Abstract
Mast cells have been implicated in various diseases that are accompanied by neovascularization. The exact mechanisms by which mast cells might mediate an angiogenic response, however, are unclear and therefore, we have investigated the possible expression of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) in the human mast cell line HMC-1 and in human skin mast cells. Reverse transcription-polymerase chain reaction (RT-PCR) analysis revealed that mast cells constitutively express VEGF121, VEGF165, and VEGF189. After a prolonged stimulation of cells for 24 h with phorbol 12-myristate 13-acetate (PMA) and the ionophore A23187, an additional transcript representing VEGF206 was detectable, as could be verified by sequence analysis. These results were confirmed at the protein level by Western blot analysis. When the amounts of VEGF released under unstimulated and stimulated conditions were compared, a significant increase was detectable after stimulation of cells. Human microvascular endothelial cells (HMVEC) responded to the supernatant of unstimulated HMC-1 cells with a dose-dependent mitogenic effect, neutralizable up to 90% in the presence of a VEGF-specific monoclonal antibody. Flow cytometry and postembedding immunoelectron microscopy were used to detect VEGF in its cell-associated form. VEGF was exclusively detectable in the secretory granules of isolated human skin mast cells. These results show that both normal and leukemic human mast cells constitutively express bioactive VEGF. Furthermore, this study contributes to the understanding of the physiological role of the strongly heparin-binding VEGF isoforms, since these were found for the first time to be expressed in an activation-dependent manner in HMC-1 cells.
INTRODUCTION
Mast cells have been implicated in the generation of an angiogenic response in various in vitro and in vivo studies (Kessler et al., 1976; Azizkhan et al., 1980; Wilson, 1985; Clinton et al., 1988; Starkey et al., 1988; Norrby et al., 1989; Meininger et al., 1995). The intimate anatomic association between mast cells and the vasculature and the increased rate of appearance of the cells during tumor growth, wound healing, and inflammation, processes that are all accompanied by neovascularization, support this assumption (Meininger and Zetter, 1992). The mechanism of mast cell-mediated angiogenesis has been mainly ascribed to the effects of released heparin and histamine (Azizkhan et al., 1980; Marks et al., 1986). Heparin, however, seems not to mediate a direct angiogenic effect, but it potentiates the mitogenic effects of heparin-binding growth factors, such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) (Gitay-Goren et al., 1992). Recently, the expression of various cytokines was identified in human mast cells (Okayama et al., 1995). These include tumor necrosis factor-α (TNF-α) and interleukin (IL)-8, which were both identified as angiogenic growth factor in vivo and in vitro (Leibovich et al., 1987; Strieter et al., 1995). While IL-8 is produced only after appropriate stimulation in mast cells (Möller et al., 1993), TNF-α is stored already preformed and therefore available for immediate release (Walsh et al., 1991).
bFGF, another potent angiogenic growth factor, has also been recently identified in human mast cells (Reed et al., 1995; Qu et al., 1995), but it lacks the typical signal peptide region necessary for its secretion. Mast cell-associated granules are a rich source of heparin and, consequently, it may be suspected that they serve as depots that are released during degranulation (Reed et al., 1995; Qu et al., 1995).
In the present report, we provide evidence that mast cells express VEGF and discuss their role in VEGF-dependent angiogenic processes. VEGF, also known as vascular permeability factor (VPF), is characterized by its highly specific mitogenic activity for endothelial cells and its angiogenic effect observed in vitro and in vivo (Ferrara et al., 1992). The VEGF family consists of at least four isoforms arising from alternative mRNA splicing (Tischer et al., 1991). VEGF121 and VEGF165 were found to be secreted by a wide spectrum of cell types, including smooth muscle cells (Ferrara et al., 1991), fibroblasts and epithelial cells (Pertovaara et al., 1994), keratinocytes (Brown et al., 1992), macrophages (Berse et al., 1992), cardiac myocytes (Ladoux and Frelin, 1993), and various tumor cells (Ferrara et al., 1992). The two larger isoforms, VEGF189 and VEGF206, share a strong heparin-binding site and, consequently, they predominantly occur as cell-associated isoforms. The specific biological significance of VEGF189 and VEGF206 is unclear since only a few indications for their precise tissue expression exist (Houck et al., 1991; Bacic et al., 1995). Using cells derived from a patient with mast cell leukemia (HMC-1 cells, Butterfield et al., 1988) and mast cells isolated from human foreskin, we investigated the expression of VEGF isoforms on the level of RNA by Reverse transcription-polymerase chain reaction (RT-PCR) and on the level of protein by Western blotting, flow cytometry, enzyme-linked immunosorbent assay (ELISA), and immunoelectron microscopy. In addition to the identification of VEGF in mast cells, these methods allowed the discrimination between VEGF stored intracellularly and VEGF released after appropriate cell stimulation.
MATERIALS AND METHODS
Cell Culture
HMC-1 cells (kindly provided by Dr. Butterfield, Rochester, MN), which are immature human leukemic mast cells (Butterfield et al., 1988; Hamann et al., 1994), were cultured in Iscove’s medium (Seromed, Berlin, Germany), supplemented with 10% fetal calf serum (FCS) (Seromed), 10 μM monothiolglycerol (Sigma Chemical, Deisenhofen, Germany), and antibiotics (streptomycin and penicillin). Stimulation of HMC-1 cells was performed in 24-multiwell plates (Falcon, Lincoln Park, NJ), as previously described (Möller et al., 1993). Before stimulation, cells were cultured for 24 h in medium without FCS. After this time, 2 × 106 cells were incubated for the times indicated in medium without FCS containing 25 ng/ml PMA (Sigma) and 250 nM calcium ionophore A23187 (Sigma). Stimulation periods longer than 12 h induced a decrease in cell viability to 85–90% as determined by trypan blue exclusion. To inhibit protein synthesis, in some experiments cycloheximide (CHX, Sigma) at a concentration of 1 μM was added during stimulation.
For human microvascular endothelial cell (HMVEC) proliferation studies, the conditioned medium (CM) of unstimulated cells was used alone. For this purpose, HMC-1 cells were cultured for 24 h under the same conditions as described above for cell stimulation, but in the absence of PMA and A23187. CM was concentrated 10-fold by ultrafiltration in Centriprep-10 filter units (Amicon, Beverly, MA), and supernatants were stored frozen at −80°C for up to 2 wk.
HMVEC were obtained from Clonetics Cell Systems (Remagen, Germany). These cells were routinely grown in endothelial cell basal medium (EBM, Clonetics), supplemented with 5% FCS, 10 ng/ml recombinant human epidermal growth factor, 1.0 μg/ml hydrocortisone, 50 μg/ml gentamicin, 50 ng/ml amphotericin-B, and 12 μg/ml bovine brain extract. All supplements were provided by Clonetics. Cells were passaged by trypsinization when they reached 80–90% confluency.
HMVEC Proliferation Assay
Second- to fifth-passage HMVEC were seeded at 5 × 103 cells/cm2 in 24-multiwell plates (Falcon) as triplicates in EBM, supplemented as described above. After 8 h, medium was changed and adherent cells were cultured for 4 d in EBM medium without hydrocortisone and bovine brain extract, but containing various amounts of VEGF165 (RD Systems, Wiesbaden-Nordenstadt, Germany), or concentrated conditioned HMC-1 medium. To demonstrate VEGF-specific effects, a neutralizing monoclonal antibody (mAb, clone 26503.11, RD Systems) was used in the proliferation assay at a concentration of 1 μg/ml. As a control, an inappropriate isotype antibody (mouse immunoglobulin [Ig]G2b, Dianova, Hamburg, Germany) was used at the same concentration. Cells were harvested by trypsinization (0.025% trypsin/0.01% EDTA, Clonetics), fixed with 1% paraformaldehyde, and counted with a cell counter/analyzer (CASY 1/TT, Schärfe System, Reutlingen, Germany).
Enrichment of Human Skin Mast Cells by Counterflow Centrifugal Elutriation (CCE)
The epidermis of human foreskin was enzymatically detached by an overnight incubation at 4°C with dispase (Boehringer, Mannheim, Germany) at a concentration of 1 mg/ml. The remaining dermis was dispersed by an incubation with collagenase I (Worthington Biochemical, Freehold, NJ) for 2 h at 37°C. Elutriation was performed at 10°C and constant rotor speed (2300 ± 10 rpm) using a Beckmann JE5-elutriator rotor equipped with a Sanderson chamber (Beckmann Instruments, Palo Alto, CA). Phosphate-buffered saline (PBS) containing 5 mM EDTA and 0.25% BSA (wt/vol) was used as elutriation medium. Single fractions were separated by a stepwise increase in flow rate (10–30 ml/min). The mast cell-enriched fraction was collected at 30 ml/min by decreasing rotor speed down to 1500 rpm. Isolated cell population consisted of 20–30% mast cells, as determined by flow cytometric analysis of the cell-specific surface marker c-kit (mAb YB5B8, kindly provided by Dr. Ashman, Adelaide, Australia) and the high-affinity IgE-receptor (mAb 29C6, kindly provided by Dr. Hakimi, Hoffmann La Roche, Nutley, NJ).
RT-PCR Amplification and Sequence Analysis
Semiquantitative RT-PCR analysis was performed as previously described (Krüger-Krasagakes et al., 1994). Briefly, 3 μg of total cellular RNA, extracted from HMC-1 cells by applying an RNeasy total RNA kit (Quiagen, Hilden, Germany), was transcribed into cDNA using random priming. For comparison of VEGF mRNA levels in different samples, cDNAs were first adjusted to equal concentrations of β-actin by the use of a β-actin control fragment (Krüger-Krasagakes et al., 1994) and then analyzed for their content of VEGF mRNA. Primer sequences for β-actin (bases 103–122 and 642–619) were taken from Yamamura et al. (1991), and those for VEGF were obtained from Weindel et al. (1992) and from Morii et al. (1993), respectively. Cycles were 5 min at 94°C, 45 s at 94°C, 60 s at 60°C, 1 min at 72°C, and 5 min at 72°C in a temperature cycler (Hybaid, Middlesex, England). Thirty-five cycles were performed.
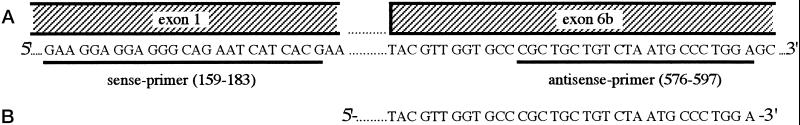
For the amplification of a VEGF206-specific cDNA fragment, sense and antisense primers were designed as shown in Figure 2A, and the same conditions as described above were applied. The corresponding band was cut from the gel, eluted, and automatically sequenced by an ABI sequencer (model 377; Applied Biosystems, Weiterstadt, Germany).
Figure 2.
(A) Design of the PCR primers used to amplify a VEGF-specific sequence (438 bp) containing 67% of the VEGF206-specific exon 6b. (B) The product amplified was directly sequenced using the sense primer of PCR amplification. The 3′-end of the amplicon sequenced showed the expected exon 6b-specific nucleotide sequence.
Western Blot Analysis of CM
The CM of unstimulated cells and HMC-1 cells stimulated for 24 h with PMA and calcium ionophore A23187 was concentrated by ultrafiltration in Centriprep-10 filter units (Amicon) and incubated overnight at 4°C with precleaned heparin-acrylic beads (Sigma). The heparin beads were collected by centrifugation, washed two times, and boiled for 10 min in sample buffer containing 2.5% 2-mercaptoethanol. Equal amounts of protein were electrophoresed under reducing and nonreducing conditions on a 12% SDS polyacrylamide gel. Separated proteins were blotted to nitrocellulose membranes (Schleicher & Schüll, Dassel, Germany). The membranes were blocked with PBS containing 0.1% Tween 20 and 5% skimmed milk powder for 1 h at room temperature. The primary rabbit anti-human VEGF polyclonal antibody (Pepro Tech, Rocky Hill, NY) and a rabbit control IgG (Dianova, Hamburg, Germany) were diluted at a concentration of 2 μg/ml and incubated with the membranes overnight at 4°C. After intense washing, the membranes were incubated with the secondary horseradish peroxidase-conjugated goat anti-rabbit IgG (Dako, Hamburg, Germany) at a concentration of 0.125 μg/ml for 2 h at room temperature. Thereafter, the membranes were washed once again and incubated in enhanced chemiluminescence (ECL, Amersham, Little Chalfont, England) substrate reagent for 1 min. The blot was exposed to film (ECL Hyperfilm, Amersham) for 30–90 s, and the molecular weights of the immunodetected bands were compared with low molecular weight standards (Sigma).
ELISA
The time-dependent secretion of VEGF and bFGF under nonstimulating and stimulating conditions was measured with growth factor-specific ELISA kits (Quantikine, RD Systems), according to the instructions of the manufacturer. The lower detection limit for VEGF was 15 pg/ml and 5 pg/ml for bFGF. Concentrations of VEGF in the samples were calculated by interpolation from the standard curve.
Flow Cytometric Quantification of Intracellular VEGF
VEGF stored intracellularly in HMC-1 cells was monitored after cell fixation, partial cell permeabilization, and staining with a VEGF-specific mAb (clone 26503.11, RD Systems) (Grützkau et al., 1997). Briefly, 5 × 105 cells were fixed at 4°C for 10 min in a mixture of 4% paraformaldehyde and 0.1% glutaraldehyde. After intense washing, cells were permeabilized with 50 μl PBS containing 5% BSA (Sigma) and 0.03% Saponin (Sigma) and were incubated for 30 min on ice. Thereafter, the VEGF-specific mAb was added at a concentration of 50 μg/ml, and cells were once again incubated for 30 min on ice. After washing, a secondary dichlorotriazinylamino-fluorescein-conjugated F(ab′)2 fragment of goat anti-mouse IgG antibodies (Dianova) was added at a concentration of 20 μg/ml, and cells were incubated for an additional 30 min at 4°C. Finally, cells were washed and fixed in PBS containing freshly prepared 1% paraformaldehyde. At least 10,000 cells were analyzed using an EPICS XL flow cytometer (Coulter Electronics, Krefeld, Germany). Results were expressed as percent positive cells, taking into account the amount of unspecific binding of the corresponding isotype control antibody.
Postembedding Immunoelectron Microscopy
The identification and ultrastructural localization of VEGF in human skin mast cells, enriched by CCE as described above, were performed by postembedding immunoelectron microscopy. The mast cell-enriched cell preparation was fixed in 4% paraformaldehyde for 10 min at room temperature, after which cells were centrifuged in molten agar (1% in 0.1 M PBS at 45°C) to form cell pellets that were sliced and processed as small blocks. Dehydration performed in a graded series of ethanol solutions at 4°C was followed by an infiltration with LR-White resin (London Resin, Berkshire, England) without accelerator (Newman and Hobot, 1987). Polymerization was induced by the addition of the manufacturer’s accelerator for 2 h at 4°C. Ultrathin sections (70 nm) were picked up on formvar-coated nickel grids.
Immunostaining was performed on 50 μl-droplets in a moist chamber using a VEGF-specific mAb (clone 26503.11, RD Systems) at a concentration of 30 μg/ml and a secondary colloidal gold (10 nm)-labeled goat anti-mouse IgG (AuroProbe, Amersham) at a 1:20 dilution. Poststaining was performed with 5% aqueous uranyl acetate (Merck, Darmstadt, Germany) for 15 min. Staining specificity was checked by substituting the primary antibody with an inappropriate isotype antibody. Specimens were examined with a Zeiss EM906 transmission electron microscope (Carl Zeiss, Thornwood, NY) at 80 kV.
RESULTS
Detection of VEGF mRNA from HMC-1 Cells by Semiquantitative RT-PCR
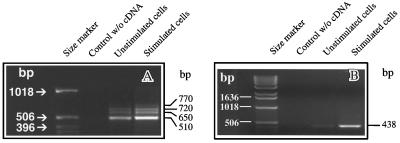
Amplification of cDNA from unstimulated HMC-1 cells and cells stimulated for 24 h with PMA/A23187 with two different pairs of primers gave rise to up to four bands of 510 base pairs (bp), 650 bp, 720 bp, and 770 bp (Figure 1A) and 400 bp, 540 bp, 610 bp, and 660 bp, respectively (our unpublished results)—the sizes predicted for VEGF121, VEGF165, VEGF189, and VEGF206 (Weindel et al., 1992; Morii et al., 1993). While the three smaller VEGF isoforms were already expressed in unstimulated cells, the largest, strongly heparin-binding isoform was only detectable in cells stimulated for at least 24 h in the presence of 25 ng/ml PMA and 250 nM calcium ionophore A23187. Since equal amounts of cDNAs of unstimulated and stimulated cells were used for RT-PCR, it was feasible to compare the intensities of products amplified. The total amount of VEGF mRNA expressed in unstimulated cells was obviously lower in comparison to stimulated cells. RT-PCR performed with higher amounts of cDNA of unstimulated cells did not result in the appearance of the VEGF206-specific amplicon, indicating that the expression of the VEGF206-transcript is stimulus dependent in HMC-1 cells (our unpublished results).
Figure 1.
Expression of VEGF transcripts in HMC-1 cells. Semiquantitative RT-PCR analysis of mRNA from unstimulated HMC-1 cells (lane 3) and cells stimulated for 24 h with PMA/A23187 (lane 4). (A) The primers chosen for this amplification allowed detection of all VEGF isoforms. (B) A VEGF206-specific primer pair was chosen (Figure 2A), and the product amplified was cut from the gel and identified by direct sequencing (Figure 2B). lane 1, size marker; lane 2, negative control (sample without cDNA). The positions of the size markers are on the left side and the sizes of the amplified products are indicated on the right side.
For the unambiguous identification of VEGF206, we used a VEGF206-specific pair of primers (Figure 2A) and identified the resulting amplicon by sequence analysis. In accordance with the results described in Figure 1A, we could detect a single PCR product of 438 bp, the size predicted for VEGF206, only in stimulated HMC-1 cells (Figure 1B). Sequence analysis revealed that the amplified product contained the VEGF206-specific nucleotide sequence with complete homology (Figure 2B).
Detection of VEGF Isoforms in the CM of HMC-1 Cultures by Western Blot Analysis
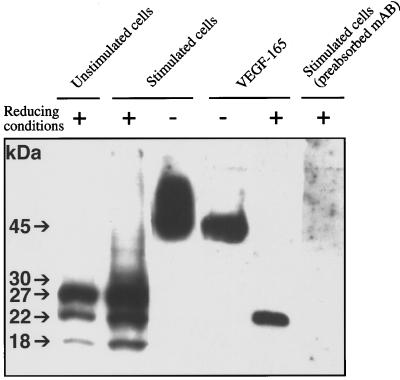
To clarify whether VEGF isoforms are expressed at the protein level and which isoforms are released after appropriate stimulation, Western blot analysis was performed with a rabbit anti-human VEGF polyclonal antibody. For this purpose, concentrated supernatants of HMC-1 cells cultured for 24 h in the presence of PMA and the calcium ionophore A23187, together with an unstimulated control, were preadsorbed with heparin coupled to acrylic beads to enrich heparin-binding VEGF isoforms. Precleaning of supernatants with heparin beads was necessary to minimize background staining, but during this step, VEGF121 was lost to a large extent. Under nonreducing conditions, a broad band between 45 kDa and 60 kDa was visible in all supernatants investigated (Figure 3, lane 3). In the presence of 2-mercaptoethanol, three bands (18 kDa, 22 kDa, and 27 kDa) were detectable in the supernatant of unstimulated and stimulated cells (Figure 3, lanes 1 and 2), which correspond to the predicted molecular size for VEGF121 (17 kDa), VEGF165 (23 kDa), and VEGF189 (26 kDa) in reducing conditions. Since identical cell numbers were used to prepare the CM from unstimulated and stimulated cells, it is possible to compare the amounts of the various isoforms released. In accordance with the data obtained by the VEGF-specific ELISA, stimulated cells released significantly higher amounts of VEGF121, VEGF165, and VEGF189. In the supernatant of stimulated cells, an additional protein of 30 kDa, most probably representing VEGF209, was detectable (Figure 3, lane 2).
Figure 3.
Immunoblot analysis of HMC-1 cell-derived supernatants. Concentrated supernatants of unstimulated cells (lane 1) and HMC-1 cells stimulated for 24 h with PMA/A23187 (lanes 2, 3, and 6) were used for SDS-PAGE analysis and blotting on nitrocellulose membranes. Precleaning of supernatants with heparin beads was necessary to minimize background staining, but during this step, VEGF121 was lost to a large extent. Recombinant human VEGF165 was used as positive control (lanes 4 and 5). Lanes 1–5 were probed with an VEGF-specific antibody, and lane 6 was probed with an isotype-specific control antibody.
These results demonstrate that HMC-1 cells possess the capability of synthesizing and secreting all known VEGF isoforms.
Time- and Stimulus-dependent Secretion of VEGF Determined by an ELISA
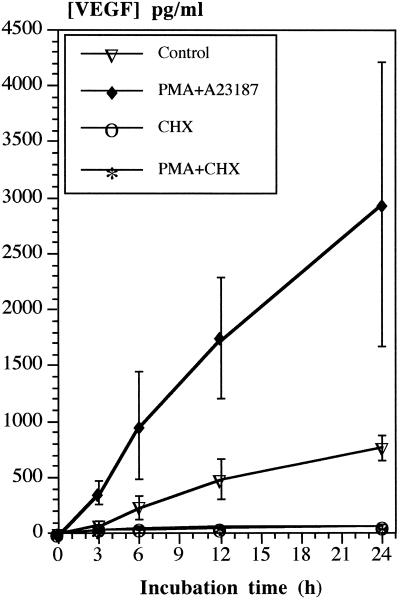
The time course of VEGF synthesis and secretion was monitored in unstimulated HMC-1 cells and in cells costimulated with PMA and calcium ionophore A23187. HMC-1 cells cultured for 24 h in the absence of any stimulus released VEGF continuously into the surrounding medium (Figure 4). Stimulation of cells caused a significant increase in VEGF secretion that was already detectable after 3 h. The continuously stimulated as well as the unstimulated release of VEGF was completely stopped in the presence of CHX (Figure 4).
Figure 4.
Time-dependent secretion of VEGF under nonstimulating and stimulating conditions as measured by a VEGF-specific ELISA. Additionally, the stimulated and the unstimulated release of VEGF was monitored in the presence of 1 μM cycloheximide (CHX), an inhibitor of protein synthesis. The values are given as means ± SD of five independent experiments.
In parallel, we determined the release of bFGF in the supernatants of stimulated and unstimulated cells but detected only minimal amounts (5 pg/ml) in cells stimulated for 48 h with PMA and A23187 (our unpublished results).
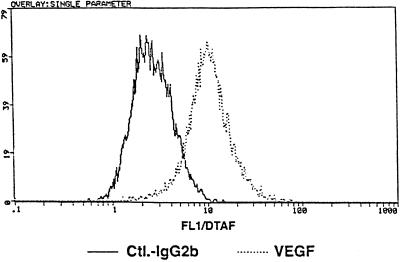
Determination of Cell-associated VEGF by Flow Cytometry
Flow cytometry was used to determine whether HMC-1 cells not only synthesize and release VEGF but are also capable of storing this factor intracellularly. After appropriate cell fixation and permeabilization, labeling with an anti-VEGF mAb resulted in 75% ± 20% positively stained cells (Figure 5). The efficiency of permeabilization was routinely checked by staining for CD68, an intracellularly located antigen expressed by nearly 100% of HMC-1 cells. Stimulation of HMC-1 cells showed no significant effect on the amount of cell-associated VEGF (our unpublished data). The intracellular amount of VEGF remained also unchanged after cells had been treated with 1 μM CHX (our unpublished data).
Figure 5.
Detection of intracellular VEGF in unstimlated HMC-1 cells by flow cytometry. Before antibody staining cells were fixed in a mixture of 4% paraformaldehyde and 0.1% glutaraldehyde and permeabilized with 0.03% saponin. The dotted line of the fluorescence histogram overlay illustrates the binding of the VEGF-specific mAb, and the solid line represents the unspecific binding of an isotype-specific control mAb.
To detect VEGF possibly expressed on the plasma membrane, we analyzed unpermeabilized cells that were not fixed before staining. Under these conditions, however, no VEGF was detectable on the surface of unstimulated cells and cells stimulated with PMA and A23187 (our unpublished data).
Identification of Bioactive VEGF in the CM of HMC-1 Cells
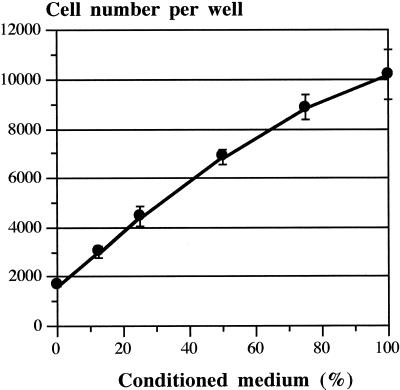
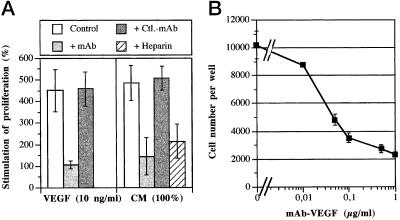
The CM of unstimulated HMC-1 cells was tested for its mitogenic activity in an HMVEC proliferation assay. Addition of various amounts of CM induced a dose-dependent increase in HMVEC proliferation rate and at 100% CM (i.e., 0.1 volume of 10-fold concentrated HMC-1 supernatant was added to the proliferation assay), an effect comparable to 10 ng/ml VEGF165 was observed (Figures 6 and 7A). This effect was suppressed up to 90% in the presence of a neutralizing VEGF-specific mAb or up to 75% by a preincubation of the CM with heparin-conjugated agarose beads (Figure 7A). The antibody used showed its half-maximal inhibitory effect at a concentration of 25 ng/ml (Figure 7B). A control antibody showed no inhibitory effect (Figure 7A).
Figure 6.
Proliferation of HMVEC in response to HMC-1 cell-derived supernatants. The CM of unstimulated HMC-1 cells was concentrated 10-fold by ultrafiltration, and dilutions were tested for its mitogenic activity in an HMVEC proliferation assay. A dose-dependent increase in HMVEC proliferation rate was detectable. Results are shown for one representative experiment performed in triplicate. Similar results were obtained in four independent experiments (our unpublished data).
Figure 7.
Inhibition of the HMC-1- and VEGF-induced HMVEC proliferation with a neutralizing VEGF-specific antibody. (A) The mitogenic effects of VEGF (10 ng/ml) and of 100% CM were suppressed up to 100% and 90%, respectively, in the presence of a neutralizing VEGF-specific mAb or up to 75% by a preincubation of the CM with heparin-conjugated agarose beads. Data are expressed as percentage of stimulation of HMVEC proliferation in comparison to control medium. (B) This Figure demonstrates the VEGF-specific mAb-induced dose-dependent neutralization of the mitogenic effect of CM. The antibody used showed its half-maximal inhibitory effect at a concentration of 25 ng/ml. A control antibody showed no inhibitory effect. In both figures mean values ± SD from three separate experiments are shown.
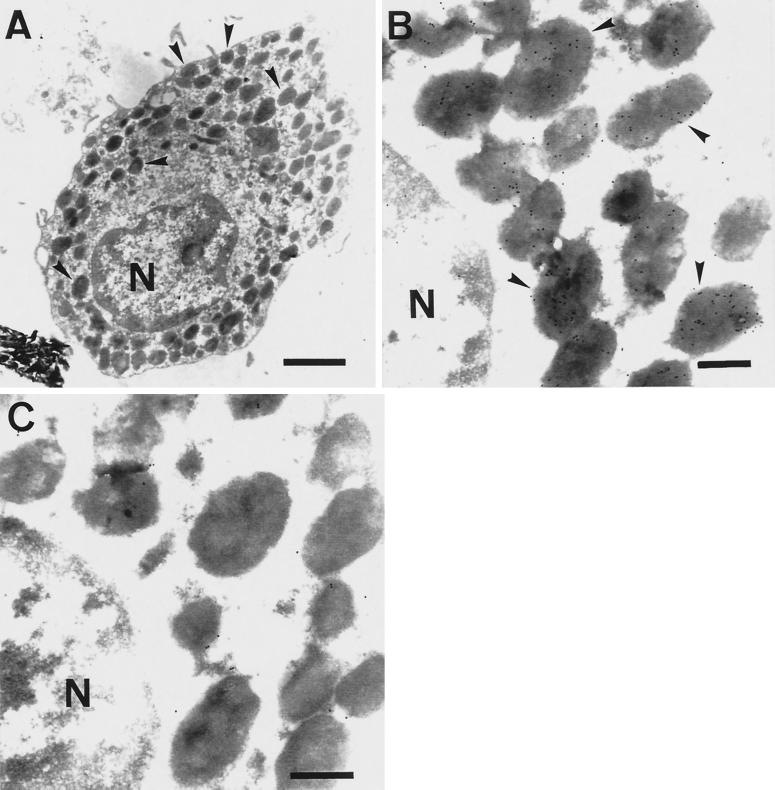
Identification and Ultrastructural Localization of VEGF in Human Skin Mast Cells
Under physiological conditions, mast cells are only found scarcely scattered in the tissue, a circumstance that makes it difficult to analyze a sufficient amount of mast cells during immunoelectron microscopic evaluation of VEGF expression. Therefore, we decided to enrich human skin mast cells by counterflow elutriation, which allows the identification of VEGF expression, and the ultrastructural localization of the molecule in a significant number of mast cells by postembedding immunoelectron microscopy. By this method, we could enrich mast cells up to 30%, as estimated after flow cytometric analysis of the cell-specific surface receptor for c-kit and IgE (our unpublished data). In addition to mast cells, macrophages, endothelial cells, and fibroblasts were also present in this cell population. For electron microscopic evaluation, mast cells were unambiguously identifiable by their characteristic ultrastructure, namely a monolobed nucleus with partially condensed peripheral chromatin and numerous granules filled with electron-dense material (Figure 8A). As can be seen, the isolation method caused almost no piecemeal or anaphylactic degranulation of cells. As shown in Figure 8B, positive staining for VEGF was detected exclusively in mast cell-specific granules. Immunolabeling varied, however, among granules on a quantitative basis, and some granules exhibited no immunogold labeling at all. The cytoplasm in the vicinity of the granules, the plasma membrane, and the nucleus showed no significant immunostaining. In other dermal cells present in the cell suspension, no VEGF was detectable at all. Background labeling was minimal, and no immunoreactive structures were seen in mast cells processed in the presence of an isotype-matched irrelevant mAb (Figure 8C).
Figure 8.
Immunoelectron microscopic localization of VEGF in ultrathin sections of human skin mast cells embedded in LR-White resin. (A) A typical mast cell isolated from human foreskin by enzymatic dispersion and enrichment by elutriation is shown. Arrowheads indicate mast cell-specific granules. N, Nucleus. Bar, 3 μm. (B) Immunolabeling was detected exclusively in mast cell-specific granules (arrowheads). N, Nucleus. Bar, 1 μm. (C) In the presence of an isotype-matched irrelevant mAb, almost no immunoreactive structures were ascertained. N, Nucleus. Bar, 1 μm.
DISCUSSION
In this report, we present for the first time evidence indicating that human mast cells express all known isoforms of VEGF. Additionally, we were able to shed light on the hitherto unknown physiological role of the strongly heparin-binding isoforms VEGF189 and VEGF206. To our knowledge, the expression of VEGF in mast cells has only been investigated up to now at the level of mRNA in HMC-1 cells (Ito et al., 1995). In this report, however, no VEGF mRNA was detectable in unstimulated cells by Northern blot analysis. Semiquantitative RT-PCR experiments performed in the present study allowed us to determine a relative abundance of VEGF121, VEGF165, VEGF189, and VEGF206 mRNA in HMC-1 cells. While the mRNA for the three major VEGF forms (121, 165, and 189 amino acids) was apparent already in unstimulated cells, the largest, strongly heparin-binding isoform was only detectable in HMC-1 cells stimulated with a combination of the calcium ionophore A23187 and PMA for at least 24 h. The obvious discrepancy between our results and the results of Ito et al. (1995) is possibly based on the different sensitivity of the detection methods applied.
Similar to bovine smooth muscle cells (Ferrara et al., 1991), human keratinocytes (Brown et al., 1992), glioma cells (Tsai et al., 1995), and retinal epithelial cells (Shima et al., 1995), HMC-1 cells already constitutively expressed measurable amounts of VEGF in vitro, as could be shown not only at the level of mRNA (PCR), but also at the protein level by ELISA and Western blotting. The rate of secretion was increased 2.5-fold in response to the combination of PMA and the calcium ionophore A23187. Therefore, it is reasonable to predict that mast cells may enhance synthesis of VEGF in vivo when activated under inflammatory conditions. Since HMC-1 cells only variably express the high-affinity receptor for IgE (Hamann et al., 1994; Nilsson et al., 1994), an IgE-like activation of these cells was achieved by triggering calcium influx, combined with PKC activation (Sagi-Eisenberg, 1993). HMC-1 cells activated in this way start de novo synthesis of various mediators, such as IL-1β, IL-6, IL-8, and bFGF (Grabbe et al., 1995; Qu et al., 1995). VEGF expression is inducible in many different cell types by a direct pharmacological activation of PKC, which phosphorylates AP-1, the transcription factor thought to be mainly responsible for the regulation of VEGF expression (Kolch et al., 1995). For IL-1β, also known to activate AP-1, a stimulating effect on VEGF gene transcription in rat aortic smooth muscle cells has been ascertained as well (Li et al., 1995).
The high contents of heparin found in the secretory granules of mast cells (Stevens et al., 1988) made it reasonable to assume that these cells are a major source for the strongly heparin-binding isoforms VEGF189 and VEGF206. This assumption was confirmed by our findings that in human skin mast cells, VEGF was exclusively detectable within the specific mast cell granules. The observation that in stimulated HMC-1 cells, an increase in VEGF release (ELISA data) was not accompanied by a decrease in intracellular VEGF-contents (FACS data) also indicates that a distinct cell-associated VEGF-pool exists in HMC-1 cells that is not completely releasable. This pool probably represents VEGF bound to heparin proteoglycans. No VEGF immunoreactivity was found on the plasma membrane of skin mast cells (immuno EM data) and of HMC-1 cells (FACS data), although heparin proteoglycans are present also as integral membrane proteins of almost all cell surfaces (Yanagishita and Hascall, 1992). VEGF bound to extracellular matrix components is releasable in its soluble form by heparin or enzymatically by plasmin without restriction of its mitogenic activity (Park et al., 1993).
So far, few reports exist about the physiological significance and the way in which these strongly cell-binding isoforms are released (Park et al., 1993; Bacic et al., 1995). While VEGF189 is distributed in the majority of cells and tissues expressing the VEGF gene, VEGF206 represents a very rare isoform that has only been detected in a human fetal liver cDNA library so far (Houck et al., 1991). Our findings demonstrate for the first time a cell in which VEGF206-expression could be verified at the mRNA and protein levels, although this was possible only when HMC-1 cells were activated for at least 24 h with PMA and the calcium ionophore A23187. This result indicates that in HMC-1 cells, splicing of VEGF206 is dependent on an appropriate PKC- and Ca2+-dependent cell stimulation. This process is clearly distinguishable from the hypoxic stimulation of VEGF expression in fibroblasts, which results primarily in the induction of VEGF121 and VEGF165 (Minchenko et al., 1994).
The role of VEGF189 and VEGF206 as paracrine mediator of angiogenesis in vivo has been disputed because so far, no secretory mechanism is known for these isoforms. Identification of mast cells as a source for the complete spectrum of VEGF isoforms provides secretion via degranulation or by direct cell-cell transfer (transgranulation) as a plausible mechanism by which this cytokine is released into the tissue or into other cells. Evidence for at least the first possibility is provided by the finding that in the supernatant of PMA/A23187-stimulated HMC-1 cells, the strongly heparin-binding isoforms were detectable by Western blotting. Similar assumptions were made for the release of bFGF, which lacks a signal sequence necessary for its secretion (Qu et al., 1995; Reed et al., 1995). In the supernatant of stimulated HMC-1 cells, where bFGF was identified on the mRNA and protein level (Qu et al., 1995), we could however detect only minor amounts of bFGF (≤ 5 pg/ml), which are insufficient to provoke a mitogenic response in endothelial cells or to synergize with VEGF for its mitogenic activity (Goto et al., 1993).
Both unstimulated and stimulated secretion of VEGF were dependent on an active protein biosynthesis because, in the presence of CHX, no release of VEGF was detectable in the cell supernatants. Intracellular staining for VEGF by flow cytometry indicated that the CHX-induced effect was mainly related to an inhibition of the secretion, since VEGF was still detectable at comparable amounts within CHX-treated and untreated cells.
The VEGF identified in the CM of unstimulated HMC-1 cells by Western blotting and ELISA was bioactive, as determined by its capacity to stimulate HMVEC proliferation in an in vitro assay. With the help of RT-PCR experiments, we could show that these endothelial cells express both types of VEGF receptors (KDR and Flt-1; our unpublished data) and respond to recombinant human VEGF165 in a dose-dependent manner. Depletion experiments performed with a VEGF-specific mAB resulted in an inhibition of the mitogenic response by up to 90%. The extent of this inhibition was unexpected because unstimulated HMC-1 cells are already known to produce a wide range of angiogenic factors such as histamine, heparin, and TNF-α (Meininger and Zetter, 1992). Obviously, under the culture conditions described, VEGF represents the major endothelial cell mitogenic activity in the CM of unstimulated HMC-1 cells.
The bulk of experiments performed in this study was done with the cell line HMC-1, representing the only established human cell line exhibiting a phenotype similar to that of normal human tissue mast cells (Hamann et al., 1994; Nilsson et al., 1994). Since VEGF is overexpressed in several transformed cell lines (White et al., 1995), we cannot exclude the possibility that the expression of VEGF in HMC-1 cells is less a mast cell-associated than a tumor-specific characteristic. But tumor cell lines exhibiting constitutive overexpression of VEGF in general behave in a refractile manner toward phorbol esther activation and do not respond with an additional increase in VEGF abundance (White et al., 1995), a phenomenon not observed in HMC-1 cells. Furthermore, HMC-1 cells differ from other tumor cell lines in their ability to synthesize all known major VEGF-isoforms. In addition to the neoplastic phenotype of HMC-1 cells, also the grade of maturity of these cells must be taken into account when judging the results presented. HMC-1 cells exhibit a more immature phenotype and, therefore, qualitative and quantitative changes in the expression of VEGF isoforms may occur during mast cell differentiation.
In summary, this study supports the significance of mast cells in angiogenesis. It is evident from the results presented here that mast cells do not only exert an indirectly acting angiogenic response, but that they also may contribute to a direct effect mediated via the expression of VEGF. Finally, the localization of VEGF to a cell that synthesizes heparin in large amounts may help to elucidate the physiological significance of the strongly heparin-binding isoforms VEGF189 and VEGF206.
ACKNOWLEDGMENTS
The authors are grateful to Professor Schnoy from the Department of Pathology, Virchow Clinics, Berlin, for making available the facilities of transmission electron microscopy, to Ms. Lajous-Petter for helpful suggestions and advice, and to Ms. Pröhl for photographic assistance. We also thank Dr. Bernhard Gibbs, Universitätsklinikum Lübeck, for critical reading of the manuscript and valuable discussion. This work was supported by Deutsche Forschungsgemeinschaft grant Mo 462/2–3 and in part by a “Forschungsprojektschwerpunkt” on mast cells of the Free University of Berlin.
Footnotes
Abbreviations used: bFGF, basic fibroblast growth Factor; CCE, counterflow centrifugal elutriation; CM, conditioned medium; CHX, cycloheximide; HMC-1, human mast cell line-1; HMVEC, human microvascular endothelial cells; VEGF/VPF, vascular endothelial growth factor/vascular permeability factor.
REFERENCES
- Azizkhan RG, Azizkhan JC, Zetter BR, Folkman J. Mast cell heparin stimulates migration of capillary endothelial cells in vitro. J Exp Med. 1980;152:931–944. doi: 10.1084/jem.152.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacic M, Edwards NA, Merrill MJ. Differential expression of vascular endothelial growth factor (vascular permeability factor) forms in rat tissue. Growth Factor. 1995;12:11–15. doi: 10.3109/08977199509003209. [DOI] [PubMed] [Google Scholar]
- Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Am J Pathol. 1992;3:211–220. doi: 10.1091/mbc.3.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LF, Yeo K-T, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield JH, Weiler D, Dewald G, Gleich G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12:345–350. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- Clinton M, Long WF, Williamson FB, Duncan JI, Thompson WD. Effect of the mast cell activator compound 48/80 and heparin on angiogenesis in the chick chorioallantoic membrane. Int J Microcirc Clin Exp. 1988;7:315–326. [PubMed] [Google Scholar]
- Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Winer J, Burton T. Aortic smooth muscle cells express and secrete vascular endothelial growth factor. Growth Factors. 1991;5:141–148. doi: 10.3109/08977199109000278. [DOI] [PubMed] [Google Scholar]
- Gitay-Goren H, Soker S, Vlodavsky I, Neufeld G. The binding of vascular endothelial growth factor to its receptor is dependent on cell surface-associated heparin-like molecules. J Biol Chem. 1992;267:6093–6098. [PubMed] [Google Scholar]
- Goto F, Goto K, Weindel K, Folkman JF. Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels. Lab Invest. 1993;69:508–517. [PubMed] [Google Scholar]
- Grabbe J, Welker P, Möller A, Dippel E, Ashman LK, Czarnetzki BM. Comparative cytokine release from human monocytes, monocyte-derived immature mast cells, and a human mast cell line (HMC-1) J Invest Dermatol. 1995;103:504–508. doi: 10.1111/1523-1747.ep12395649. [DOI] [PubMed] [Google Scholar]
- Grützkau A, Krüger-Krasagakes S, Kögel H, Möller A, Lippert U, Henz BM. Detection of interleukin-8 in human mast cells, flow cytometry as a guide for immunoelectron microscopy. J Histochem Cytochem. 1997;45:935–945. doi: 10.1177/002215549704500703. [DOI] [PubMed] [Google Scholar]
- Hamann K, Grabbe J, Welker P, Haas N, Algermissen B, Czarnetzki BM. Phenotypic evaluation of cultured human mast and basophilic cells and of normal human skin mast cells. Arch Dermatol Res. 1994;286:380–385. doi: 10.1007/BF00371797. [DOI] [PubMed] [Google Scholar]
- Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- Ito A, Hirota S, Mizuno H, Kawasaki Y, Takemura T, Nishiura T, Kanakura Y, Katayama Y, Nomura S, Kitamura Y. Expression of vascular permeability factor (VPF/VEGF) messenger RNA by plasma cells: possible involvement in the development of edema in chronic inflammation. Pathol Int. 1995;45:715–720. doi: 10.1111/j.1440-1827.1995.tb03387.x. [DOI] [PubMed] [Google Scholar]
- Kessler DA, Langer RS, Pless NA, Folkman J. Mast cells and tumor angiogenesis. Int J Cancer. 1976;18:703–709. doi: 10.1002/ijc.2910180520. [DOI] [PubMed] [Google Scholar]
- Kolch W, Martiny-Baron G, Kieser A, Marmé D. Regulation of the expression of the VEGF/VPF and its receptors: role in tumor angiogenesis. Breast Cancer Res Treat. 1995;36:139–155. doi: 10.1007/BF00666036. [DOI] [PubMed] [Google Scholar]
- Krüger-Krasagakes S, Krasagakes K, Garbe C, Schmitt E, Hüls C, Blankenstein T. Expression of interleukin-10 in human melanoma. Br J Cancer. 1994;70:1182–1185. doi: 10.1038/bjc.1994.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladoux A, Frelin C. Hypoxia is a strong inducer of vascular endothelial growth factor mRNA expression in the heart. Biochem Biophys Res Commun. 1993;195:1005–1010. doi: 10.1006/bbrc.1993.2144. [DOI] [PubMed] [Google Scholar]
- Leibovich SJ, Polverini PJ, Shepard HM, Wisemann DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329:630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- Li J, Perrella MA, Tsai J-C, Yet S-F, Hsieh C-M, Yoshizumi M, Patterson C, Endege WO, Zhou F, Lee M-E. Induction of vascular endothelial growth factor gene expression by interleukin-1b in rat smooth muscle cells. J Biol Chem. 1995;270:308–312. doi: 10.1074/jbc.270.1.308. [DOI] [PubMed] [Google Scholar]
- Marks RM, Roche WR, Czerniecki M, Penny R, Nelson DS. Mast cell granules cause proliferation of human microvascular endothelial cells. Lab Invest. 1986;55:289–294. [PubMed] [Google Scholar]
- Meininger CJ, Brightman SE, Kelly KA, Zetter BR. Increased stem cell factor release by hemangioma-derived endothelial cells. Lab Invest. 1995;72:48–52. [PubMed] [Google Scholar]
- Meininger CJ, Zetter BR. Mast cells and angiogenesis. Semin Cancer Biol. 1992;3:73–79. [PubMed] [Google Scholar]
- Minchenko A, Bauer T, Salceda S, Caro J. Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab Invest. 1994;71:374–379. [PubMed] [Google Scholar]
- Möller A, Lippert U, Lessmann D, Kolde G, Hamann K, Welker P, Schadendorf D, Rosenbach T, Luger T, Czarnetzki BM. Human mast cells produce IL-8. J Immunol. 1993;151:3261–3266. [PubMed] [Google Scholar]
- Morii K, Tanaka R, Washiyama K, Kumanishi T, Kuwano R. Expression of vascular endothelial growth factor in capillary hemangioblastoma. Biochem Biophys Res Commun. 1993;194:749–755. doi: 10.1006/bbrc.1993.1885. [DOI] [PubMed] [Google Scholar]
- Newman GR, Hobot JA. Modern acrylics for post-embedding immunostaining techniques. J Histochem Cytochem. 1987;35:971–981. doi: 10.1177/35.9.3302021. [DOI] [PubMed] [Google Scholar]
- Nilsson G, Blom T, Kusche-Gullberg M, Kjellen L, Butterfield JH, Sundström C, Nilsson K, Hellman L. Phenotypic characterization of the human mast-cell line HMC-1. Scand J Immunol. 1994;39:489–498. doi: 10.1111/j.1365-3083.1994.tb03404.x. [DOI] [PubMed] [Google Scholar]
- Norrby K, Jakobsson A, Sörbo J. Mast-cell secretion and angiogenesis, a quantitative study in rats and mice. Virchows Arch B Cell Pathol. 1989;57:251–256. doi: 10.1007/BF02899089. [DOI] [PubMed] [Google Scholar]
- Okayama Y, Bradding P, Tunon-de-Lara JM, Holgate ST, Church MK. Cytokine production by human mast cells. Human basophils and mast cells: biological aspects. Chem Immunol. 1995;61:114–134. [PubMed] [Google Scholar]
- Park JE, Keller G-A, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara L, Kaipainen A, Mustonen T, Orpana A, Ferrara N, Saksela O, Alitalo K. Vascular endothelial growth factor is induced in response to transforming growth factor-b in fibroblastic and epithelial cells. J Biol Chem. 1994;269:6271–6274. [PubMed] [Google Scholar]
- Qu Z, Liebler JM, Powers MR, Galey T, Ahmadi P, Huang X-N, Ansel JC, Butterfield JH, Planck SR, Rosenbaum JT. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol. 1995;147:564–573. [PMC free article] [PubMed] [Google Scholar]
- Reed JA, Albino AP, McNutt NS. Human cutaneous mast cells express basic fibroblast growth factor. Lab Invest. 1995;72:215–222. [PubMed] [Google Scholar]
- Sagi-Eisenberg R. Immunopharmacology of mast cells and basophils. In: Immunopharmacology of Mast Cells and Basophils. J.C. Foreman, New York: Academic Press; 1993. Signal-transmission pathways in mast cell exocytosis; pp. 71–88. [Google Scholar]
- Shima DT, Adamis AP, Ferrara N, Yeo K-T, Yeo TK, Allende R, Folkman J, D’Amore A. Hypoxic induction of endothelial cell growth factors in retinal cells: identification and characterization of vascular endothelial growth factor (VEGF) as the mitogen. Mol Med. 1995;1:182–193. [PMC free article] [PubMed] [Google Scholar]
- Starkey JR, Crowle PK, Taubenberger S. Mast-cell-deficient W/W mice exhibit a decreased rate of tumor angiogenesis. Int J Cancer. 1988;42:48–52. doi: 10.1002/ijc.2910420110. [DOI] [PubMed] [Google Scholar]
- Stevens RL, Fox CC, Lichtenstein LM, Austen KF. Identification of chondroitin sulfate E proteoglycans and heparin proteoglycans in the secretory granules of human lung mast cells. Proc Natl Acad Sci USA. 1988;85:2284–2287. doi: 10.1073/pnas.85.7.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Polverini PJ, Arenberg DA, Walz A, Opdenakker G, van Damme J, Kunkel SL. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J Leukocyte Biol. 1995;57:752–762. doi: 10.1002/jlb.57.5.752. [DOI] [PubMed] [Google Scholar]
- Tischer E, Mitchell R, Hartmann T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- Tsai J-C, Goldman CK, Gillespie GY. Vascular endothelial growth factor in human glioma cell lines: induced secretion by EGF, PDGF-BB and bFGF. J Neurosurg. 1995;82:864–873. doi: 10.3171/jns.1995.82.5.0864. [DOI] [PubMed] [Google Scholar]
- Walsh LJ, Trinchiery G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factor a, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci USA. 1991;88:4220–4224. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindel K, Marmé D, Weich HA. AIDS-associated Kaposi’s sarcoma cells in culture express vascular endothelial growth factor. Biochem Biophys Res Commun. 1992;183:1167–1174. doi: 10.1016/s0006-291x(05)80313-4. [DOI] [PubMed] [Google Scholar]
- White FC, Carroll SM, Kamps MP. VEGF mRNA is reversibly stabilized by hypoxia and persistently stabilized in VEGF-overexpressing human tumor cell lines. Growth Factors. 1995;12:289–301. doi: 10.3109/08977199509028967. [DOI] [PubMed] [Google Scholar]
- Wilson DJ. Mast cells are present during angiogenesis in the chick extraembryonic vascular system. Experientia. 1985;41:9–11. doi: 10.1007/BF02002631. [DOI] [PubMed] [Google Scholar]
- Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Yanagishita M, Hascall VC. Cell surface heparan sulfate proteoglycans. J Biol Chem. 1992;267:9451–9454. [PubMed] [Google Scholar]