Abstract
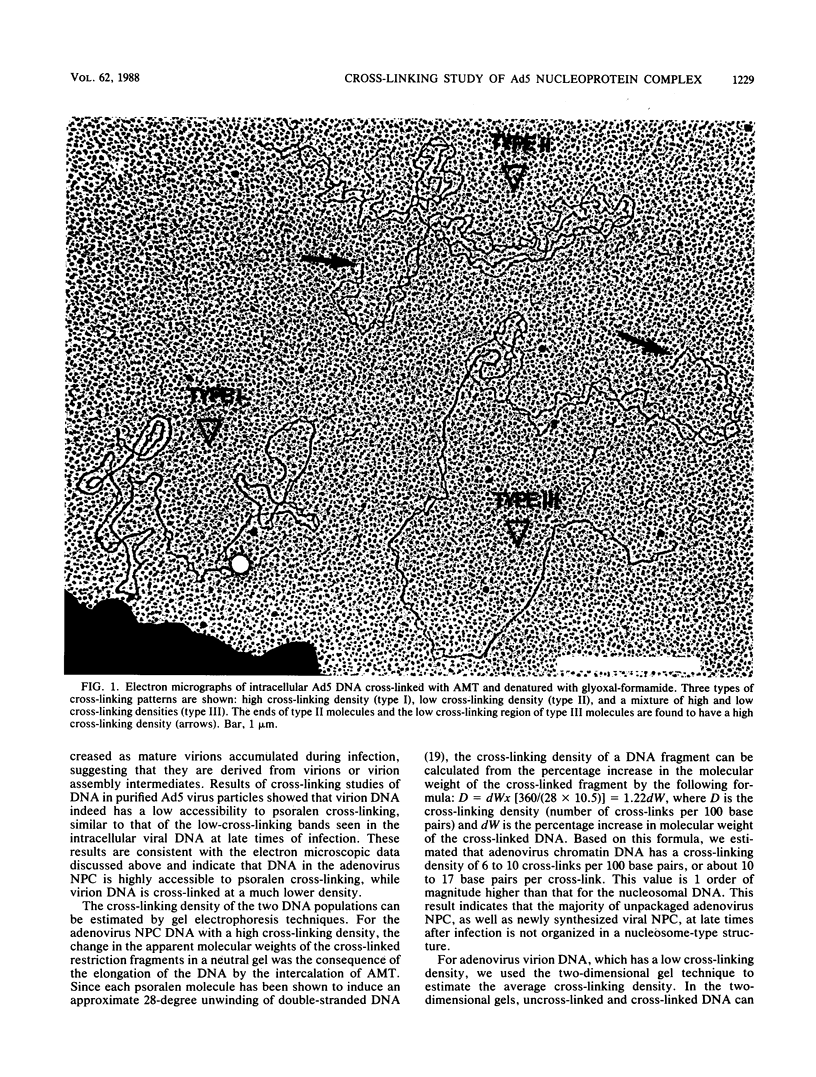
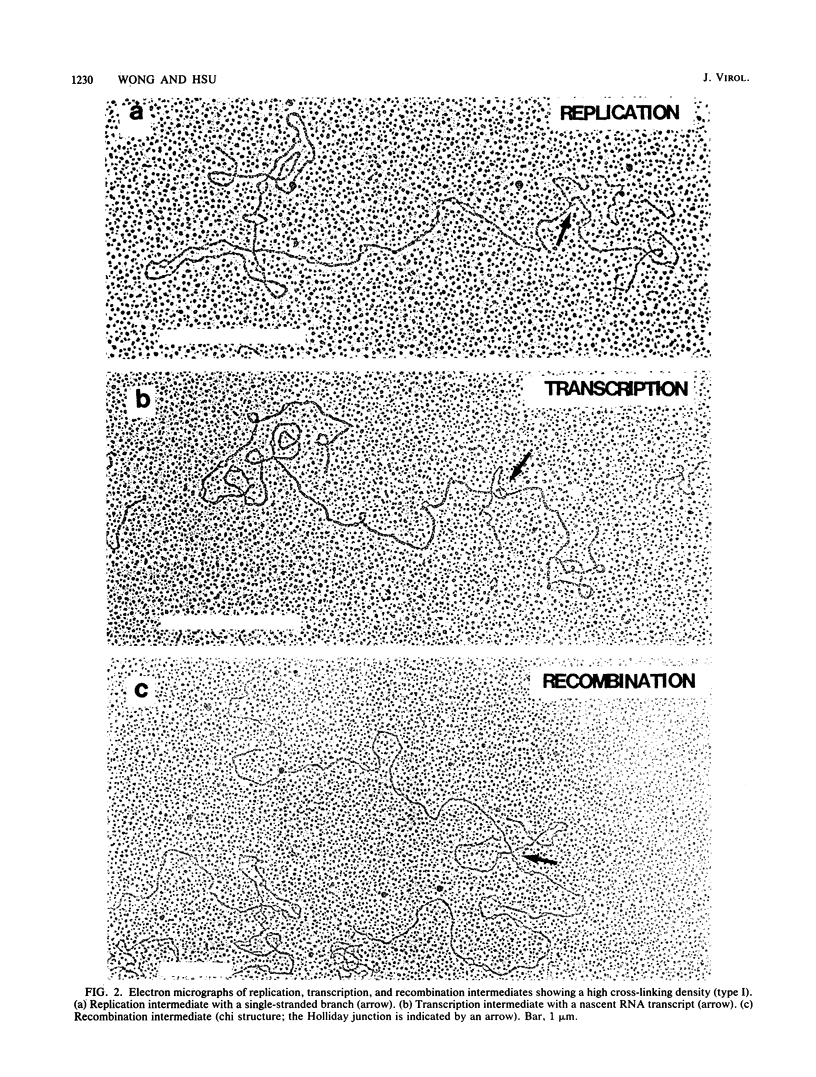
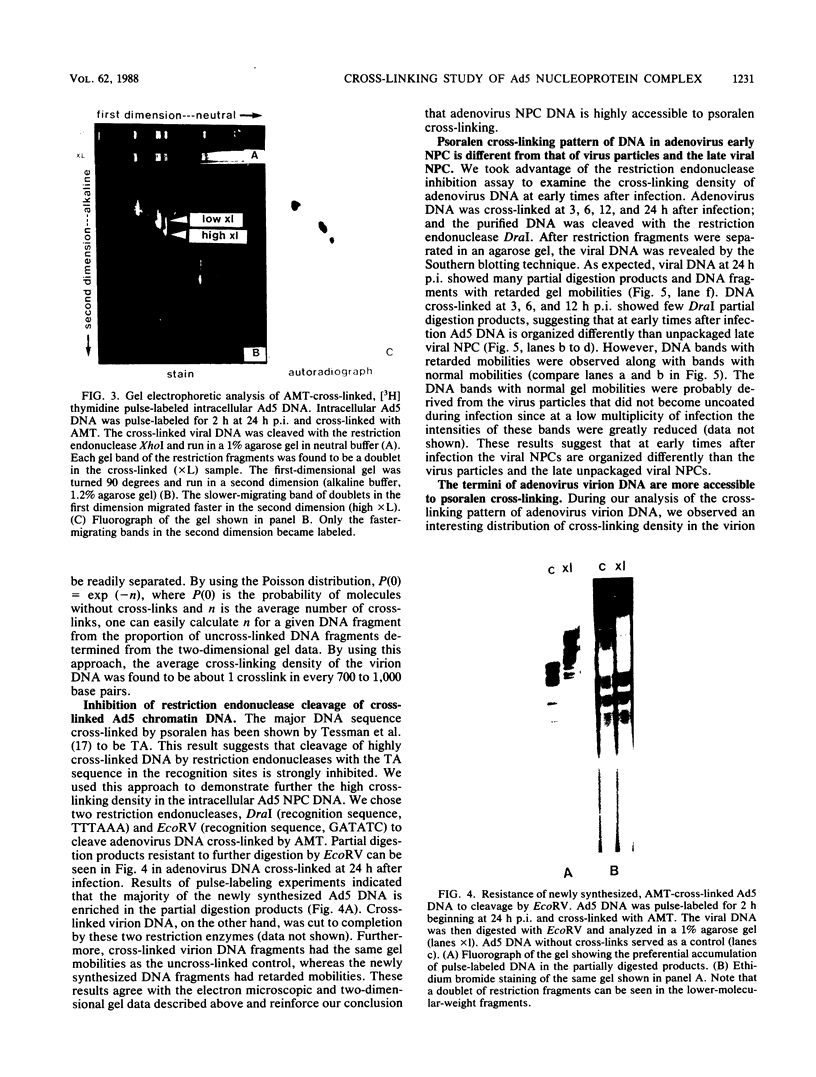
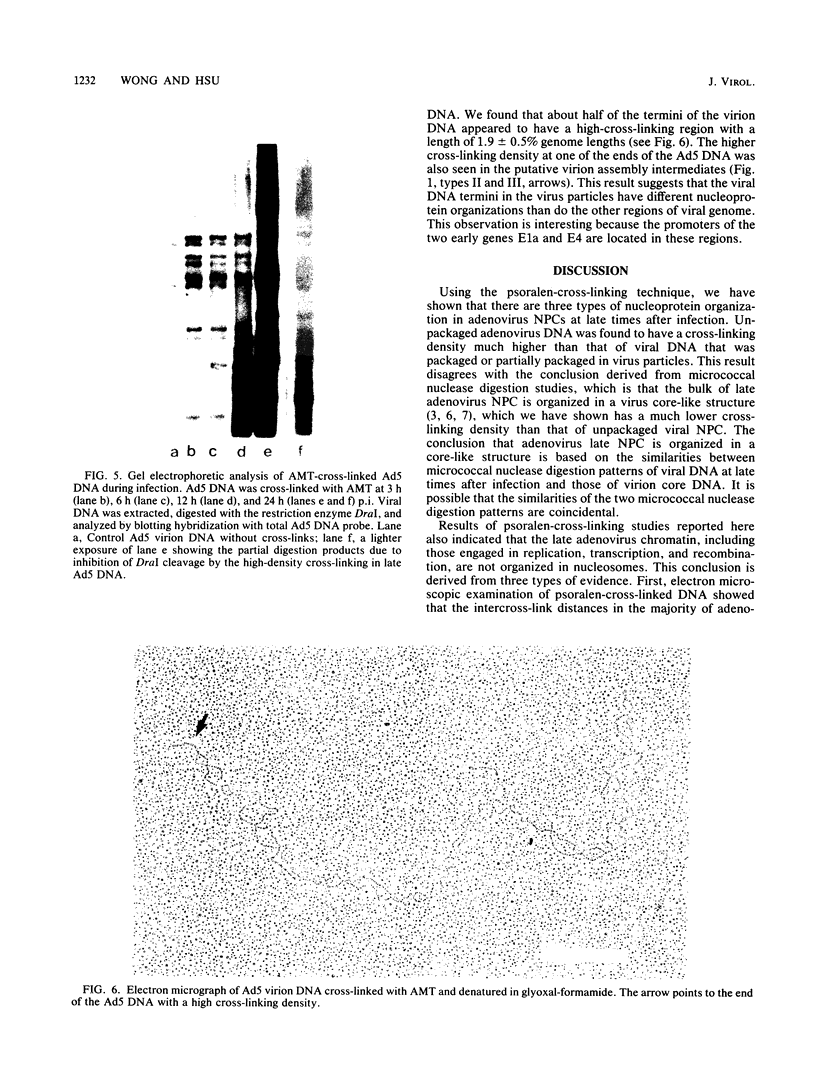
We used the psoralen-cross-linking technique to investigate the structures of adenovirus nucleoprotein complexes during infection. At late times after infection, three types of psoralen cross-linking patterns were observed. A high cross-linking pattern (type I), with about one cross-link in every 10 to 17 base pairs, was found for the newly synthesized and the bulk of the adenovirus late chromatin. Viral templates involved in replication, transcription, and recombination were all found to exhibit this cross-linking pattern. These results suggest that there is no nucleosome-like organization in the unpackaged late adenovirus nucleoprotein complexes. The second type of cross-linking pattern (type II) had a low cross-linking density of about one cross-link in every 700 to 1,000 base pairs. This cross-linking pattern was found to be associated with the viral DNA in the mature virus particles. The sequences at the termini of the virion DNAs, however, were found to have higher cross-linking densities, as shown by electron microscopy. The third type of cross-linking pattern (type III) was composed of a mixture of various proportions of type I and type II patterns in a single molecule. This mixed cross-linking pattern suggests that these molecules are virion assembly intermediates, with viral DNA being partially packaged in the virus particles. The organization of adenovirus nucleoprotein complexes at early times after infection was analyzed by the gel electrophoresis technique following digestion of the DNA with a restriction enzyme that was inhibited by cross-links. Our data suggest that the viral nucleoprotein complexes at early times after infection have accessibility to psoralen cross-linking between the virion DNA and the late viral nucleoprotein complexes. The observed cross-linking density of the early nucleoprotein complex DNA, however, was inconsistent with the nucleosomelike organization suggested by previous investigators.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Bouton A. H., Hodge L. D., Miller O. L., Jr Visualization of the major late R strand transcription unit of adenovirus serotype 2. J Mol Biol. 1981 Apr 5;147(2):269–295. doi: 10.1016/0022-2836(81)90441-1. [DOI] [PubMed] [Google Scholar]
- Brown M., Weber J. Virion core-like organization of intranuclear adenovirus chromatin late in infection. Virology. 1980 Nov;107(1):306–310. doi: 10.1016/0042-6822(80)90297-4. [DOI] [PubMed] [Google Scholar]
- Chatterjee P. K., Vayda M. E., Flint S. J. Adenoviral protein VII packages intracellular viral DNA throughout the early phase of infection. EMBO J. 1986 Jul;5(7):1633–1644. doi: 10.1002/j.1460-2075.1986.tb04406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Daniell E., Groff D. E., Fedor M. J. Adenovirus chromatin structure at different stages of infection. Mol Cell Biol. 1981 Dec;1(12):1094–1105. doi: 10.1128/mcb.1.12.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déry C. V., Toth M., Brown M., Horvath J., Allaire S., Weber J. M. The structure of adenovirus chromatin in infected cells. J Gen Virol. 1985 Dec;66(Pt 12):2671–2684. doi: 10.1099/0022-1317-66-12-2671. [DOI] [PubMed] [Google Scholar]
- Hsu M. T. Electron microscopic evidence for the cruciform structure in intracellular SV40 DNA. Virology. 1985 Jun;143(2):617–621. doi: 10.1016/0042-6822(85)90400-3. [DOI] [PubMed] [Google Scholar]
- Kedinger C., Brison O., Perrin F., Wilhelm J. Structural analysis of viral replicative intermediates isolated from adenovirus type 2-infected HeLa cell nuclei. J Virol. 1978 May;26(2):364–379. doi: 10.1128/jvi.26.2.364-379.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuguchi M., Puvion-Dutilleul F., Moyne G. Late transcription and simultaneous replication of simian adenovirus 7 DNA as revealed by spreading lytically infected cell cultures. J Gen Virol. 1979 Mar;42(3):443–456. doi: 10.1099/0022-1317-42-3-443. [DOI] [PubMed] [Google Scholar]
- Mirza M. A., Weber J. Structure of adenovirus chromatin. Biochim Biophys Acta. 1982 Jan 26;696(1):76–86. doi: 10.1016/0167-4781(82)90012-4. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Control of cellular and viral transcription during adenovirus infection. CRC Crit Rev Biochem. 1986;19(4):307–322. doi: 10.3109/10409238609082543. [DOI] [PubMed] [Google Scholar]
- Rinaldy A., Feunteun J., Rosenberg B. H. Prereplicative events involving simian virus 40 DNA in permissive cells. J Virol. 1982 Jan;41(1):237–243. doi: 10.1128/jvi.41.1.237-243.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant A., Tigges M. A., Raskas H. J. Nucleosome-like structural subunits of intranuclear parental adenovirus type 2 DNA. J Virol. 1979 Mar;29(3):888–898. doi: 10.1128/jvi.29.3.888-898.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo J. M., Ness P. J., Widmer R. M., Parish R. W., Koller T. Psoralen-crosslinking of DNA as a probe for the structure of active nucleolar chromatin. J Mol Biol. 1984 Oct 5;178(4):897–919. doi: 10.1016/0022-2836(84)90318-8. [DOI] [PubMed] [Google Scholar]
- Tate V. E., Philipson L. Parental adenovirus DNA accumulates in nucleosome-like structures in infected cells. Nucleic Acids Res. 1979 Jun 25;6(8):2769–2785. doi: 10.1093/nar/6.8.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman J. W., Isaacs S. T., Hearst J. E. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. Conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985 Mar 26;24(7):1669–1676. doi: 10.1021/bi00328a015. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Mathews M. B. DNA replication and the early to late transition in adenovirus infection. Cell. 1980 Nov;22(2 Pt 2):523–533. doi: 10.1016/0092-8674(80)90362-1. [DOI] [PubMed] [Google Scholar]
- Wiesehahn G., Hearst J. E. DNA unwinding induced by photoaddition of psoralen derivatives and determination of dark-binding equilibrium constants by gel electrophoresis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2703–2707. doi: 10.1073/pnas.75.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv M., Cereghini S. Structure of transcriptionally active chromatin. CRC Crit Rev Biochem. 1986;21(1):1–26. doi: 10.3109/10409238609113607. [DOI] [PubMed] [Google Scholar]