Abstract
The carboxyl-terminal domain of thrombospondin-1 enhances the migration and proliferation of smooth muscle cells. Integrin-associated protein (IAP or CD47) is a receptor for the thrombospondin-1 carboxyl-terminal cell-binding domain and binds the agonist peptide 4N1K (kRFYVVMWKk) from this domain. 4N1K peptide stimulates chemotaxis of both human and rat aortic smooth muscle cells on gelatin-coated filters. The migration on gelatin is specifically blocked by monoclonal antibodies against IAP and a β1 integrin, rather than αvβ3 as found previously for 4N1K-stimulated chemotaxis of endothelial cells on gelatin. Both human and rat smooth muscle cells displayed a weak migratory response to soluble type I collagen; however, the presence of 4N1K peptide or intact thrombospondin-1 provoked a synergistic chemotactic response that was partially blocked by antibodies to α2 and β1 integrin subunits and to IAP. A combination of antiα2 and IAP monoclonal antibodies completely blocked chemotaxis. RGD peptide and antiαvβ3 mAb were without effect. 4N1K and thrombospondin-1 did not augment the chemotactic response of smooth muscle cells to fibronectin, vitronectin, or collagenase-digested type I collagen. Complex formation between α2β1 and IAP was detected by the coimmunoprecipitation of both α2 and β1 integrin subunits with IAP. These data suggest that IAP can associate with α2β1 integrin and modulate its function.
INTRODUCTION
For more than a decade, thrombospondin-1 has been implicated as a positive effector of smooth muscle cell proliferation. Thrombospondin-1 stimulates smooth muscle cell growth in vitro (Majack et al., 1985, 1986, 1988), and the protein is associated with sites of smooth muscle cell proliferation in vivo in atherosclerotic lesions (Liau et al., 1993; Van Zanten et al., 1994) and in wounds (DiPietro et al., 1996). Thrombospondin-1 synergizes with epidermal growth factor to potentiate the growth response of smooth muscle cells (Majack et al., 1986). Platelet-derived growth factor (PDGF) or angiotensin II treatment of smooth muscle cells results in the rapid synthesis of thrombospondin-1 on the same time scale as “immediate early” genes such as myc (Majack et al., 1987; Scott-Burden et al., 1990; Kobayashi and Yamamoto, 1991). Cycloheximide treatment of smooth muscle cells potentiates the induction of thrombospondin-1 mRNA as it does mRNAs of immediate early genes (Majack et al., 1987). Antibodies and heparin, which inhibit the association of secreted thrombospondin-1 with the cell surface, attenuate the response of the smooth muscle cells to PDGF (Majack et al., 1988). Furthermore, mAb C6.7 directed against the thrombospondin-1 carboxyl- terminal domain can inhibit the stimulation of proliferation of smooth muscle cells by thrombospondin-1 (Majack et al., 1988) and the chemotaxis of smooth muscle cells toward the intact thrombospondin-1 molecule (Yabkowitz et al., 1993). The sum of these experiments establishes thrombospondin-1 as a potentially important factor in the pathogenesis of atherosclerosis and restenosis and provides a rationale for pursuing the mechanism of action of thrombospondin-1 on smooth muscle cells.
An important step in this direction is to determine the receptors on smooth muscle cells with which thrombospondin-1 interacts. This has been hindered by the complex structure of the thrombospondin-1 molecule, which contains several domains harboring peptide sequences that interact with distinct cellular receptors. For example, the N-terminal heparin-binding domain binds sulfated glycosaminoglycans and glycolipids such as sulfatides (Sun et al., 1989; Abedi et al., 1995) and destabilizes focal adhesions in some types of adherent cells (Murphy-Ullrich et al., 1993). The type 1 repeat peptides bind CD36 (Asch et al., 1987, 1992, 1993; Tolsma et al., 1993) and inhibit the stimulatory effects of angiogenic factors such as basic fibroblast growth factor and vascular endothelial growth factor on endothelial cell chemotaxis and proliferation (Dawson et al., 1997). The RGD sequence of thrombospondin-1 resides in the last of the type 3 or calcium-binding repeats and binds to integrins such as αvβ3 and αIIbβ3 (Lawler and Hynes, 1989). Recently we have localized the cell-binding activity of the carboxyl-terminal cell-binding domain of thrombospondin-1 to two homologous peptides, RFYVVM and IRVVM (Gao and Frazier, 1994). Derivatives of these peptides were used to affinity label a receptor candidate, a membrane glycoprotein of 52 kDa, which proved to be integrin-associated protein or IAP (CD47) (Gao et al., 1996b). IAP is known to associate with β3 integrins and when stimulated with thrombospondin-1, the recombinant cell-binding domain, or a VVM-containing peptide such as 4N1K (kRFYVVMWKk), IAP initiates a signaling pathway resulting in up-regulation of integrin-mediated functions such as cell spreading (Gao et al., 1996a), cell migration on RGD-containing matrices (Gao et al., 1996b), and platelet aggregation (Chung et al., 1997). All of these functions involve the activation of β3 integrins. However, effects of anti-IAP monoclonal antibodies (mAbs) in assays of leukocyte transmigration (Cooper et al., 1995; Parkos et al., 1996) and phagocytosis (Blystone et al., 1995) suggest that β2 and perhaps β1 integrins could also be modulated by thrombospondin-1–IAP interactions.
The purpose of the present study was to investigate the possible role of IAP in the effects of thrombospondin-1 on smooth muscle cells. In culture, human aortic smooth muscle cells undergo a transition from their in situ “contractile” phenotype to the proliferative, migratory “synthetic” phenotype thought to be analogous to the activated state of smooth muscle cells found at sites of vessel injury (Ross and Kariya, 1980; Skinner et al., 1994). This transition includes the down-regulation of the expression of α1β1 integrin and the reciprocal up-regulation of α2β1 integrin expression, which mediates migration of these cells on collagen-I (Skinner et al., 1994). Here we have used the thrombospondin-1–derived 4N1K peptide as an agonist of IAP to investigate the role of IAP in modulating smooth muscle cell migration. Curiously, rat and human smooth muscle cells utilize α2β1 for adhesion and migration on both gelatin and native collagen-I. Other cell types use αv integrins when attaching and migrating on gelatin, an RGD-dependent process (Leavesley et al., 1992; Felding-Habermann and Cheresh, 1993; Gao et al., 1996b). While 4N1K ligation of IAP itself is a relatively weak chemotactic stimulus, IAP can modulate the activity of α2β1, resulting in enhanced migration toward soluble collagen. Finally, we present data indicating that IAP can associate with α2β1.
MATERIALS AND METHODS
Reagents
All peptides used were synthesized by the Protein and Nucleic Acid Chemistry Laboratory of Washington University School of Medicine as described previously (Kosfeld and Frazier, 1993). Peptides were evaluated by mass spectrometry before and after purification by high-pressure liquid chromatography. The amino acid sequences of the thrombospondin-1 peptides and preparation of human platelet thrombospondin-1 were as described (Santoro and Frazier, 1987). Rat tail collagen-I, human vitronectin, and fibronectin were obtained from Collaborative Biochemical Products (Bedford, MA). Anti-human IAP mAbs, 2D3, B6H12, 1F7, and anti αv (L230) mAbs were supplied by Dr. E. Brown (Washington University School of Medicine, St. Louis MO) (Brown et al., 1990; Lindberg et al., 1993, 1994). mAbs P4C10 (anti-β1) and P1E6 (anti-α2) were obtained from Life Technologies (Grand Island, NY); anti-αvβ3 mAb 4C1 was from Monsanto-Searle, St. Louis, MO; mAb BHA2.1 (anti-α2β1) and Western blotting polyclonal antibodies against α2 and β1 were from Chemicon International (Temecula, CA). Goat anti-rabbit (Fab)2 antibody conjugated with horseradish peroxidase was from Jackson ImmunoResearch Labs (West Grove, PA). Enhanced chemiluminescence Western blotting detection kit was from Amersham (Arlington Heights, IL). Anti-mouse immunoglobulin G (IgG) agarose and other reagents were from Sigma Chemical (St. Louis, MO).
Cell Culture
Human arterial smooth muscle cells from the aorta of a 4-y-old boy and rat aorta smooth muscle cells were isolated by the explant method and cultured as described (Ross and Kariya, 1980). Cells were maintained in a humidified 37°C and 5% CO2 environment in minimal essential medium (MEM) with 20% fetal calf serum and identified by immunostaining of α-actin (Janat and Liau, 1992). Passages 2–10 were used for experiments.
Cell Adhesion
Assays were performed in 96-well plates as previously described (Kosfeld and Frazier, 1993). Synthetic peptides were solubilized in Tris-buffered saline (TBS) (25 mM Tris, 150 mM NaCl, pH 7.4) and 50 μl of solution was added to each well of 96-well plates (Nunc Immuno Plate Maxisorp, Naperville, IL), and incubated at 4°C overnight. Wells were rinsed with TBS and blocked with 1% bovine serum albumin (BSA) for 1 h at room temperature. Cells were harvested from near-confluent cultures by brief treatment with trypsin/EDTA and were immediately washed and resuspended in Ca2+-free TBS with 0.4% BSA. Cell suspension (100 μl) was added to each well. After incubation at 37°C for 2 h, the plates were rinsed three times with TBS. Cell attachment was quantified with a colorimetric reaction using endogenous cellular phosphatase activity by adding 100 μl of the following substrate/lysis solution to each well: 1% Triton X-100, 6 mg/ml p-nitrophenyl phosphate, in 50 mM sodium acetate, pH 5.0. Wells were incubated for 1–2 h at 37°C, after which the reaction was stopped by the addition of 50 μl 1 N NaOH and read in an enzyme-linked immunosorbent assay plate reader (Dynatech Laboratories, Cambridge, MA) with a 410-nm filter. Wells were set up in triplicate, and all experiments were repeated at least three times.
Cell Migration
Chemotaxis assays were conducted in microBoyden chambers (Neuroprobe, Cabin John, MD) using 8 μm PVP-free, polycarbonate filters (Nuclepore, Pleasanton, CA). Filters were precoated by soaking them in 100 μg/ml gelatin at 37°C overnight, followed by washing twice in phosphate-buffered saline (PBS). Smooth muscle cells were harvested with trypsin/EDTA and diluted in MEM with 0.1% BSA to a final concentration of 3–5×105 cells/ml; chemoattractants were diluted in the same solution. The assembled chamber was incubated for 6 h at 37°C. Filters were fixed, stained, and mounted. Cells were counted in five high-power fields in each of the triplicate wells. Checkerboard assays were used to distinguish between chemotaxis (directed migration) and chemokinesis (random migration) (Zigmond and Hirsch, 1973; Wilkinson and Allan, 1978).
Fluorescence-activated Cell Sorter (FACS) Analysis
Human smooth muscle cell were harvested by trypsin/EDTA and resuspended in culture medium with 10% fetal calf serum. Primary mAbs (5 μg/ml) were added to cell suspensions and incubated for 2 h at 4°C with rocking. After several washes in PBS, the cells were stained with fluoroisothiocyanate-labeled anti-mouse secondary antibody (Pierce, Rockford, IL) for another 1 h in the cell culture medium, washed again with PBS, and analyzed by flow cytometry.
Immunoprecipitation and Western Blotting
Cells were lysed in 30 mM n-octyl-β-d-glucopyranoside in TBS with proteinase inhibitors (10 μg/ml each of antipain, pepstatin A, chymostatin, leupeptin, soybean trypsin inhibitor, aprotinin, and 1 mM phenylmethylsulfonylfluoride) by rocking for 30 min at 4°C followed by microcentrifugation at top speed (13,000 rpm) for 30 min. The soluble material from equal amounts of protein was incubated with the specified monoclonal antibody overnight at 4°C and immunoprecipitated with anti-mouse IgG-agarose in the presence of 3% goat serum. The precipitates were extensively washed with lysis buffer, dissolved, and boiled in a small volume of SDS-sample buffer. Proteins were separated by SDS-PAGE on 10% precast Tris-Glycine gels (NOVEX, San Diego, CA) and transferred to nitrocellulose membranes. Blots were blocked with 3% BSA plus 3% dried milk in TBST (0.1% Tween-20 in TBS) for at least 1 h and probed with the indicated antibodies overnight at 4°C, washed, and incubated with 1:25,000 dilution of horseradish peroxidase-conjugated goat anti-rabbit IgG(Fab)2 for another 2 h. Detection was by chemiluminescence with an enhanced chemiluminescence kit.
RESULTS
Smooth Muscle Cells Bind to an IAP-Agonist Peptide from the Carboxyl-terminal Domain of Thrombospondin
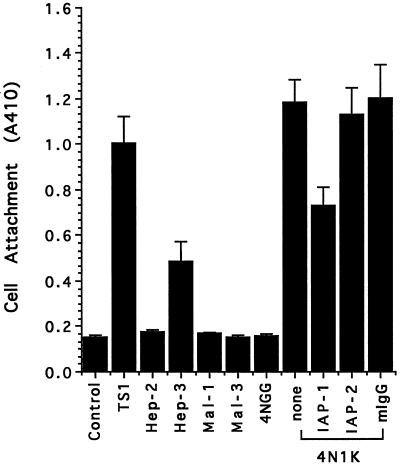
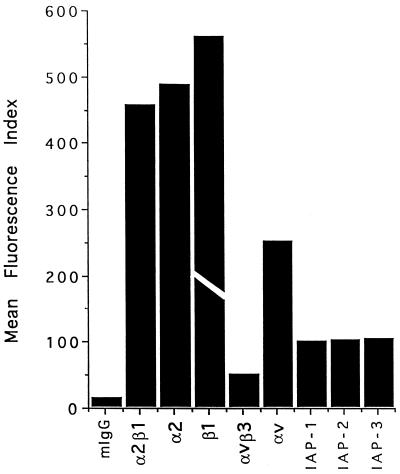
To confirm the interaction of smooth muscle cell with the thrombospondin-1 cell-binding domain and to identify other potential regions of smooth muscle cell interaction with thrombospondin-1, we screened a number of peptides derived from different domains of thrombospondin-1 for their ability to bind human and rat aortic smooth muscle cells. As seen in Figure 1, peptide 4N1K from the cell-binding domain of thrombospondin-1 bound human smooth muscle cells as well as intact thrombospondin-1. The control peptide 4NGG (kRFYGGMWKk) has a sequence identical with that of 4N1K except for the two glycine residues replacing the VV sequence. Thus, even though the heparin-binding peptide Hep-3 from the N-terminal heparin-binding domain of thrombospondin-1 binds the smooth muscle cell to a limited extent, the positively charged 4NGG binds the smooth muscle cells not at all. Identical results were obtained with rat aortic smooth muscle cells (not shown). The binding of cells to 4N1K was partially inhibited by the function blocking anti-IAP mAb 1F7, but not by 2D3, which binds to IAP but does not inhibit its function in a number of assays (Brown et al., 1990; Gao et al., 1996a,b; Chung et al., 1997). These binding data suggest that the newly identified thrombospondin-1 receptor IAP (Gao et al., 1996b) is at least partially responsible for the interaction of the smooth muscle cells with thrombospondin-1 in this assay. The expression of IAP on the surface of cultured human smooth muscle cells was confirmed by flow cytometry using three anti-IAP mAbs (Figure 2) as well as by specific affinity labeling with 125I-4N1K, which revealed the expected 52-kDa labeled protein (our unpublished results).
Figure 1.
Direct attachment of human smooth muscle cells to thrombospondin-1 and peptides from different domains of thrombospondin-1. Microtiter plates were coated with 50 μg/ml thrombospondin-1 or 50 μM peptides at 4°C overnight. Cells were suspended in 0.1% BSA in TBS or pretreated with 100 μg/ml antibodies in the same solution for 15 min at 37°C before being added to the wells. After 2 h at 37°C, the attached cells were quantified by the absorbance at 410 nm due to endogenous cellular phosphatase hydrolysis of p-nitrophenyl phosphate.
Figure 2.
Expression of IAP and integrins on human smooth muscle cells. The bars represent the mean fluorescence index observed for each of the antibodies indicated. Mouse IgG was used as a control. IAP-1 is 1F7; IAP-2 is 2D3; IAP-3 is B6H12. The experiment was repeated twice. The actual value of the mean fluorescence index for the anti-β1 mAb P4C10 was 1298. The bar is truncated in the figure to emphasize differences among the values for the other mAbs.
The IAP Agonist Peptide Is a Chemoattractant of Smooth Muscle Cells
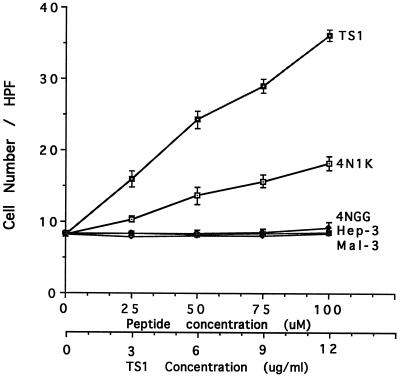
Intact thrombospondin-1 has previously been shown to mediate the chemotactic migration of calf pulmonary artery smooth muscle cells (Yabkowitz et al., 1993). We thus tested intact thrombospondin-1 as a chemoattractant of human and rat aortic smooth muscle cells (Figure 3), and found it to be a relatively potent attractant of both cell types. Since Yabkowitz et al. (1993) reported that the chemotaxis of calf pulmonary artery smooth muscle cells toward thrombospondin-1 was blocked by mAb C6.7 against the thrombospondin-1 cell-binding domain, we tested the 4N1K peptide from this domain as a chemoattractant of human and rat smooth muscle cells in the Boyden chamber assay using gelatin-coated filters as for whole thrombospondin-1. For both human (Figure 3) and rat (not shown) cells, 4N1K is an attractant while 4NGG, the control peptide, is devoid of activity. Other peptides from the heparin-binding domain and the type 1 repeats of thrombospondin-1 are also inactive (Figure 3), including Hep-3, which had a low level of cell binding activity (Figure 1). It should be noted that on an M basis, thrombospondin-1 is much more potent than 4N1K. This could be due to the fact that in thrombospondin-1 the 4N1K peptide is presumably in its native conformation as well as being trimeric. A checkerboard analysis of the migration of human smooth muscle cells in the Boyden chamber assay with 4N1K is shown in Table 1. This indicates that no matter what the absolute concentration of 4N1K peptide above or below the filter, the cells always respond with migration up the gradient of peptide, the hallmark of a true chemotactic, as opposed to chemokinetic or random response. These results indicate that the chemotaxis of smooth muscle cells toward thrombospondin-1 reported by Yabkowitz et al. (1993) is probably due to the activity of the cell-binding domain, specifically the IAP agonist sequence contained within peptide 4N1K.
Figure 3.
Thrombospondin-1 and its peptides stimulate human smooth muscle cell chemotaxis on gelatin-coated filters. The indicated concentrations of thrombospondin-1, 4N1K, 4NGG, Hep-3, and Mal-3 peptides were added to the lower compartments of the Boyden chamber. Data are mean ± SE of the number of migrated cells counted per high-power field determined over five fields for each of triplicate wells.
Table 1.
Checkerboard analysis of the chemotactic activity of 4N1K
| 4N1K in lower chamber (μM) | 4N1K in upper chamber (μM)
|
|||
|---|---|---|---|---|
| 0 | 25 | 50 | 10 | |
| 0 | 22 ± 3 | 18 ± 2 | 16 ± 3 | 11 ± 3 |
| 25 | 30 ± 4 | 24 ± 2 | 20 ± 3 | 18 ± 4 |
| 50 | 40 ± 5 | 32 ± 3 | 26 ± 4 | 16 ± 3 |
| 100 | 54 ± 7 | 39 ± 5 | 32 ± 4 | 23 ± 4 |
Different gradient conditions were created by adding various concentrations of 4N1K to the upper and lower compartment of the chamber as indicated. The motility response is expressed as mean ± SE cells per high-power field (five fields for each of triplicate wells were counted).
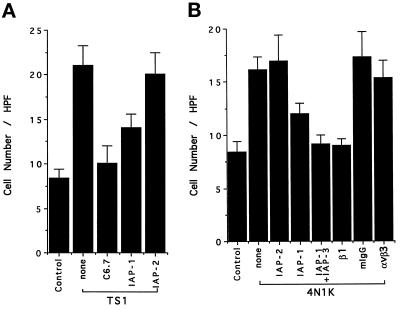
To determine the receptors responsible for the migration of the smooth muscle cells, they were tested in the Boyden chamber assay with thrombospondin-1 or 4N1K as the attractant and challenged with mAbs. Figure 4A shows that mAb C6.7, which binds to the cell-binding domain of thrombospondin-1, virtually eliminated migration toward thrombospodin-1, and mAb 1F7, a function blocking anti-IAP mAb, substantially reduced the migration of the cells toward thrombospondin-1 while the nonfunction-blocking anti-IAP mAb 2D3 had no effect. Thus, the cell-binding domain region of thrombospondin-1 is responsible for its chemotactic activity, which is mediated via IAP. We next characterized the chemotactic response of human smooth muscle cells to peptide 4N1K (Figure 4B). As with whole thrombospondin-1, mAb 2D3 had no effect while mAb 1F7 significantly inhibited migration. The addition of a second function blocking anti-IAP mAb B6H12 along with 1F7 resulted in total inhibition of directed migration.
Figure 4.
(A) Human smooth muscle cell migration to thrombospondin-1 is inhibited by anti-IAP and thrombospondin-1 antibodies. Smooth muscle cells were preincubated with 100 μg/ml C6.7 or 1F7, 2D3 at 37°C for 15 min before being added to the upper compartment of the Boyden chamber. 4N1K (100 μM) was present in the lower compartment, and TBS was used as control. (B) Human smooth muscle cell migration to 4N1K is inhibited by antibodies against IAP and β1 integrin. Smooth muscle cells were preincubated in the presence or absence of β1 mAb (1:1500) or other mAbs as indicated (100 μg/ml) at 37°C for 15 min before being added to the upper compartment of the Boyden chamber.
α2β1 Is Required for Chemotaxis to 4N1K Peptide
In previous studies with endothelial cells we found that chemotaxis to 4N1K on gelatin-coated filters required functional αvβ3 integrin. Thus, we challenged the smooth muscle cell response to 4N1K with an anti-αvβ3 function-blocking mAb 4C1 and found no effect (Figure 4B). FACS analysis revealed relatively little αvβ3 expressed on these cells (Figure 2). In view of the huge amount of β1 present on smooth muscle cells and previous reports of β1 integrin expression on them (Skinner et al., 1994; Liaw et al., 1995), we tested the anti-β1 mAb P4C10 and found that it completely blocked chemotaxis (Figure 4B). Since α2β1 integrin has previously been shown to be the major collagen-binding integrin on cultured smooth muscle cells (Davis, 1992; Coso et al., 1995; Liaw et al., 1995), we determined the expression of α2 (mAb P1E6) and α2β1 (mAb BHA2.1) on the human aortic smooth muscle cells. These two mAbs gave virtually identical staining and indicate robust expression of α2β1 on the cells. In addition, mAb BHA2.1 directed against α2β1 completely inhibited adhesion of the cells to immobilized collagen I and gelatin (our unpublished results).
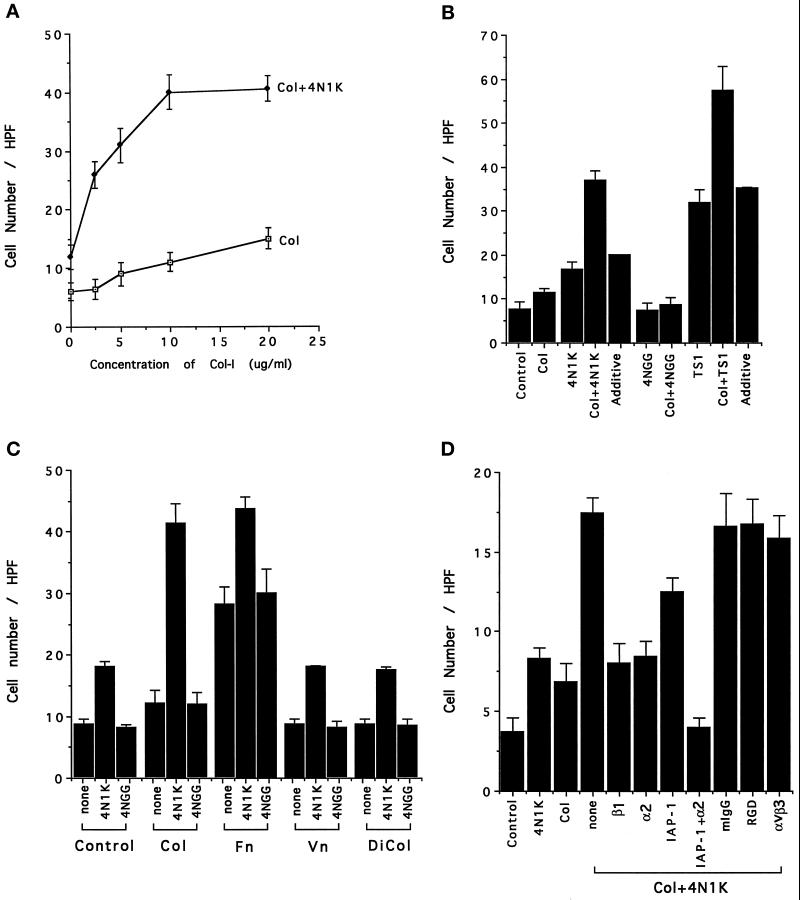
4N1K and Soluble Collagen Stimulate Chemotaxis Synergistically
We next tested the ability of the aortic smooth muscle cells to migrate toward soluble, native collagen type I (Nelson et al., 1996) in the Boyden chamber assay and found that these cells displayed a relatively weak but significant chemotactic response (Figure 5A) similar to that with 4N1K (Figures 3 and 4). However, when soluble collagen I was tested in combination with 4N1K peptide (Figure 5A), the stimulation of cell migration was synergistic (compare the Col + 4N1K with the calculated “additive” response in Figure 5B). As before, the control peptide 4NGG was completely inactive and was unable to synergize with soluble collagen to give an increased response. Intact thrombospondin-1 gave the expected strong response and was also synergistic with native collagen (Figure 5B). To determine whether 4N1K could stimulate the response of smooth muscle cells to other matrix proteins, soluble collagen type I (Col), fibronectin (Fn), vitronectin (Vn), and collagenase-digested collagen-I (DiCol) were each tested as chemoattractants in the presence of no additives, 4N1K, or 4NGG (both at 100 μM). As seen in Figure 5C, native collagen-I and 4N1K together gave a much stronger response than 4NGG plus native collagen-I, while in the cases of fibronectin, vitronectin, and digested collagen, the amount of additional migration in the presence of 4N1K is only that expected from the action of 4N1K alone. Thus the 4N1K synergy response seems to be limited to native soluble collagen-I.
Figure 5.
(A) The synergistic effect of 4N1K and soluble collagen-I on rat smooth muscle cell chemotaxis. 4N1K (100 μM) and increasing concentrations of soluble collagen-I were used as chemoattractant. (B) Both 4N1K and thrombospondin-1 are synergistic with collagen-I. Collagen-I was present at 5 μg/ml along with 100 μM 4N1K or 4NGG, or 6 μg/ml thrombospondin-1 as chemoattractants. The experimentally observed values for the concerted effect of 4N1K, thrombospondin-1, and collagen (4N1K±Col, TS1±Col) were significantly greater than the calculated additive effect (additive). (C) Comparison of the effect of soluble collagen-I (Col), fibronectin (Fn), vitronectin (Vn), and digested collagen-I (DiCol) on 4N1K- induced rat smooth muscle cell chemotaxis. Collagen-I (5 μg/ml) was digested with 0.4 U/ml collagenase type I at 37°C overnight. 4N1K or 4NGG (100 μM) and 5 μg/ml of the different matrix proteins in MEM with 0.1% BSA were added to the lower compartment of Boyden chamber. (D) The effect of mAbs on collagen- and 4N1K- induced human smooth muscle cell chemotaxis. Smooth muscle cells were preincubated with mAbs at 37°C for 15 min before being added to the upper compartment of the Boyden chamber. The concentrations of mAbs were: anti-β1 mAb P4C10 (1:1500 dilution), anti-α2 mAb P1E6 (1:3000 dilution), anti-IAP-1 mAb 1F7 100 μg/ml, mIgG 100 μg/ml, RGD peptide 50 μM, anti-αvβ3 mAb 4C1 100 μg/ml.
To determine the nature of this response, the effect of mAbs against integrins and IAP was examined. As seen in Figure 5D, 4N1K again synergizes with soluble native type I collagen (none = no inhibitor added). MAbs specific for both the β1 (P4C10) and α2 (P1E6) integrin subunits reduce the migration to that seen for 4N1K alone. MAb 1F7 against IAP partially reduces migration, but in combination with mAb P1E6 against the α2 integrin subunit, it reduces migration to background levels. Mouse IgG, RGD peptide, and mAb 4C1, a function-blocking mAb against αvβ3, were all without effect on smooth muscle cell migration stimulated by collagen plus 4N1K.
IAP and α2β1 Form a Stable Complex
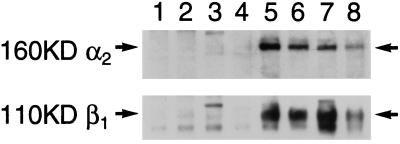
When αvβ3 or αIIbβ3 functions are modulated by 4N1K/IAP, the IAP is found in a detergent-stable complex with the integrins (Gao et al., 1996a,b; Chung et al., 1997). Thus we asked whether IAP could associate with α2β1 in these smooth muscle cells. Human smooth muscle cells were lysed in n-octyl-β-d-glucopyranoside, and the clarified lysate immunoprecipitated with a number of control antibodies as well as mAbs against IAP. The immunoprecipitates were then run on SDS gels, blotted onto nitrocellulose, and probed with antibodies specific for the α2 and β1 integrin chains. Figure 6 shows that both α2 and β1 integrin subunits are recovered in IAP immunoprecipitates performed with two different antiIAP mAbs, but not in those using control antibodies from two different suppliers (lanes 1 and 4) or anti-HLA (lane 2) or anti-TNF receptor (lane 3) mAbs. The same experiment was performed with Triton X-100 detegent lysates of human smooth muscle cell with comparable results, indicating that the complex of the α2β1 integrin with IAP is not detergent-dependent and probably does not require the integrity of “detergent resistant” domains (Brown and Rose 1992; Hanada et al., 1995).
Figure 6.
Association of IAP and α2β1 integrin. Human smooth muscle cells were harvested by trypsin/EDTA and lysed in 30 mM n-octy-β-d-glucopyranoside as described in MATERIALS AND METHODS. Soluble material from equal numbers of cells was immunoprecipitated with the following mAbs: mouse IgG from Sigma (lane 1), anti-HLA (lane 2), anti-TNF receptor (lane 3), mouse IgG from Pierce (lane 4), anti-α2β1 BHA2.1 (lane 5), anti-IAP 2D3 and 1F7 (lanes 6 and 7). Lane 8 is a total cell lysate. α2 and β1 subunits were identified by Western blotting with polyclonal antibodies and ran at the expected sizes of 160 and 110 kDa, as indicated by the arrows.
DISCUSSION
The present data indicate that thrombospondin-1, through its IAP-binding motif, the 4N1K peptide, is able to modulate the activity of the α2β1 integrin such that it can promote chemotaxis of arterial smooth muscle cells to soluble collagen-I. The enhanced ability of integrins to bind soluble ligands is often associated with “affinity modulation” or “inside-out” signaling (Shattil et al., 1994; Chung et al., 1997). In cells in which IAP activates a β3 integrin such as αvβ3 in C32 cells (Gao et al., 1996a) and αIIbβ3 in platelets (Chung et al., 1997), protein kinase C (PKC) and PI-3 kinase activation are required. Our preliminary data indicate that both PKC and PI-3 kinase are also involved in the stimulation of α2β1-dependent chemotaxis in smooth muscle cells. These results are in agreement with the β3 systems and suggest that IAP is able to activate the α2β1 integrin in such a way that it can bind soluble collagen and transduce signals leading to directional migration. The αIIbβ3 integrin is maintained in a low-affinity/avidity state in circulating platelets such that it cannot bind its primary ligand, soluble fibrinogen, which is present at a high concentration in plasma. Upon activation by “inside-out” signaling originating with IAP or other costimulatory receptors, i.e., thrombin, ADP, or epinephrine receptors, the integrin is able to bind the soluble ligand, and platelet aggregation ensues (Shattil et al., 1994; Chung et al., 1997). The fact that chemotaxis toward soluble collagen is augmented by the 4N1K-IAP interaction suggests that it is the affinity of α2β1 for a soluble ligand that is being modulated here. It is well known that α2β1 can exist in three states depending upon the cell type in which the integrin is expressed (Santoro and Zutter, 1995); perhaps these states are determined by the expression levels of IAP and/or thrombospondin-1, or the competition for available IAP by the integrin population expressed on the different cells.
The human smooth muscle cells used in these studies express relatively little αvβ3 (as determined by FACS with an anti-αvβ3 mAb, Figure 2). Their interaction with both native and denatured collagen-I is mediated by α2β1 (as determined in cell adhesion assays, our unpublished results). This is consistent with reports from other laboratories which show that, although apparently not present on normal aortic smooth muscle cell in situ, α2β1 is expressed abundantly on cultured smooth muscle cells (Skinner et al., 1994; Liaw et al., 1995). While the cells used in our studies probably express lower levels of other collagen-binding β1 integrins such as α3β1 (Skinner et al., 1994), the fact that the anti-α2 mAb reduces the chemotactic response to the same low level as the anti-β1 mAb (Figure 6D) indicates that the response to collagen, which is amplified by 4N1K, is mediated entirely by α2β1. Thus it appears that the previously reported chemotactic activity of thrombospondin-1 for smooth muscle cells (Yabkowitz et al., 1993) can be explained by the activity of the 4N1K peptide, which resides in the cell-binding domain. This conclusion is strengthened by the observation of Yabkowitz et al. (1993) that, of our panel of domain-specific anti-thrombospondin-1 mAbs, only mAb C6.7 against the cell-binding domain could inhibit the stimulation of chemotaxis. However, their study used bovine pulmonary artery smooth muscle cells, and found that migration of those cells on gelatin-coated polycarbonate filters was dependent on αvβ3 integrin (Yabkowitz et al., 1993), as we have found for human umbilical vein endothelial cells, and not α2β1 as seen here for human aortic smooth muscle cells. The types and levels of integrins expressed by the bovine smooth muscle cells used in that study were not investigated (Yabkowitz et al., 1993). Thus, while the integrin may be different, the mechansim of the stimulation by thrombospondin-1 is probably the same, i.e., modulation of the integrin’s affinity/avidity by IAP.
An important aspect of these data is that it extends the biological relevance of the thrombospondin–IAP interaction to another subset of integrins, the β1 family. Not only does ligation of IAP with the agonist peptide 4N1K augment the function of α2β1 in sensing a gradient of soluble collagen, but we have demonstrated for the first time, the existence of a physical complex that includes IAP and α2β1 integrin (Figure 6). In addition to the coimmunoprecipitation of α2β1 and IAP, our preliminary data indicate that α2 and β1 integrin subunits coelute with IAP from a 4N1K affinity column (Wang and Frazier, unpublished). Neither of these methods can of course distinguish between a direct or indirect association. It has been previously reported that IAP can physically associate with αvβ3 (Brown et al., 1990), and we have found that IAP copurifies and coimmunoprecipitates with αIIbβ3 from platelet lysates (Chung et al., 1997). In the case of both of these β3 integrins, it appears that IAP associates with the integrin that it modulates, even though these signaling pathways require activation of PKC (Gao et al., 1996a; Chung et al., 1997), and hence would not, a priori, seem to require association. In the one case in which IAP had been shown to modulate a β1 integrin (Blystone et al., 1994, 1995), signaling emanating from a complex of αvβ3 and IAP inhibited a high-affinity state of α5β1. These observations suggest that association of IAP with the integrin may be necessary for a positive modulatory effect. The signaling pathways by which this occurs are currently under study.
The modulation of α2β1 integrin in smooth muscle cells by the IAP-binding domain of thrombospondin-1 may have physiological implications even though expression of α2β1 on smooth muscle cells in vivo has not been reported (Skinner et al., 1994; Gotwals et al., 1996). Freshly isolated smooth muscle cells from the same sources as those used in our study express large amounts of α1β1 and α3β1 (Skinner et al., 1994). If the mechanism of integrin modulation found for α2β1 in vitro is in place in smooth muscle cells in vivo, the regulated synthesis and/or secretion of thrombospondin-1 at sites of wounding, atherosclerosis and restenosis may affect the function of these β1 integrins as well. Not only is thrombospondin-1 deposited at sites of vessel injury due to platelet discharge, either chronic or acute, but thrombospondin-1 is a major biosynthetic product of endothelial cells, fibroblasts, macrophages, and PDGF-stimulated smooth muscle cells themselves (Adams et al., 1995). Thus the local deposition or biosynthesis of thrombospondin-1 could generate a positively reinforced loop, resulting in the continued attraction of smooth muscle cells into injured sites. The recent finding that α2β1 is involved in collagen-dependent cell cycle regulation in cultured smooth muscle cells (Koyama et al., 1996) suggests a role for β1 integrins in control of smooth muscle cell proliferation. It also opens the possibility that thrombospondin-IAP interactions may regulate smooth muscle cell proliferation by modulating the ability of β1 integrins to signal to the proteins that regulate the cell cycle (Mechtersheimer et al., 1994). Finally, the physiological role of thrombospondin is underscored by our recent data using a rat carotid artery balloon injury model in which mAb C6.7 against the thrombospondin-1 cell binding domain significantly inhibited both neointimal thickening and smooth muscle cell proliferative index at the site of injury (Chen et al., 1997). Whether this effect is due to integrin modulation via IAP remains to be determined.
ACKNOWLEDGMENTS
We thank Drs. Eric Brown, Samuel Santoro, Fred Lindberg, Scott Blystone, and Ai-Guo Gao for helpful discussions and advice, Dr. Eric Brown for mAbs against IAP, and Anna Goffinet for preparation of the manuscript. We thank Dr. Kevin Glenn of Monsanto-Searle for helpful discussions. This work was supported by funds from Monsanto-Searle (fellowship to X.-Q.W.).
REFERENCES
- Abedi H, Dawes KE, Zachary I. Differential effects of platelet-derived growth factor BB on p125 focal adhesion kinase and paxillin tyrosine phosphorylation and on cell migration in rabbit aortic vascular smooth muscle cells and Swiss 3T3 fibroblasts. J Biol Chem. 1995;270:11367–11376. doi: 10.1074/jbc.270.19.11367. [DOI] [PubMed] [Google Scholar]
- Adams JC, Tucker RP, Lawler J. The Thrombospondin Gene Family. Austin, TX: R.G. Landes Company; 1995. [Google Scholar]
- Asch AS, Barnwell J, Silverstein RL, Nachman RL. Isolation of the thrombospondin-1 membrane receptor. J Clin Invest. 1987;79:1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch AS, Silbiger S, Heimer E, Nachman RL. Thrombospondin sequence motif (CSVTCG) is responsible for CD 36 binding. Biochem Biophys Res Commun. 1992;182:1208–1217. doi: 10.1016/0006-291x(92)91860-s. [DOI] [PubMed] [Google Scholar]
- Asch AS. The role of CD 36 as thrombospondin-1 receptor. In: Lahav J, editor. Thrombospondin. Boca Raton, FL: CRC Press; 1993. pp. 265–275. [Google Scholar]
- Asch AS, Liu I, Briccetti FM, Barnwell JW, Kwakye-Berko F, Dokun A, Goldberger J, Pernambuco M. Analysis of CD 36 binding domains: Ligand specificity controlled by dephosphorylation of an ectodomain. Science. 1993;262:1436–1440. doi: 10.1126/science.7504322. [DOI] [PubMed] [Google Scholar]
- Blystone SD, Graham IL, Lindberg FP, Brown EJ. Integrin αvβ3 differentially regulates adhesive and phagocytic functions of the fibronectin receptor α5β1. J Cell Biol. 1994;127:1129–1137. doi: 10.1083/jcb.127.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blystone SD, Lindberg FP, LaFlamme SE, Brown EJ. Integrin β3 cytoplasmic tail is necessary and sufficient for regulation of α5β1 phagocytosis by αvβ3 and integrin-associated protein. J Cell Biol. 1995;130:745–754. doi: 10.1083/jcb.130.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brown E, Hooper L, Ho T, Gresham H. Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol. 1990;111:2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D., Asahara, T., Krasinski, K., Witzenbllcher, B., Yang, J., Magner, M., Kearney, M., Frazier, W.A., and Isner, J.M. (1998). Antibody blockade of thrombospondin-1 in rat carotid artery balloon injury model. Circulation (in press). [DOI] [PubMed]
- Chung J, Gao A-G, Frazier WA. Thrombospondin acts via integrin-associated protein to activate the platelet integrin αIIbβ3. J Biol Chem. 1997;272:14740–14746. doi: 10.1074/jbc.272.23.14740. [DOI] [PubMed] [Google Scholar]
- Cooper D, Lindberg FP, Gamble GR, Brown EJ, Vadas MA. Transendothelial migration of neutrophils involves integrin-associated protein (CD47) Proc Natl Acad Sci USA. 1995;92:3978–3982. doi: 10.1073/pnas.92.9.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coso OA, Chiariello M, Yu J-C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins rac 1 and cdc 42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Davis GE. Affinity of integrins for damaged extracellular matrix: αvβ3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun. 1992;182:1025–1031. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- Dawson DW, Pearce FA, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD 36 mediates the inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ. Thrombospondin 1 synthesis and function in wound repair. Am J Pathol. 1996;148:1851–1860. [PMC free article] [PubMed] [Google Scholar]
- Felding-Habermann B, Cheresh DA. Vitronectin and its receptors. Curr Opin Cell Biol. 1993;5:864–868. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- Gao AG, Frazier WA. Identification of a receptor candidate for the carboxyl-terminal cell binding domain of thrombospondins. J Biol Chem. 1994;269:29650–29657. [PubMed] [Google Scholar]
- Gao AG, Lindberg FP, Dimitry JM, Brown EJ, Frazier WA. Thrombospondin modulates αvβ3 function through integrin associated protein. J Cell Biol. 1996a;135:533–544. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin-1. J Biol Chem. 1996b;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- Gotwals PJ, Chi-Rosso G, Linder V, Yang J, Ling L, Fawell SE, Koteliansky VE. The α1β1 integrin is expressed during neointima formation in rat arteries and mediates collagen matrix reorganization. J Clin Invest. 1996;97:2469–2477. doi: 10.1172/JCI118693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Nishijima M, Akamatsu Y, Pagano RE. Both sphingolipids and cholesterol participate in the detergent insolubility of alkaline phosphatase, a glycosylphosphatidylinositol-anchored protein, in mammalian membranes. J Biol Chem. 1995;270:6254–6260. doi: 10.1074/jbc.270.11.6254. [DOI] [PubMed] [Google Scholar]
- Janat MF, Liau G. Transforming growth factor β1 is a powerful modulator of platelet-derived growth factor action in vascular smooth muscle cells. J Cell Physiol. 1992;150:232–242. doi: 10.1002/jcp.1041500203. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Yamamoto T. The molecular biologic study of the expression of thrombospondin-1 in vascular smooth muscle cells and mesangial cells. J Diabetic Complications. 1991;5:121–123. doi: 10.1016/0891-6632(91)90040-v. [DOI] [PubMed] [Google Scholar]
- Kosfeld MD, Frazier WA. Identification of a new cell adhesion motif in two homologous peptides from the COOH-terminal cell binding domain of human thrombospondin-1. J Biol Chem. 1993;268:8808–8814. [PubMed] [Google Scholar]
- Koyama H, Raines EW, Eornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- Lawler J, Hynes RO. An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin-1 [see comments] Blood. 1989;74:2022–2027. [PubMed] [Google Scholar]
- Leavesley DI, Ferguson GD, Wayner EA, Cheresh DA. Requirement of the integrin β3 subunit for carcinoma cell spreading or migration on vitronectin and fibrinogen. J Cell Biol. 1992;117:1101–1107. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau G, Winkles JA, Cannon MS, Kuo L, Chilian WM. Dietary-induced atherosclerotic lesions have increased levels of acidic FGF mRNA and altered cytoskeletal and extracellular matrix mRNA expression. J Vasc Res. 1993;30:327–332. doi: 10.1159/000159014. [DOI] [PubMed] [Google Scholar]
- Liaw L, Skinner MP, Raines EW, Ross R, Cheresh DA, Schwartz SM. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. J Clin Invest. 1995;95:713–724. doi: 10.1172/JCI117718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg FP, Gresham HD, Schwarz E, Brown EJ. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in alpha v beta 3-dependent ligand binding. J Cell Biol. 1993;123:484–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg FP, Lublin DM, Telen MJ, Veile RA, Miller YE, Donis-Keller H, Brown EJ. Rh-related antigen CD 47 is the signal-transducer integrin-associated protein. J Biol Chem. 1994;269:1567–1570. [PubMed] [Google Scholar]
- Majack RA, Cook SC, Bornstein P. Platelet-derived growth factor and heparin-like glycosaminoglycans regulate thrombopsondin synthesis and deposition in the matrix by smooth muscle cells. J Cell Biol. 1985;101:1059–1070. doi: 10.1083/jcb.101.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack RA, Cook SC, Bornstein P. Control of smooth muscle cell growth by components of the extracellular matrix: autocrine role for thrombospondin-1. Proc Natl Acad Sci USA. 1986;83:9050–9054. doi: 10.1073/pnas.83.23.9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack RA, Mildbrandt J, Dixit VM. Induction of thrombospondin-1 messenger RNA levels occurs as an immediate primary response to platelet-derived growth factor. J Biol Chem. 1987;262:8821–8825. [PubMed] [Google Scholar]
- Majack RA, Goodman LV, Dixit VM. Cell surface thrombospondin-1 is functionally essential for vascular smooth muscle cell proliferation. J Cell Biol. 1988;106:415–422. doi: 10.1083/jcb.106.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechtersheimer G, Barth T, Quentmeier A, Moller P. Differential expression of β1 integrin in nonneoplastic smooth and striated muscle cells and in tumors derived from these cells. Am J Pathol. 1994;114:1172–1182. [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Gurusiddappa S, Frazier WA, Hook M. Heparin-binding peptides from thrombospondins 1 and 2 contain focal adhesion-labilizing activity. J Biol Chem. 1993;268:26784–26789. [PubMed] [Google Scholar]
- Nelson PR, Yamamura S, Kent KC. Extracellular matrix proteins are potent agonists of human smooth muscle cell migration. J Vasc Surg. 1996;24:25–33. doi: 10.1016/s0741-5214(96)70141-6. [DOI] [PubMed] [Google Scholar]
- Parkos CA, Colgan SP, Liang TW, Nusrat A, Bacarra AE, Carnes DK, Madara JL. CD 47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. J Cell Biol. 1996;132:437–450. doi: 10.1083/jcb.132.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Kariya B. Circulation, vascular smooth muscle. In: Bohr DF, Somlyo AP, Sparks V, editors. Handbook of Physiology. The Cardiovascular System. Bethesda, MD: American Physiological Society; 1980. pp. 69–91. [Google Scholar]
- Santoro SA, Frazier WA. Isolation and characterization of thrombospondin-1. Methods Enzymol. 1987;144:438–446. doi: 10.1016/0076-6879(87)44193-1. [DOI] [PubMed] [Google Scholar]
- Santoro SA, Zutter MM. The α2β1 integrin: a collagen receptor on platelets and other cells. Thromb Haemostasis. 1995;74:813–821. [PubMed] [Google Scholar]
- Scott-Burden, T., Resink, T.J., Hahn, A.W.A., and Buhler, F.R. (1990). Induction of thrombospondin-1 expression in vascular smooth muscle cells by angiotension II. J. Cardiovasc. Pharmacol. 16(suppl 7), S17–S20. [PubMed]
- Shattil SJ, Ginsberg MH, Brugge JS. Adhesive signaling in platelets. Curr Opin Cell Biol. 1994;6:695–704. doi: 10.1016/0955-0674(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Skinner MP, Raines EW, Ross R. Dynamic expression of α1β1 and α2β1 integrin receptors by human vascular smooth muscle cells. α2β1 integrin is required for chemotaxis across type I collagen-coated membranes. Am J Pathol. 1994;145:1070–1081. [PMC free article] [PubMed] [Google Scholar]
- Sun X, Mosher DF, Rapraeger A. Heparan sulfate-mediated binding of epithelial cell surface proteoglycan to thrombospondin-1. J Biol Chem. 1989;264:2885–2889. [PubMed] [Google Scholar]
- Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zanten GH, de Graaf S, Slootweg PJ, Heijnen HFG, Connolly TM, de Groot PG, Sixma JJ. Increased platelet deposition on atherosclerotic coronary arteries. J Clin Invest. 1994;93:615–632. doi: 10.1172/JCI117014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson PC, Allan RB. Leukocyte Chemotaxis. J.I. Gallin and P.G. Quie, New York: Raven Press; 1978. Assay systems for measuring leukocyte locomotion: an overview; pp. 1–7. [Google Scholar]
- Yabkowitz R, Mansfield PJ, Ryan US, Suchard SJ. Thrombospondin mediates migration and potentiates platelet-derived growth factor-dependent migration of calf pulmonary artery smooth muscle cells. J Cell Physiol. 1993;157:24–32. doi: 10.1002/jcp.1041570104. [DOI] [PubMed] [Google Scholar]
- Zigmond SH, Hirsch JG. Leukocyte locomotion and chemotaxis. J Exp Med. 1973;137:387–391. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]