Abstract
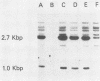
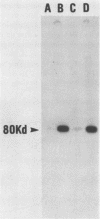
Vesicular stomatitis virus (VSV) leader RNA and a synthetic oligodeoxynucleotide of the same sequence were found to inhibit the replication of adenovirus DNA in vitro. In contrast, the small RNA transcribed by the VSV defective interfering particle DI-011 did not prevent adenovirus DNA replication. The inhibition produced by leader RNA was at the level of preterminal protein (pTP)-dCMP complex formation, the initiation step of adenovirus DNA replication. Initiation requires the adenovirus pTP-adenovirus DNA polymerase complex (pTP-Adpol), the adenovirus DNA-binding protein, and nuclear factor I. Specific replication in the presence of leader RNA was restored when the concentration of adenovirus-infected or uninfected nuclear extract was increased or by the addition of purified pTP-Adpol or HeLa cell DNA polymerase alpha-primase to inhibited replication reactions. Furthermore, the activities of both purified DNA polymerases could be inhibited by the leader sequence. These results suggest that VSV leader RNA is the viral agent responsible for inhibition of adenovirus and possibly cellular DNA replication during VSV infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centrella M., Lucas-Lenard J. Regulation of protein synthesis in vesicular stomatitis virus-infected mouse L-929 cells by decreased protein synthesis initiation factor 2 activity. J Virol. 1982 Mar;41(3):781–791. doi: 10.1128/jvi.41.3.781-791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro: origin and direction of daughter strand synthesis. J Mol Biol. 1979 Dec 25;135(4):999–1012. doi: 10.1016/0022-2836(79)90524-2. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Complete nucleotide sequence of the leader RNA synthesized in vitro by vesicular stomatitis virus. Cell. 1978 Sep;15(1):93–101. doi: 10.1016/0092-8674(78)90085-5. [DOI] [PubMed] [Google Scholar]
- Emerson S. U. Vesicular stomatitis virus: structure and function of virion components. Curr Top Microbiol Immunol. 1976;73:1–34. doi: 10.1007/978-3-642-66306-2_1. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Lichy J. H., Ikeda J. E., Hurwitz J. Adenovirus DNA replication in vitro: purification of the terminal protein in a functional form. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6779–6783. doi: 10.1073/pnas.78.11.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friefeld B. R., Lichy J. H., Field J., Gronostajski R. M., Guggenheimer R. A., Krevolin M. D., Nagata K., Hurwitz J., Horwitz M. S. The in vitro replication of adenovirus DNA. Curr Top Microbiol Immunol. 1984;110:221–255. doi: 10.1007/978-3-642-46494-2_8. [DOI] [PubMed] [Google Scholar]
- Grinnell B. W., Wagner R. R. Comparative inhibition of cellular transcription by vesicular stomatitis virus serotypes New Jersey and Indiana: role of each viral leader RNA. J Virol. 1983 Oct;48(1):88–101. doi: 10.1128/jvi.48.1.88-101.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Wagner R. R. Inhibition of DNA-dependent transcription by the leader RNA of vesicular stomatitis virus: role of specific nucleotide sequences and cell protein binding. Mol Cell Biol. 1985 Oct;5(10):2502–2513. doi: 10.1128/mcb.5.10.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Wagner R. R. Nucleotide sequence and secondary structure of VSV leader RNA and homologous DNA involved in inhibition of DNA-dependent transcription. Cell. 1984 Feb;36(2):533–543. doi: 10.1016/0092-8674(84)90246-0. [DOI] [PubMed] [Google Scholar]
- Ikeda J. E., Enomoto T., Hurwitz J. Replication of adenovirus DNA-protein complex with purified proteins. Proc Natl Acad Sci U S A. 1981 Feb;78(2):884–888. doi: 10.1073/pnas.78.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson L. E., Rose J. K. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell. 1981 Feb;23(2):477–484. doi: 10.1016/0092-8674(81)90143-4. [DOI] [PubMed] [Google Scholar]
- Iverson L. E., Rose J. K. Sequential synthesis of 5'-proximal vesicular stomatitis virus mRNA sequences. J Virol. 1982 Oct;44(1):356–365. doi: 10.1128/jvi.44.1.356-365.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L. M., Ariga H., Hurwitz J., Horwitz M. S. Complementation of the temperature-sensitive defect in H5ts125 adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5534–5538. doi: 10.1073/pnas.76.11.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilla M. G., Keene J. D. The leader RNA of vesicular stomatitis virus is bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1983 Oct;34(3):837–845. doi: 10.1016/0092-8674(83)90541-x. [DOI] [PubMed] [Google Scholar]
- Kurilla M. G., Piwnica-Worms H., Keene J. D. Rapid and transient localization of the leader RNA of vesicular stomatitis virus in the nuclei of infected cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5240–5244. doi: 10.1073/pnas.79.17.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Field J., Horwitz M. S., Hurwitz J. Separation of the adenovirus terminal protein precursor from its associated DNA polymerase: role of both proteins in the initiation of adenovirus DNA replication. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5225–5229. doi: 10.1073/pnas.79.17.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Horwitz M. S., Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2678–2682. doi: 10.1073/pnas.78.5.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGowan J. J., Emerson S. U., Wagner R. R. The plus-strand leader RNA of VSV inhibits DNA-dependent transcription of adenovirus and SV40 genes in a soluble whole-cell extract. Cell. 1982 Feb;28(2):325–333. doi: 10.1016/0092-8674(82)90350-6. [DOI] [PubMed] [Google Scholar]
- McGowan J. J., Wagner R. R. Inhibition of cellular DNA synthesis by vesicular stomatitis virus. J Virol. 1981 Apr;38(1):356–367. doi: 10.1128/jvi.38.1.356-367.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Specific binding of a cellular DNA replication protein to the origin of replication of adenovirus DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6177–6181. doi: 10.1073/pnas.80.20.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnders A. W., van Bergen B. G., van der Vliet P. C., Sussenbach J. S. Specific binding of the adenovirus terminal protein precursor-DNA polymerase complex to the origin of DNA replication. Nucleic Acids Res. 1983 Dec 20;11(24):8777–8789. doi: 10.1093/nar/11.24.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlabach A., Fridlender B., Bolden A., Weissbach A. DNA-dependent DNA polymerases from HeLa cell nuclei. II. Template and substrate utilization. Biochem Biophys Res Commun. 1971 Aug 20;44(4):879–885. doi: 10.1016/0006-291x(71)90793-5. [DOI] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A., Emerson S. U. The complete sequence of a unique RNA species synthesized by a DI particle of VSV. Cell. 1978 Sep;15(1):103–112. doi: 10.1016/0092-8674(78)90086-7. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Topp W. C., Engler J. A. Conserved sequences at the origin of adenovirus DNA replication. J Virol. 1982 Nov;44(2):530–537. doi: 10.1128/jvi.44.2.530-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Carroll A. R., Shattuck D. M., Wagner R. R. Use of UV irradiation to identify the genetic information of vesicular stomatitis virus responsible for shutting off cellular RNA synthesis. J Virol. 1979 Jun;30(3):746–753. doi: 10.1128/jvi.30.3.746-753.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Wagner R. R. Inhibition of RNA synthesis in mouse myeloma cells infected with vesicular stomatitis virus. J Virol. 1978 Mar;25(3):770–780. doi: 10.1128/jvi.25.3.770-780.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Wagner R. R. Transcription of vesicular stomatitis virus is required to shut off cellular RNA synthesis. J Virol. 1979 Apr;30(1):410–413. doi: 10.1128/jvi.30.1.410-413.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz J., Youngner J. S., Keene J. D. Base mutations in the terminal noncoding regions of the genome of vesicular stomatitis virus isolated from persistent infections of L cells. Virology. 1985 Jan 30;140(2):249–256. doi: 10.1016/0042-6822(85)90363-0. [DOI] [PubMed] [Google Scholar]