of cancer by only 1%, in this country alone, would equate to saving about 5,600 lives. If one assumes that these lives saved resulted in an average of 15 years of additional life, this would account for 82,500 life-years saved (a frequently used metric) per year of dietary change or intervention. Thus although it may be next to impossible to measure as small a risk reduction as 1 or 2% (placebo-controlled clinical trials with a tumor endpoint would be impossible to conduct), the significance of even this magnitude of preventive effect should not be lost on policy makers. The risks in this example are essentially non-existent.
Conversely, there are risks in a supplementation or food fortification strategy (e.g., interactions with medications; reviewed recently by Ohnishi (35)). These risks must be weighed against a higher real or possibly perceived ability to reduce the risk of a variety of chronic and degenerative diseases. This pot of gold at the end of the proverbial rainbow has lured a multitude of companies of all sizes and qualities to move into this business area. Many of the smaller companies make products rapidly. Some of them make claims that are allowed under the Food and Drug Administration (FDA) guidelines and create high quality products, some of them pose spurious claims, or they ride the popular media’s latest “cure-de-jour” publicity, and some make no attempt whatsoever to differentiate or distinguish their products. Many of the larger companies insist on rigorous review of claims language, and insist on some degree of clinical trial support. As a result of this risk-averse positioning, they are frequently slow to create products in reaction to high profile scientific studies. When they do, however, there is almost always significant patent protection surrounding their product introductions, protecting either processes, formulations, or in some cases putative mode-of-action. Once issued, these patents create a formidable barrier to other companies because contesting or litigating against them is very costly and very difficult. Few small companies can afford such protracted litigation.
Food vs. Supplement
Most scientists have now come to accept the views of Doll and Peto (36) that a sizeable fraction of cancer mortality is attributable to diet. However, acceptance of this epidemiologic reality leads to one of the most perplexing issues in nutrition education: Should we address the prevention of chronic disease primarily through encouraging better diet and more exercise, or yield to the realities of reduced energy expenditure in the modern world and support the rational development of supplements which might not require, for example, the consumption of at least 5 servings per day of fruits and vegetables (37)? As already discussed (see Fig. 2), the net energy expenditure in industrialized society has plummeted. It is now about half of what it was a century ago. This has led to what many people point out, is a disconnect between dietary recommendations (e.g., eat 5 or more servings per day of fruits and vegetables) and a dramatically reduced caloric requirement for what has become a much more sedentary population than even its grandparents’ generation. As a result, some make the case that it may be very difficult to maintain a healthy body weight AND maintain an optimal “protected” state vis-à-vis intake of phytochemical inducers of chemoprotective enzymes plus the appropriate levels of vitamins and minerals. Are such people who wish to practice preventive behavior and control their weight, candidates for pills or other concentrated doses of phytochemical supplements, or for foods and food products reinforced with the same bioactive ingredients? Perhaps they should be? We are unfortunately still at a state scientifically whereby it is exceptionally difficult to study experimentally, or even to model mathematically, the health effects of multiple phytochemicals. One must study the effect of an extract on animal or human metabolism, or one must examine the effects of isolated active compounds. Experimentally, a combinatorial approach, even in cell culture, is problematic. There have been hints and anecdotes from many quarters of the effects of, for example, plant extracts or plant preparations, without concomitant efficacy of isolated components from those extracts. Globally there is an abundance of both written and verbal anecdotal evidence on the efficacy of a huge variety of folk medicines – so much so that clearly there are effects worth pursuing with scientific rigor. To our knowledge, although much descriptive work has been generated, nobody has yet solved the issue of chemically dissecting the multitude of bioactive compounds from such medicines, and biologically evaluating them in such a manner that proves the synergies rumored to exist in such concoctions, decoctions, preparations, elixirs, extracts, and tonics. Are there truly synergies or matrix effects? We have no answers to these questions, but propose that they deserve to be the substrate for creative minds over the next decade.
Importance of Using Scientific Principles: Evidence-Based Medicine
There is tremendous appeal to the consumer for dietary supplements: perceived efficacy coupled with low cost, ease of distribution, culturally acceptability and presumed safety. Globally, the business of dietary supplements is booming. In the U.S., business has become unfettered by the deregulatory effect of the 1994 Dietary Supplement Health and Education Act (DSHEA). DSHEA precludes the FDA from regulating dietary supplements as a drug solely because of any statements on the products label regarding health claims. Whereas the manufacturing and clinical evaluation of drugs is well defined by the FDA approval process, there are no clear guidelines for creating or evaluating supplements. Despite the popularity of botanical dietary supplements, many of these materials are not well characterized in terms of their mechanisms of action, toxicity or efficacy in humans. Moreover, as discussed elsewhere in this Forum, there has been a lack of standardization of the composition of some dietary supplements.
It is certainly our view, and one shared by much of the scientific community, that the development of chemopreventive foods (supplements, nutraceuticals) should follow a pathway designed to create knowledge about composition and pharmacology. There are a number of key issues that need to be considered. Unlike many botanical products where efficacy is inferred on the basis of limited or no information on mechanisms of action, the use of a molecular target such as Nrf2 signaling for identification and evaluation of chemopreventive foods provides a fast track to meeting the essential scientific requirements typical of drug development. Chang (38) and Talalay (39) have recently reviewed the critical issues underlying the development of either a plant extract or whole plant to yield a product with a consistent pharmacological activity. Briefly, they include (i) quality control issues related to chemical composition (e.g., plant selection, source, constituents and contaminants), (ii) standard protocols for formulation and testing (both chemical and pharmacological profiles); (iii) safety testing, which is usually assumed, but not proven; (iv) identification of mechanism-based biomarkers to evaluate beneficial/harmful effects; and (v) prospective clinical trials. Other pertinent reviews of this topic are also noted (40, 41). While it is beyond the scope of this article to document application of these tenets to development of specific phytochemicals, supplements or foods for cancer chemoprevention, the reader is referred to the literature regarding curcumin and sulforaphane (in glucosinolate-rich broccoli) as lead examples of the application of evidence-based approaches (39, 42, 43). Fulfillment of these tenets is essential in establishing the true value of food-derived chemopreventive interventions.
Acknowledgments
The authors acknowledge support from the following grants: CA094076 and CA093780.
References
- 1.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition and the Prevention of Cancer: a Global Perspective. AICR; Washington, D.C: 1997. [DOI] [PubMed] [Google Scholar]
- 2.IARC. Fruit and Vegetables 8. IARC Press; Lyon: 2003. [Google Scholar]
- 3.Halsted CH. Dietary supplements and functional foods: 2 sides of a coin? Am J Clin Nutr. 2003;77:1001S–1007S. doi: 10.1093/ajcn/77.4.1001S. [DOI] [PubMed] [Google Scholar]
- 4.Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol. 2005;18:1779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 5.Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, Sporn MB, Talalay P. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci U S A. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kensler TW. Chemoprevention by inducers of carcinogen detoxication enzymes. Environ Health Perspect. 1997;105(Suppl 4):965–970. doi: 10.1289/ehp.97105s4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Ann Rev Pharm Tox. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 8.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 9.Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr. 2001;131:3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 10.Dinkova-Kostova AT, Fahey JW, Talalay P. Chemical structures of inducers of nicotinamide quinone oxidoreductase 1 (NQO1) Meth Enzymol. 2004;382:423–448. doi: 10.1016/S0076-6879(04)82023-8. [DOI] [PubMed] [Google Scholar]
- 11.Beecher GR. Flavonoids in Foods. In: Packer L, Hiramatsu M, Yoshikawa T, editors. Proc Int Sym Antioxidant Food Supplements in Human Health. Academic Press; NY: 1999. pp. 269–281. [Google Scholar]
- 12.Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–1100. [PubMed] [Google Scholar]
- 14.Egner PA, Munoz A, Kensler TW. Chemoprevention with chlorophyllin in individuals exposed to dietary aflatoxin. Mutat Res 523–. 2003;524:209–216. doi: 10.1016/s0027-5107(02)00337-8. [DOI] [PubMed] [Google Scholar]
- 15.Egner PA, Wang JB, Zhu YR, Zhang BC, Wu Y, Zhang QN, Qian GS, Kuang SY, Gange SJ, Jacobson LP, Helzlsouer KJ, Bailey GS, Groopman JD, Kensler TW. Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc Natl Acad Sci USA. 2001;98:14601–14606. doi: 10.1073/pnas.251536898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahey JW, Stephenson KK, Dinkova-Kostova AT, Egner PA, Kensler TW, Talalay P. Chlorophyll, chlorophyllin and related tetrapyrroles are significant inducers of mammalian phase 2 cytoprotective genes. Carcinogenesis. 2005;26:1247–1255. doi: 10.1093/carcin/bgi068. [DOI] [PubMed] [Google Scholar]
- 17.Fahey JW, Stephenson KK. Pinostrobin from honey and Thai ginger (Boesenbergia pandurata): a potent flavonoid inducer of mammalian phase 2 chemoprotective and antioxidant enzymes. J Agric Food Chem. 2002;50:7472–7476. doi: 10.1021/jf025692k. [DOI] [PubMed] [Google Scholar]
- 18.Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, Sporn MB, Talalay P. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prochaska HJ, Santamaria AB, Talalay P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc Natl Acad Sci USA. 1992;89:2394–2398. doi: 10.1073/pnas.89.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fahey JW, Dinkova-Kostova AT, Stephenson KK, Talalay P. The “Prochaska” microtiter plate bioassay for inducers of NQO1. Meth Enzymol. 2004;382:243–258. doi: 10.1016/S0076-6879(04)82014-7. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Posner GH, Cho CG, Green JV, Zhang Y, Talalay P. Design and synthesis of bifunctional isothiocyanate analogs of sulforaphane: correlation between structure and potency as inducers of anticarcinogenic detoxication enzymes. J Med Chem. 1994;37:170–176. doi: 10.1021/jm00027a021. [DOI] [PubMed] [Google Scholar]
- 23.Pratt MM, Reddy AP, Hendricks JD, Pereira C, Kensler TW, Bailey GS. The importance of carcinogen dose in chemoprevention studies: quantitative interrelationships between dibenzo[a,l]pyrene, chlorophyllin dose, target organ DNA adduct biomarkers, and final tumor outcome. Carcinogenesis. 2006 doi: 10.1093/carcin/bgl174. in press. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 25.Motohashi H, O’Connor T, Katsuoka F, Engel JD, Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene. 2002;294:1–12. doi: 10.1016/s0378-1119(02)00788-6. [DOI] [PubMed] [Google Scholar]
- 26.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Tox. 2005;18:1779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 29.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Hong F, Freeman ML, Liebler DC. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem Res Toxicol. 2005;18:1917–1926. doi: 10.1021/tx0502138. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: A clinical Phase I study. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 32.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, Ye L, Coady JL, Wang JB, Wu Y, Sun Y, Zhang QN, Zhang BC, Zhu YR, Qian GS, Carmella SG, Hecht SS, Benning L, Gange SJ, Groopman JD, Talalay P. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 34.USDA Agricultural Economics Research Service. Sugar and Sweetener: Situation and Outlook Report. SSS. 2001;232:1–88. [Google Scholar]
- 35.Ohnishi N, Yokoyama T. Interactions between medicines and functional foods or dietary supplements. Keio J of Med. 2004;53:137–150. doi: 10.2302/kjm.53.137. [DOI] [PubMed] [Google Scholar]
- 36.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 37.2005 Dietary Guidelines Advisory Committee. 2005 Dietary Guidelines Advisory Committee Report. US Department of Health and Human Services and US Department of Agriculture; Washington, DC: 2004. [Google Scholar]
- 38.Chang J. Medicinal herbs: drugs or dietary supplements? Biochem Pharm. 2000;59:211–219. doi: 10.1016/s0006-2952(99)00243-9. [DOI] [PubMed] [Google Scholar]
- 39.Talalay P. The importance of using scientific principles in the development of medicinal agents from plants. Acad Med. 2001;76:238–247. doi: 10.1097/00001888-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Palou A, Serra F, Pico C. General aspects on the assessment of functional foods in the European Union. European journal of clinical nutrition. 2003;57(Suppl 1):S12–17. doi: 10.1038/sj.ejcn.1601822. [DOI] [PubMed] [Google Scholar]
- 41.Schilter B, Andersson C, Anton R, Constable A, Kleiner J, O’Brien J, Renwick AG, Korver O, Smit F, Walker R. Guidance for the safety assessment of botanicals and botanical preparations for use in food and food supplements. Food Chem Toxicol. 2003;41:1625–1649. doi: 10.1016/s0278-6915(03)00221-7. [DOI] [PubMed] [Google Scholar]
- 42.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 43.Dinkova-Kostova AT, Talalay P. Relation of structure of curcumin analogs to their potencies as inducers of Phase 2 detoxification enzymes. Carcinogenesis. 1999;20:911–914. doi: 10.1093/carcin/20.5.911. [DOI] [PubMed] [Google Scholar]
- 44.Kang YH, Pezzuto JM. Induction of quinone reductase as a primary screen for natural product anticarcinogens. Meth Enzymol. 2004;382:380–414. doi: 10.1016/S0076-6879(04)82021-4. [DOI] [PubMed] [Google Scholar]
- 45.Dinkova-Kostova AT. Protection against cancer by plant phenylpropenoids: induction of mammalian anticarcinogenic enzymes. Mini Rev Med Chem. 2002;2:595–610. doi: 10.2174/1389557023405558. [DOI] [PubMed] [Google Scholar]
- 46.Khachik F, Bertram JS, Huang M-T, Fahey JW, Talalay P. Dietary carotenoids and their metabolites as potentially useful chemoprotective agents against cancer. In: Packer L, Hiramatsu M, Yoshikawa T, editors. Proc Int Symp Antioxidant Food Supplements in Human Health. Academic Press; N.Y: 1999. pp. 203–229. [Google Scholar]


