Abstract
A chemo- and regioselective copper-catalyzed cross-coupling procedure for amination of 2-bromobenzoic acids is described. The method eliminates the need for acid protection and produces N-aryl and N-alkyl anthranilic acid derivatives in up to 99%. N-(1-Pyrene)anthranilic acid has been employed in metal ion-selective fluorosensing. Titration experiments showed that this pyrene-derived amino acid forms an equimolar complex with Hg(II) in water resulting in selective fluorescence quenching even in the presence of other metal ions such as Zn(II) and Cd(II).
The synthesis of N-aryl anthranilic acids such as flufenamic and mefenamic acid has received considerable attention during recent years because they are important non-steroidal anti-inflammatory drugs and candidates for the therapy of neurodegenerative and amyloid diseases.1 N-Aryl anthranilic acids are also synthetic precursors of acridines, which have been utilized as antimalarial and anticancer drugs.2 Due to excellent solubility in water, intriguing stereodynamic properties in peptide chains, and potential use in drug development N-aryl anthranilic acids and other non-natural achiral amino acids have found various biomedical applications.3 Achiral amino acids have been incorporated into biologically active peptides to alter secondary protein structures and biochemical properties or to investigate the stereochemical control of peptide folding.4 Recently, helicity of achiral peptide chains has been induced through asymmetric noncovalent domino effects using an external chiral stimulus.5
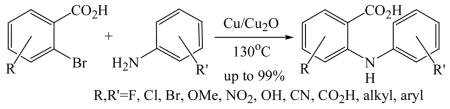
The first direct synthesis of N-aryl anthranilic acids from 2-chlorobenzoic acid was accomplished by Ullmann.6 Since then, various copper-catalyzed amination procedures suitable to ortho-chlorobenzoic acids have been described by us and others.7 Palladium-catalyzed amination of aryl halides exhibiting free carboxylic acid groups in meta- or para-position has also been explored.8 N-Aryl anthranilic acids are usually prepared from 2-chlorobenzoic acids or via coupling of anthranilic acid and aryl halides9 although a wide range of 2-bromobenzoic acid derivatives is readily available, for example through oxidation of 2-alkyl-1-bromo-benzenes10 or lithiation of dibromobenzenes and subsequent treatment with carbon dioxide.11 Common drawbacks of cross-coupling procedures using bromobenzoic acids are limited tolerance of functional groups due to very high reaction temperatures and low yields with sterically hindered aryl amines.12 We therefore wish to report a highly regioselective synthetic procedure providing convenient access to a range of N-aryl anthranilic acids exhibiting various functional groups through Cu-catalyzed amination of 2-bromobenzoic acids. The use of water-soluble N-(1-pyrene)anthranilic acid for metal ion-selective fluorosensing has also been investigated.
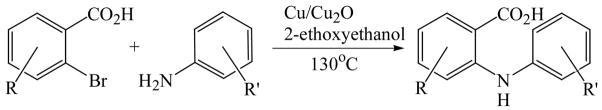
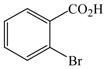
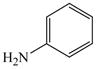
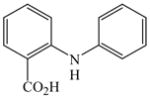
Initially, we employed CuI, Cu2O or Cu and combinations thereof as catalysts in the reaction of 2-bromobenzoic acid, 1, and aniline, 2, using n-butanol, 2-ethoxyethanol, and ethylene glycol as solvent. Further screening of bases (Na2CO3, Cs2CO3, K3PO4, NaOAc, tert-BuOK, and 2,2,6,6-tetramethylpiperidine) showed that best results for the synthesis of N-phenylanthranilic acid, 3, are obtained in the presence of potassium carbonate and catalytic amounts of Cu powder and copper(I)oxide in 2-ethoxyethanol at 130 °C (Scheme 1).
Scheme 1.
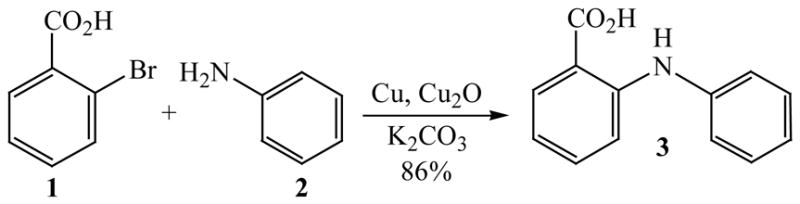
Copper-catalyzed amination of 2-bromobenzoic acid.
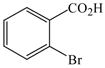
The optimized amination procedure was then applied to a variety of aryl amines and bromobenzoic acids to evaluate the synthetic potential of this method, Table 1. Reaction of bromobenzoic acid 1 with 1-aminonaphthalene, 4, 2-aminonaphthalene, 5, or 1-aminopyrene, 6, gave the corresponding N-aryl anthranilic acids 7–9 in 55–97% (entries 2–4). Importantly, the copper-catalyzed amination proceeds with remarkable chemo- and regioselectivity since only the bromide adjacent to the carboxylic acid moiety is replaced. Amination of 2-bromo-4-fluorobenzoic acid, 10, 2,5-dibromobenzoic acid, 11, and 2-bromo-4-chlorobenzoic acid, 12, with aniline yielded N-phenyl-4-fluoro-, N-phenyl-5-bromoanthranilic acids, and N-phenyl-4-chloroanthranilic acids 13 to 15, in 82–94% (entries 5 to 7). Aryl halide bonds located in the aniline ring are also not affected. N-(3-Chlorophenyl)- and N-(3-bromophenyl)anthranilic acids, 23 and 24, were obtained through cross-coupling of 1 with anilines 16 and 17, respectively, in 81 to 84% (entries 8 and 9). Comparison of the results obtained with 4-substituted anilines 18–22 reveals that incorporation of electron-donating groups facilitates the amination reaction (entries 10 to 14). In particular, formation of N-(4-nitrophenyl)anthranilic acid, 26, proved to be slow and substantial amounts of starting materials were recovered after 24 h. By contrast, coupling of 4-bromoisophthalic acid, 26, and aniline gave anthranilic acid 30 in quantitative amounts (entry 15). The amination protocol is also suitable to the synthesis of sterically crowded anthranilic acids such as 34–36, which were obtained in 53 to 78% from 2,6-dimethylaniline, 31, 2-tert-butylaniline, 32, and 2-isopropylaniline, 33, respectively (entries 16 to 18). Coupling of aniline and 2-bromo-3-methylbenzoic acid, 37, gave N-phenyl-3-methylanthranilic acid, 38, in 58% (entry 19). Noteworthy, formation of 35 in only 24% by copper(II)acetate-promoted amination of 2-bromobenzoic acid has been reported.12 The amination protocol can also applied to aliphatic amines. Employing two equivalents of primary and secondary aliphatic amines 39, 40, and 41 in the reaction with 2-bromobenzoic acid we obtained the corresponding anthranilic acids 42, 43, and 44 in 65 to 91% (entries 20–22). The results show that our amination procedure provides convenient access to a wide range of N-arylanthranilic acids from readily available, unprotected 2-bromobenzoic acids and aniline derivatives. The reaction tolerates various functionalities and proceeds with remarkable regioselectivity, which is probably due to the accelerating effect of ortho-carboxylate groups in homogeneous copper-catalyzed exchange reactions.13
Table 1.
Amination of bromobenzoic acids with aryl aminesa
 | ||||
|---|---|---|---|---|
| entry | aryl bromide | amine | product | yield (%)b |
| 1 |
1 |
2 |
3 |
86 |
| 2 |
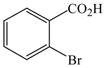
1 |
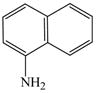
4 |
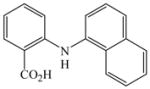
7 |
97 |
| 3 |
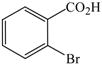
1 |
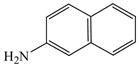
5 |
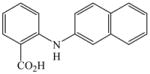
8 |
90 |
| 4 |
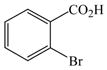
1 |
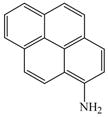
6 |
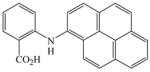
9 |
55 |
| 5 |
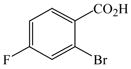
10 |
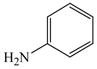
2 |
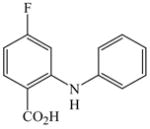
13 |
82 |
| 6 |
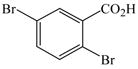
11 |
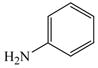
2 |
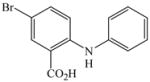
14 |
84 |
| 7 |
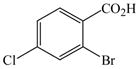
12 |
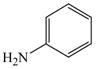
2 |
15 |
94 |
| 8 |
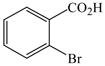
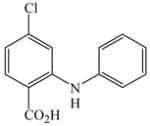
1 |
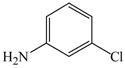
16 |
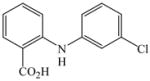
23 |
84 |
| 9 |
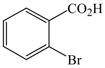
1 |
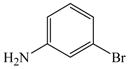
17 |
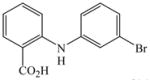
24 |
81 |
| 10 |
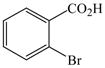
1 |
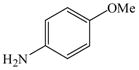
18 |
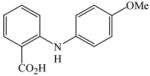
25 |
88 |
| 11 |
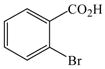
1 |
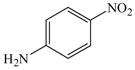
19 |
26 |
53 |
| 12 |
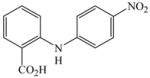
1 |
20 |
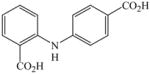
27 |
80c |
| 13 |
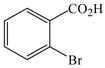
1 |
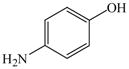
21 |
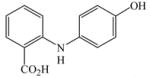
28 |
63c |
| 14 |
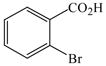
1 |
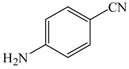
22 |
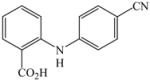
29 |
71 |
| 15 |
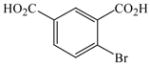
1 |
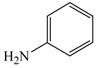
2 |
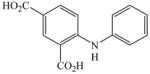
30 |
99c |
| 16 |
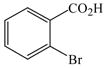
1 |
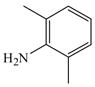
31 |
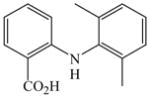
34 |
68 |
| 17 |
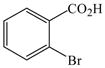
1 |
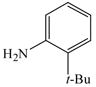
32 |
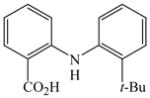
35 |
53 |
| 18 |
1 |
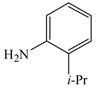
33 |
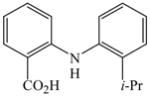
36 |
78 |
| 19 |
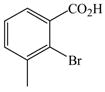
37 |
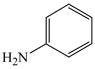
2 |
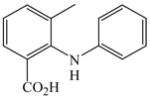
38 |
58 |
| 20 |
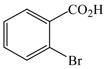
1 |
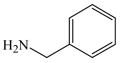
39 |
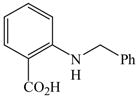
42 |
82d |
| 21 |
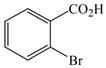
1 |
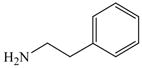
40 |
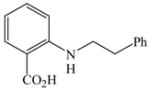
43 |
91d |
| 22 |
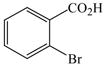
1 |
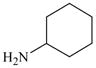
41 |
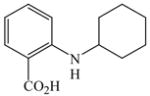
44 |
65d |
Reaction conditions: A mixture of 2-bromobenzoic acid (8.8 mmol), 1.05 equiv. of aniline derivative, 1.0 equiv. of K2CO3, 9 mol% of Cu, 4 mol% of Cu2O, was heated in 3 mL of 2-ethoxyethanol to 130 °C for 24 hours.
Isolated yields.
2 equivalents of K2CO3 were used.
two equivalents of the amine were used.
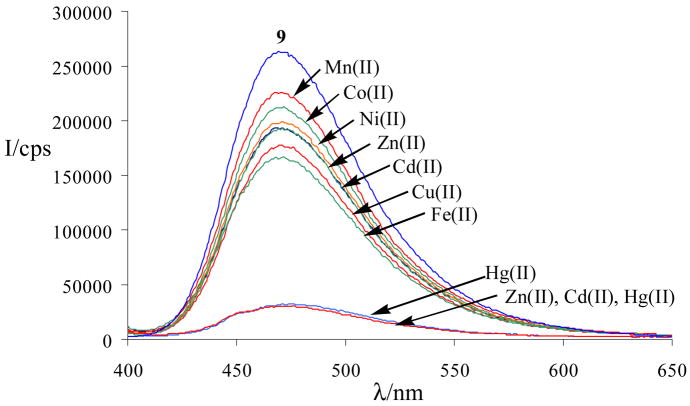
Since N-(1-pyrene)anthranilic acid, 9, has a metal binding site in close proximity to the fluorescent pyrene ring, we decided to study its use as a metal ion sensor in aqueous solution. The increasing demand for new strategies that can be employed in real-time analysis of alkali, alkaline earth, and transition metals in aqueous solutions has led to the development of numerous chemo- and biosensors.14 We have recently reported the use of highly constrained 1,8-diacridylnaphthalenes for selective fluorosensing of Cu(II), Fe(II) and Fe(III).15 While the construction of molecular sensors exhibiting a fluorophore in close proximity to a metal-chelating site has resulted in a variety of useful fluorosensors, high selectivity towards one metal ion in water has rarely been accomplished. Chang and coworkers have developed an 8-hydroxyquinoline sensor bearing an ionophoric boron-dipyrrolemethene group that proved to be highly selective for Hg(II) in dioxane-water solutions.16 A water-soluble fluorescent naphthalimide PET sensor exhibiting an iminodiacetate receptor with high selectivity for Zn(II) and an azobenzene-derived sensor for naked-eye detection of Cu(II) in water have recently been reported by Gunnlaugsson et al.17 MerR-type metal regulating proteins have been used to construct metal-ion sensitive biosensors for selective detection of Hg(II)or Cu(I), Ag(I) and Au(I).18 Spectrophotometric detection of Hg(II) in aqueous solution has also been accomplished using an optically transparent, mesoporous nanocrystalline TiO2 film sensitized with a ruthenium dye.19
Investigation of the fluorescence properties of pyrene-derived anthranilic acid 9 revealed one maximum at approximately 470 nm and a quantum yield of 0.12. Fluorescence studies using 25 μM of 9 were performed in aqueous 3 × 10−4 M K3PO4 solution at pH = 8.0. The screening of the fluorescence of 9 in the presence of 10−4 M of main group and transition metal chlorides showed selective fluorescence quenching but no shift of the emission maximum (Figure 1). No quenching was observed in the presence of main group metal ions such as Na+, K+, Mg2+, and Al3+ whereas addition of some transition metals results in a considerable decrease of the fluorescence response of 9. Increasing the metal ion concentrations above 10−4 M did not result in any further quenching. Most importantly, only Hg(II) exhibits a strong quenching effect which is not diminished in the presence of equimolar amounts of Zn(II) and Cd(II). The sensor can thus be employed for selective detection of Hg(II) in water.
Figure 1.
Fluorescence spectra of 9 in the absence and presence of various transition metal ions in aqueous 3 × 10−4 M K3PO4 solution (pH = 8.0). The concentration of 9 was 2.5 × 10−5 M and the metal ion concentration was 1.0 × 10−4 M. Excitation wavelength: 390 nm.
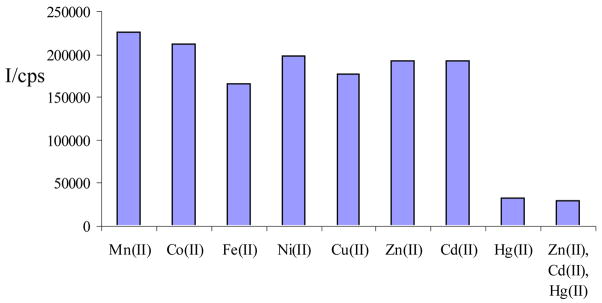
Mercury and its ionic forms are highly toxic environmental pollutants that can be introduced into the food chain by bacterial methylation and subsequent bioaccumulation. Mercury salts and Hg-derived organometallic compounds have serious neurotoxic effects and cause disruption of the central nervous system, e.g. Minamata disease. Since mercury ions are often accompanied by Zn(II) and Cd(II) it is crucial to develop Hg(II)-selective sensors for that are not compromised by the presence of these transition metal ions.20 The remarkable fluorescent response of water-soluble N-(1-pyrene)anthranilic acid, 9, to mercury chloride in the presence of both Zn(II) and Cd(II) may open new entries for a fast quantitative and qualitative analysis of Hg(II) ions in aqueous samples (Figure 2).
Figure 2.
Fluorescence of 9 in aqueous 3 × 10−4 M K3PO4 solution (pH = 8.0) in the presence of Mn(II), Co(II), Fe(II), Ni(II), Cu(II), Zn(II), Cd(II), and Hg(II)chlorides. The concentration of 9 was 2.5 × 10−5 M. The metal ion concentration was 1.0 ×10−4 M. Excitation wavelength: 390 nm. Emission wavelength: 470 nm.
We attempted to grow single crystals of 9 for X-ray analysis and conducted fluorescence titration experiments in order to reveal the 3-dimensional structure of the sensor and the stoichiometry and stability of the corresponding Hg(II) complex. We were able to grow colorless triclinic crystals of 9 belonging to the P1̄ space group from a DMF solution (Figure 3 and Table 2). Crystallographic analysis shows that the sensor does not undergo intramolecular proton transfer to form a zwitterionic structure which can be attributed to the low basicity of the diarylamine moiety. However, the amino function participates in intramolecular hydrogen bonding with the coplanar carboxylic acid group. The O1···H1-N1 hydrogen bond length is 1.975 Å and the angle (C2-N1-C8-C9) between the pyrene and the anthranilic plane was determined as 72.3°.21
Figure 3.
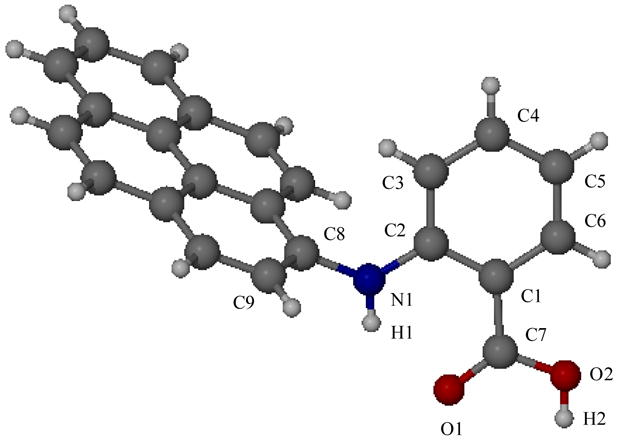
Single crystal structure of 9.
Table 2.
Selected crystallographic data of 9-DMF
| empirical formula | C26H22N2O3 |
| formula weight | 410.46 |
| crystal system | triclinic |
| space group | P1̄ |
| unit cell dimensions | a = 8.367(5) Å |
| b = 11.281(7) Å | |
| c = 13.200(5) Å | |
| α = 65.18(4)° | |
| β = 79.23(4)° | |
| γ = 69.25(4)° | |
| volume | 1056.5(10) Å3 |
| Z | 4 |
| density (calculated) | 1.290 g cm−3 |
| crystal size | 0.4 × 0.4 × 0.2 mm |
The X-ray structure and low basicity of the secondary diarylamino function suggest that only the carboxylate group of deprotonated 9 participates in the coordination to Hg(II) in aqueous solution. Job analysis of Hg(II)chloride and 9 at a total concentration of 8.0 × 10−5 M revealed the existence of one maximum at a molar ratio of 0.5 which suggests the formation of an equimolar complex (see supplemental information).22 Addition of 2.5 × 10−5 to 1.25 × 10−4 M of Hg(II) to a 25 μM solution of 9 in 3 × 10−4 M K3PO4 (pH = 8.0) results in fluorescence quenching following the Benesi-Hildebrand equation derived for a 1:1 complex.23 Benesi-Hildebrand plotting gave an association constant for Hg(II)-9 of 1262 M−1 (see supplemental information).
In summary, we have developed a Cu/Cu2O-catalyzed cross-coupling procedure that allows highly regioselective amination of 2-bromobenzoic acids. The reaction complements existing methods, tolerates various functional groups and gives N-aryl anthranilic acids in good to high yields. Pyrene-derived anthranilic acid 9 was used for fluorimetric metal ion detection in water and showed strong fluorescence quenching in the presence of Hg(II) whereas other metal ions exhibit considerably smaller effects on the fluorescence intensity. The Hg(II)-sensing ability of this non-natural amino acid is not compromised in the presence of Zn(II) and Cd(II).
Experimental Section
Typical Amination Procedure
A mixture of 1-aminopyrene (2.0 g, 9.3 mmol), 2-bromobenzoic acid (1.75 g, 8.8 mmol), K2CO3 (8.8 mmol), Cu powder (0.2–0.3 micron, 0.8 mmol), Cu2O (<5 micron, 0.4 mmol) and 3 ml of 2-ethoxyethanol was refluxed at 130 °C for 24 hours under nitrogen. The cooled reaction mixture was poured into 30 ml of water to which decolorized charcoal was added. The mixture was filtrated through Celite. The crude product was obtained by precipitation upon acidification of the filtrate with diluted HCl. The residue was dissolved in 100 ml of 5% aqueous Na2CO3. The solution was filtered through Celite and N-(1-pyrenyl)anthranilic acid 9 (1.65 g, 4.9 mmol) was obtained in 55% yield as an off-white solid by precipitation as described above. 1H NMR (300 MHz, DMSO-d6) δ = 6.94 (dd, J = 7.4 Hz, 7.4 Hz, 1H), 7.14 (d, J = 8.3 Hz, 1H), 7.43 (dd, J = 8.0 Hz, 7.4 Hz, 1H), 8.09–8.41 (m, 10H), 10.7 (bs, 1H). 13C NMR (75 MHz, DMSO-d6): δ = 114.2, 118.1, 122.0, 122.1, 124.8, 124.9, 125.4, 125.7, 125.8, 126.4, 126.7, 127.2, 128.0, 128.2, 131.4, 131.7, 133.6, 134.6, 135.2, 148.8, 172.7. Anal. calcd. for C23H15NO2: C, 81.88; H, 4.48; N, 4.15. Found: C, 81.63; H, 4.74; N, 4.32.
Supplementary Material
Synthetic procedures, UV, fluorescence and single crystal analysis data of anthranilic acid 9, and NMR spectra of all products. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We gratefully acknowledge the National Science Foundation (CAREER Award CHE-0347368), the National Institutes of Health (R01 AI060792), and the Petroleum Research Fund administered by the American Chemical Society (PRF40897-G4) for financial support.
References and Notes
- 1.(a) Oza VB, Petrassi HM, Purkey HE, Kelly JW. Bioorg Med Chem Lett. 1999;9:1–6. doi: 10.1016/s0960-894x(98)00696-9. [DOI] [PubMed] [Google Scholar]; (b) Green NS, Palaninathan SK, Sacchettini JC, Kelly JW. J Am Chem Soc. 2003;125:13404–13414. doi: 10.1021/ja030294z. [DOI] [PubMed] [Google Scholar]; (c) Oza VB, Smith C, Raman B, Koepf EK, Lashuel HA, Petrassi HM, Chiang KP, Powers ET, Sachettinni J, Kelly JW. J Med Chem. 2002;45:321–332. doi: 10.1021/jm010257n. [DOI] [PubMed] [Google Scholar]; (d) Baures PW, Oza VB, Peterson SA, Kelly JW. Bioorg Med Chem. 1999;7:1339–1347. doi: 10.1016/s0968-0896(99)00066-8. [DOI] [PubMed] [Google Scholar]; (e) Klabunde T, Petrassi HM, Oza VB, Raman P, Kelly JW, Saccettini JC. Nature Struct Biol. 2000;7:312–321. doi: 10.1038/74082. [DOI] [PubMed] [Google Scholar]
- 2.(a) Girault S, Grellier P, Berecibar A, Maes L, Mouray E, Lemiere P, Debeu MA, Davioud-Charvet E, Sergheraert C. J Med Chem. 2000;43:2646–4654. doi: 10.1021/jm990946n. [DOI] [PubMed] [Google Scholar]; (b) Demeunynck M, Charmantray F, Martelli A. Curr Pharm Des. 2001;7:1703–1724. doi: 10.2174/1381612013397131. [DOI] [PubMed] [Google Scholar]; (c) Brana MF, Cacho M, De Pascual-Teresa B, Ramos A. Curr Pharm Des. 2001;7:1745–1780. doi: 10.2174/1381612013397113. [DOI] [PubMed] [Google Scholar]; (d) Cain BF, Seelye RN, Atwee GJ. J Med Chem. 1974;17:922–928. doi: 10.1021/jm00255a003. [DOI] [PubMed] [Google Scholar]
- 3.(a) Paglialunga PM, Torrini I, Pagani GZ, Lucente G, Gavuzzo E, Mazza F, Pochetti G. Tetrahedron. 1995;51:2379–2386. [Google Scholar]; (b) Nagasawa HT, Elberling JA, Shirota FN. J Med Chem. 1973;16:823–826. doi: 10.1021/jm00265a016. [DOI] [PubMed] [Google Scholar]; (c) Breveglieri A, Guerrini R, Salvadori S, Bianchi C, Bryant SD, Attila M, Lazarus LH. J Med Chem. 1996;39:773–780. doi: 10.1021/jm950490j. [DOI] [PubMed] [Google Scholar]
- 4.(a) Inai Y, Kurokawa Y, Ida A, Hirabayashi T. Bull Chem Soc Jpn. 1999;72:55–61. [Google Scholar]; (b) Ramesh K, Balaram P. Bioorgan Med Chem. 1999;7:105–117. doi: 10.1016/s0968-0896(98)00221-1. [DOI] [PubMed] [Google Scholar]; (c) Heinonen P, Virta P, Lonnberg H. Tetrahedron. 1999;55:7613–7624. [Google Scholar]; (d) Abele S, Seebach D. Eur J Org Chem. 2000;1:1–15. [Google Scholar]
- 5.(a) Inai Y, Tagawa K, Takasu A, Hirabayashi T, Oshikawa T, Yamashita M. J Am Chem Soc. 2000;122:11731–11732. [Google Scholar]; (b) Inai Y, Ousaka N, Okabe T. J Am Chem Soc. 2003;125:8151–8162. doi: 10.1021/ja035040s. [DOI] [PubMed] [Google Scholar]
- 6.Ullmann F. Ber dt chem Ges. 1903;36:2382–2384. [Google Scholar]
- 7.(a) Kwong FY, Klapars A, Buchwald SL. Org Lett. 2002;4:581–584. doi: 10.1021/ol0171867. [DOI] [PubMed] [Google Scholar]; (b) Wolf C, Mei X. J Am Chem Soc. 2003;125:10651–10658. doi: 10.1021/ja0358145. [DOI] [PubMed] [Google Scholar]; (c) Docampo Palacios ML, Pellon Comdom RF. Synth Commun. 2003;33:1771–1775. [Google Scholar]; (d) Mei X, Wolf C. J Org Chem. 2005;70:2299–2305. doi: 10.1021/jo0479361. [DOI] [PubMed] [Google Scholar]; (e) Mei X, Wolf C. J Am Chem Soc. 2004;126:14736–14737. doi: 10.1021/ja0459781. [DOI] [PubMed] [Google Scholar]; (f) Mei X, Wolf C. Chem Comm. 2004:2078–2079. doi: 10.1039/b407718k. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Anderson KW, Zim D, Jiang L, Klapars A, Buchwald SL. J Am Chem Soc. 2003;125:6653–6655. doi: 10.1021/ja035483w. [DOI] [PubMed] [Google Scholar]
- 9.Kunz K, Scholz U, Ganzer D. Synlett. 2003:2428–2439. [Google Scholar]
- 10.(a) Hay AS, Blanchard HS. Can J Chem. 1965;43:1306–1317. [Google Scholar]; (b) Sasson Y, Zappi GD, Neumann R. J Org Chem. 1986;51:2880–2883. [Google Scholar]
- 11.Chen LS, Chen GJ, Tamborski C. J Organomet Chem. 1980;193:283–292. [Google Scholar]
- 12.Dokorou V, Kovala-Demertzi D, Jasinski JP, Galani A, Demertzis MA. Helv Chim Acta. 2004;87:1940–1950. [Google Scholar]
- 13.Couture C, Paine AJ. Can J Chem. 1985;63:111–120. [Google Scholar]
- 14.(a) Unob F, Asfari Z, Vicens J. Tetrahedron Lett. 1998;39:2951–2954. [Google Scholar]; (b) Purrello R, Gurrieri S, Lauceri R. Coord Chem Rev. 1999;192:683–706. [Google Scholar]; (c) Leray I, Valeur B, O'Reilly F, Jiwan JLH, Soumillion JP, Valeur B. Chem Commun. 1999:795–796. [Google Scholar]; (d) Valeur B, Leray I. Coord Chem Rev. 2000;205:3–40. [Google Scholar]; (e) de Silva AP, Fox DB, Huxley AJM, Moody TS. Coord Chem Rev. 2000;205:41–57. [Google Scholar]; (f) Deo S, Godwin HA. J Am Chem Soc. 2000;122:174–175. [Google Scholar]; (g) Singh A, Yao Q, Tong L, Still CW, Sames D. Tetrahedron Lett. 2000;41:9601–9605. [Google Scholar]; (h) Prodi L, Montalti M, Zaccheroni N, Dallavalle F, Folesani G, Lanfranchi M, Corradini R, Pagliari S, Marchelli R. Helv Chim Acta. 2001;84:690–706. [Google Scholar]; (i) Burdette SC, Walkup GK, Spingler B, Tsien RY, Lippard S. J Am Chem Soc. 2001;123:7831–7841. doi: 10.1021/ja010059l. [DOI] [PubMed] [Google Scholar]; (j) Baxter PNW. Chem Eur J. 2002:5250–5264. [Google Scholar]; (k) Collado D, Perez-Inestrosa E, Suau R, Desvergne J-P, Bouas-Laurent H. Org Lett. 2002;4:855–858. doi: 10.1021/ol025568m. [DOI] [PubMed] [Google Scholar]; (l) Chao CT, Huang WP. J Am Chem Soc. 2002;124:6246–6247. doi: 10.1021/ja025710e. [DOI] [PubMed] [Google Scholar]; (m) Yang NC, Jeong JK, Suh DH. Chem Lett. 2003;32:40–41. [Google Scholar]; (n) Grabchev I, Chovelon JM, Qian X. New J Chem. 2003;27:337–340. [Google Scholar]; (o) Zheng Y, Gattas-Asfura KM, Li C, Andreopoulos FM, Pham SM, Leblanc RM. J Phys Chem B. 2003;107:483–488. [Google Scholar]; (p) Kim JS, Noh KH, Lee SH, Kim SK, Yoon J. J Org Chem. 2003;68:597–600. doi: 10.1021/jo020538i. [DOI] [PubMed] [Google Scholar]; (q) Clark MA, Duffy K, Tibrewala J, Lippard SJ. Org Lett. 2003;5:2051–2054. doi: 10.1021/ol0344570. [DOI] [PubMed] [Google Scholar]
- 15.(a) Wolf C, Mei X. J Am Chem Soc. 2003;125:10651–10658. doi: 10.1021/ja0358145. [DOI] [PubMed] [Google Scholar]; (b) Wolf C, Mei X, Rokadia HK. Tetrahedron Lett. 2004;45:7867–7871. [Google Scholar]; (c) Tumambac GE, Rosencrance CM, Wolf C. Tetrahedron. 2004;60:11293–11297. [Google Scholar]
- 16.Moon SY, Cha NR, Kim YH, Chang SK. J Org Chem. 2004;69:181–183. doi: 10.1021/jo034713m. [DOI] [PubMed] [Google Scholar]
- 17.(a) Gunnlaugsson T, Lee TC, Parkesh R. Org Biomol Chem. 2003;1:3265–3267. doi: 10.1039/b309569j. [DOI] [PubMed] [Google Scholar]; (b) Gunnlaugsson T, Leonard JP, Murray NS. Org Lett. 2004;6:1557–1560. doi: 10.1021/ol0498951. [DOI] [PubMed] [Google Scholar]
- 18.Chen P, He C. J Am Chem Soc. 2004;126:728–729. doi: 10.1021/ja0383975. [DOI] [PubMed] [Google Scholar]
- 19.Palomares E, Vilar R, Durrant JR. Chem Commun. 2004:362–363. doi: 10.1039/b314138a. [DOI] [PubMed] [Google Scholar]
- 20.(a) Yoon J, Ohler NE, Vance DH, Aumiller WD, Czarnik AW. Tetrahedron Lett. 1997;38:3845–3848. [Google Scholar]; (b) Hennrich G, Sonnenschein H, Resch-Genger U. J Am Chem Soc. 1999;121:5073–5074. [Google Scholar]; (c) Prodi L, Bargossi C, Montalti M, Zaccheroni N, Su N, Bradshaw JS, Izatt RM, Savage PB. J Am Chem Soc. 2000;122:6769–6770. [Google Scholar]
- 21.O1 and H2 undergo intermolecular hydrogen bonding to co-crystallizing DMF (not shown). O1···H-C(DMF): 2.331 Å, H2···O-C(DMF): 1.818 Å.
- 22.Connors KA. Binding Constants, The Measurement of Molecular Complex Stability. Wiley; New York: 1987. [Google Scholar]
- 23.(a) Benesi HA, Hildebrand JH. J Am Chem Soc. 1949;71:2703–2707. [Google Scholar]; (b) Connors KA. The measurements of molecular complex stability. Wiley & Sons; New York: 1987. Binding constants; pp. 149–160. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthetic procedures, UV, fluorescence and single crystal analysis data of anthranilic acid 9, and NMR spectra of all products. This material is available free of charge via the Internet at http://pubs.acs.org.